Summary of mentioned lipopeptide antibiotic adjuvants
| Structure | Name (abbreviation, number in text) | Source | Application |
|---|---|---|---|
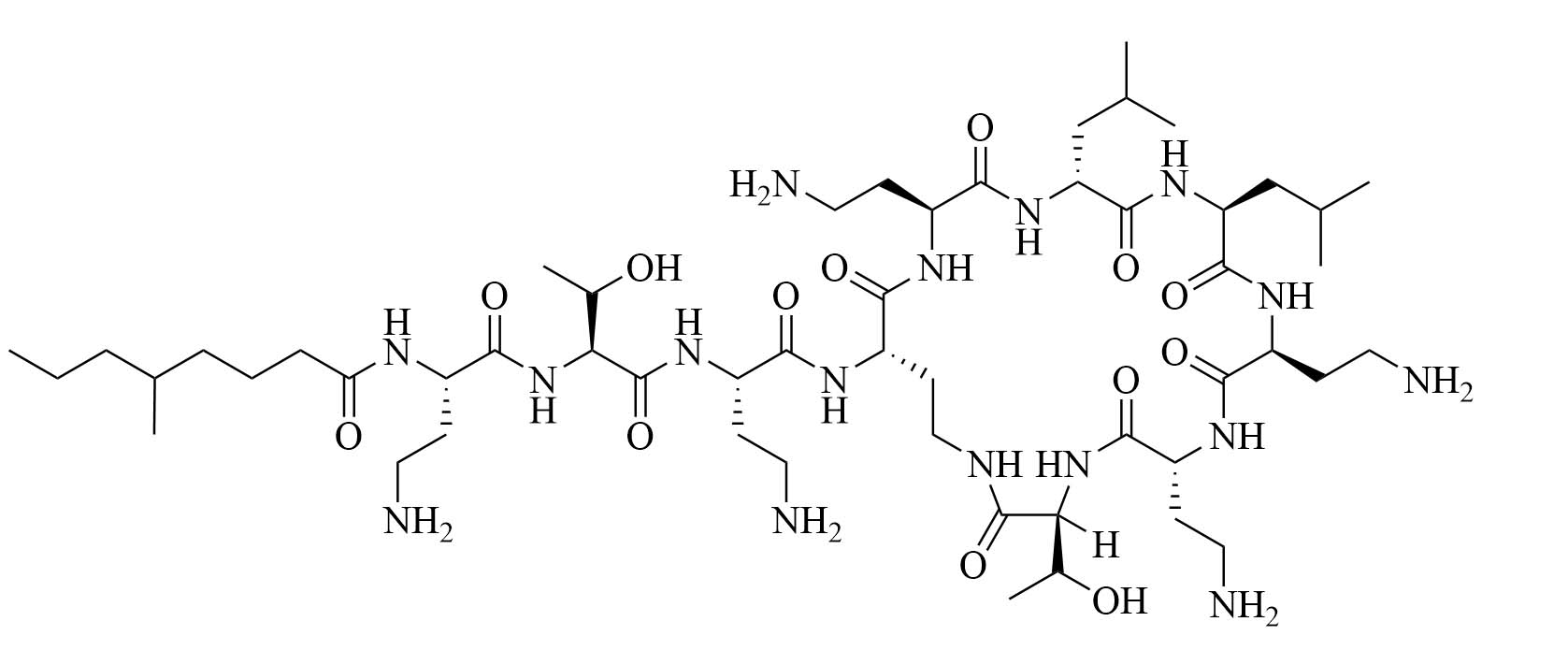 | Colistin | Paenibacillus polymyxa subspecies colistinus [31] | Last resort antibiotic for GNB infections [31, 32] |
