Class I HDACis undergoing clinical trials [23]
| ZBG class | Name | Structure | Clinical trial phase | Indications |
|---|---|---|---|---|
| Benzamide | Entinostat | 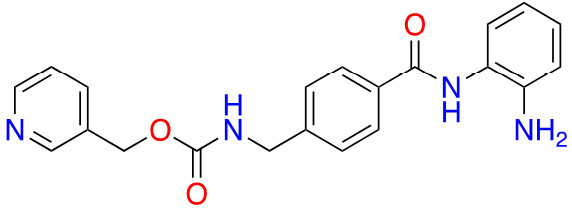 | 2 | Breast cancer [24] and elanoma [25] |
| RDN-929 | 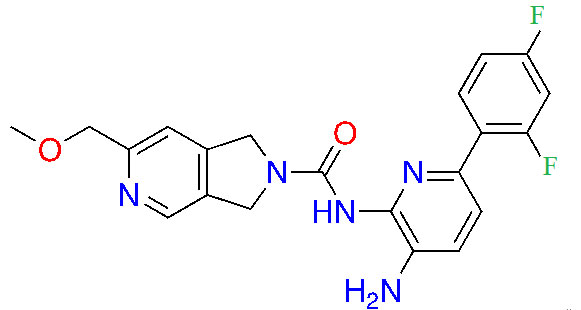 | 1 | Healthy patients [26] | |
| Mocetinostat | 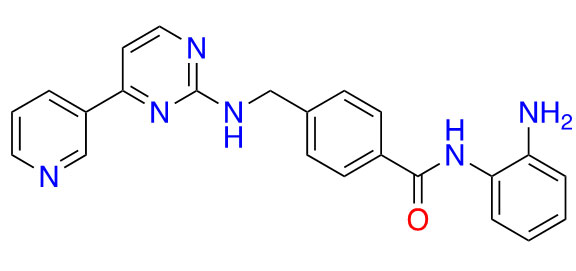 | 1 | Lymphoma [27] and lung cancer [28] | |
| Hydroxamic acid | Pracinostat | 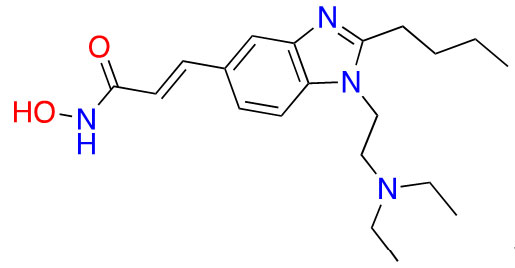 | 2 | AML [29] |
| Resminostat | 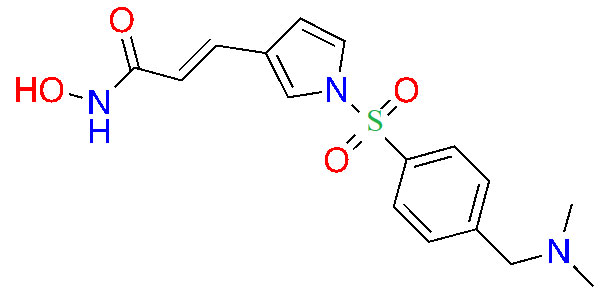 | 1/2 | Colorectal CTCL [30] | |
| Givinostat | 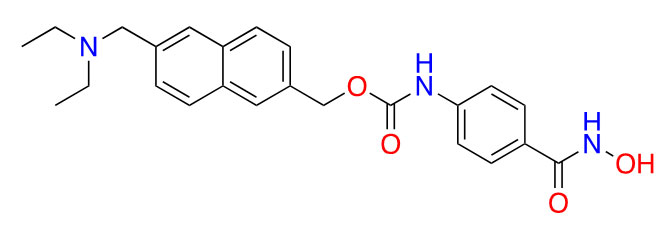 | 2 | Polycythemia vera [31], DMD [32], and arthritis [33, 34] | |
| Remetinostat | 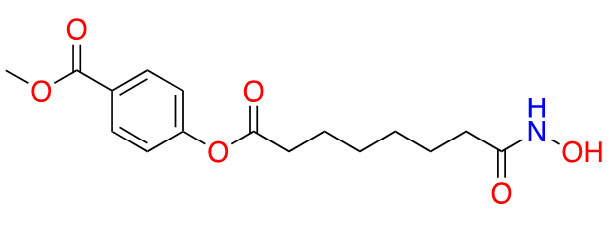 | 2 | CTCL [35] | |
| Thiol | OKI-179 | Structure undisclosed | 1 | Solid tumors [36] |
AML: acute myeloid leukemia; CTCL: cutaneous T-cell lymphoma; DMD: duchenne muscular dystrophy