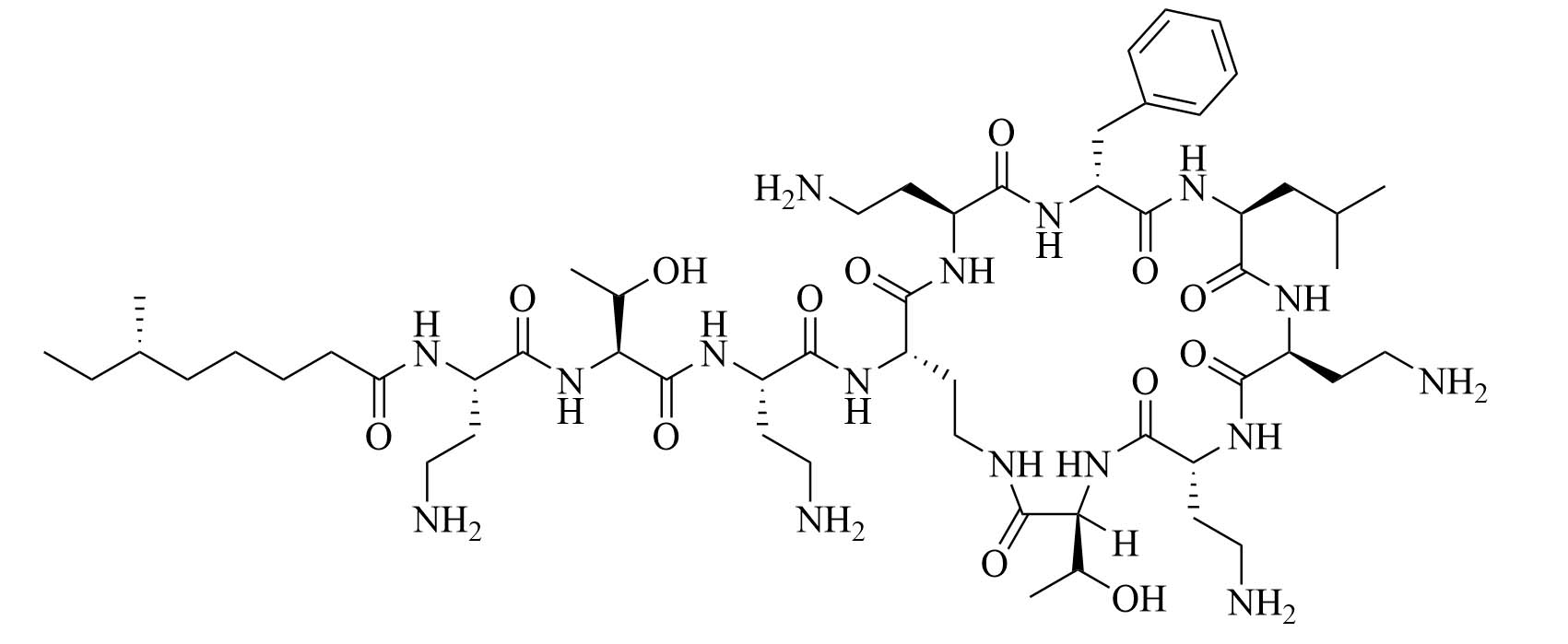 | Polymyxin B | Paenibacillus polymyxa subspecies colistinus [33] | Last resort antibiotic for GNB infections [33] |
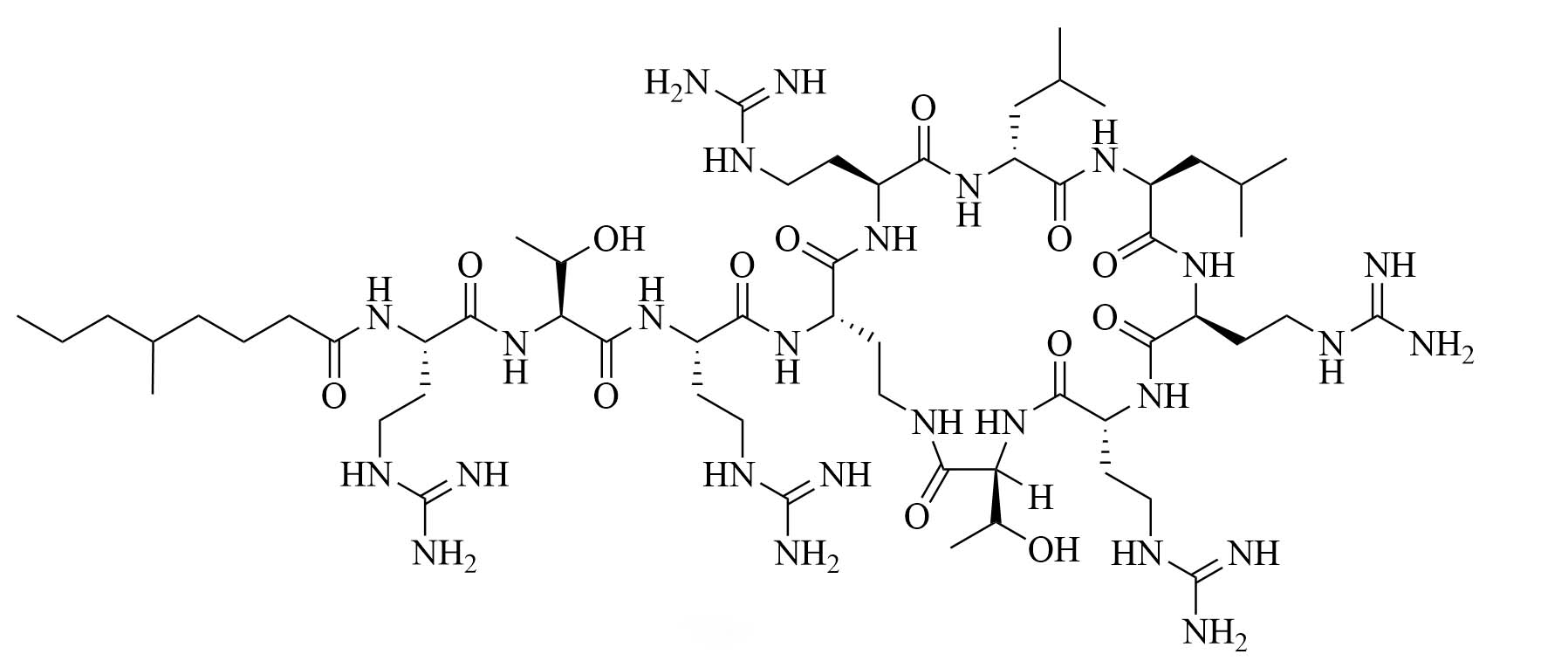 | Guanidinylated colistin (GCol) | Modification of colistin [34] | Antibiotic adjuvant, synergy with rifampicin, novobiocin, and erythromycin against Escherichia coli (E. coli), Acinetobacter baumannii (A. baumannii), and P. aeruginosa [34] |
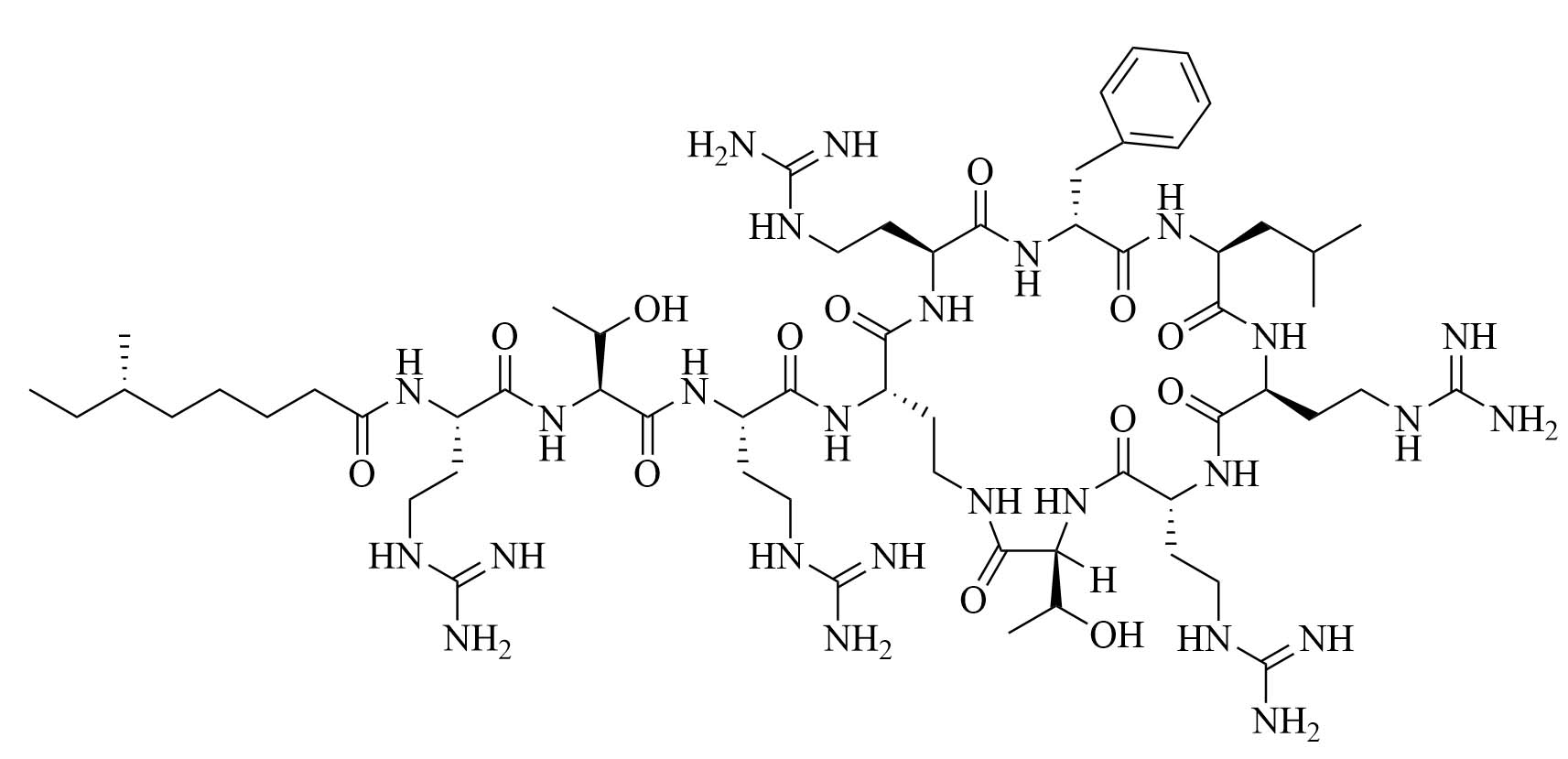 | GCol polymyxin B (GPMB) | Modification of polymyxin B [34] | Antibiotic adjuvant, synergy with vancomycin, clindamycin, and linezolid against E. coli, A. baumannii, and P. aeruginosa [34] |
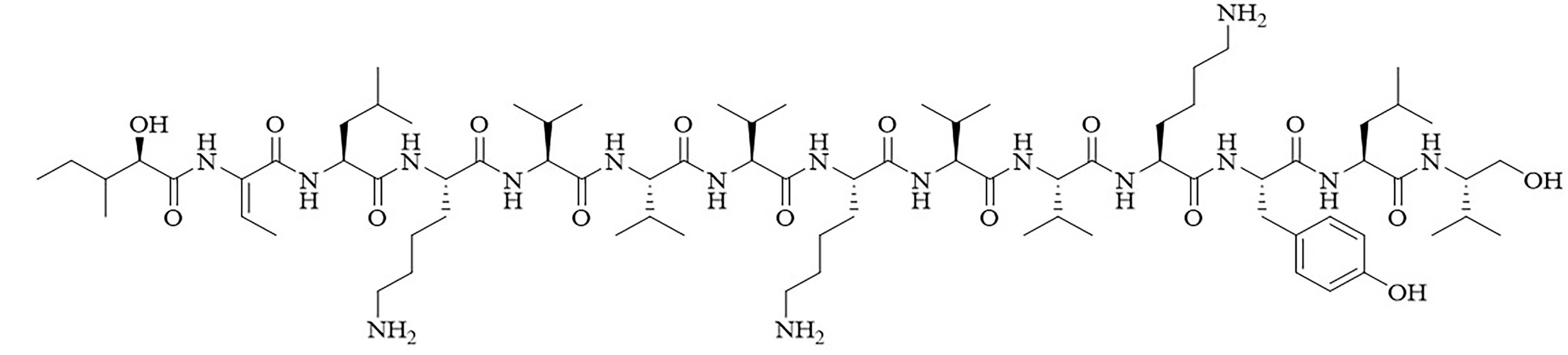 | Brevibacillin 2V | Brevibacillus laterosporus [35, 36] | Antibiotic adjuvant, synergy with nalidixic acid, azithromycin, rifampicin, and amikacin against E. coli, Klebsiella pneumoniae (K. pneumoniae), P. aeruginosa, and A. baumannii [35, 36] |
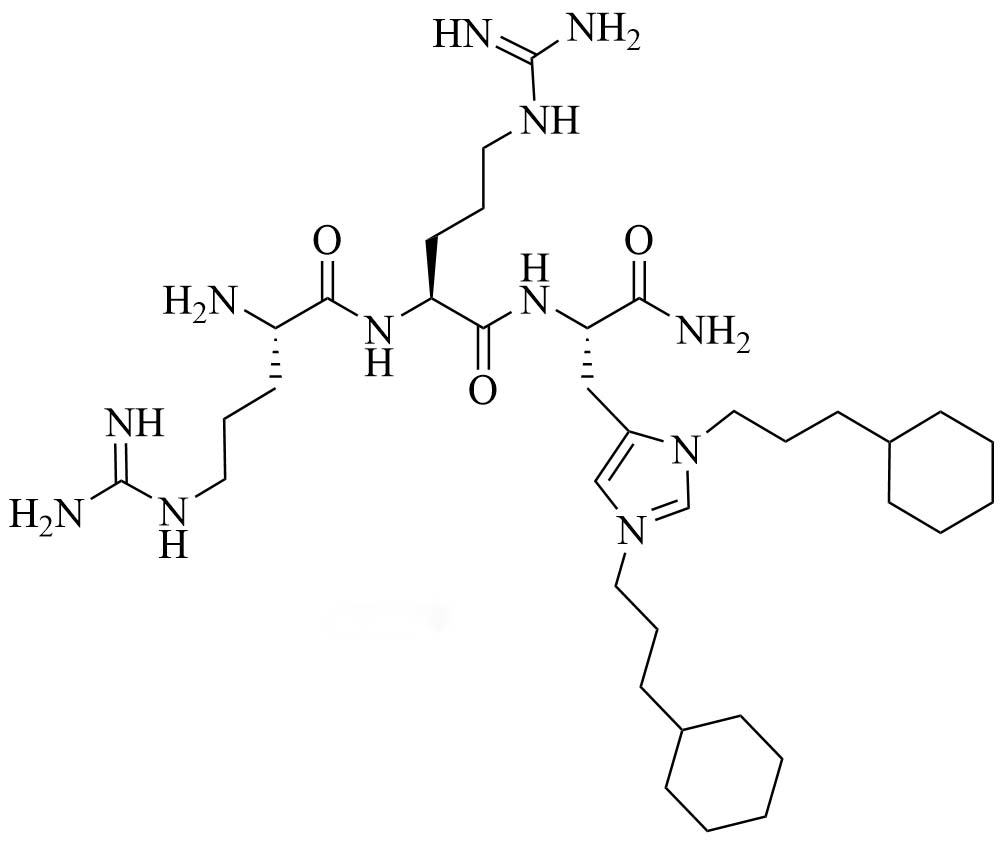 | Short antimicrobial peptidomimetic 5 (SAMP-5) | Peptidomimetics | Antibiotic with activity against E. coli and P. aeruginosa [37]; antibiotic adjuvant, synergy with ciprofloxacin, oxacillin, and chloramphenicol against P. aeruginosa [38]; antibiotic adjuvant, synergy with ciprofloxacin and oxacillin against E. coli [38] |
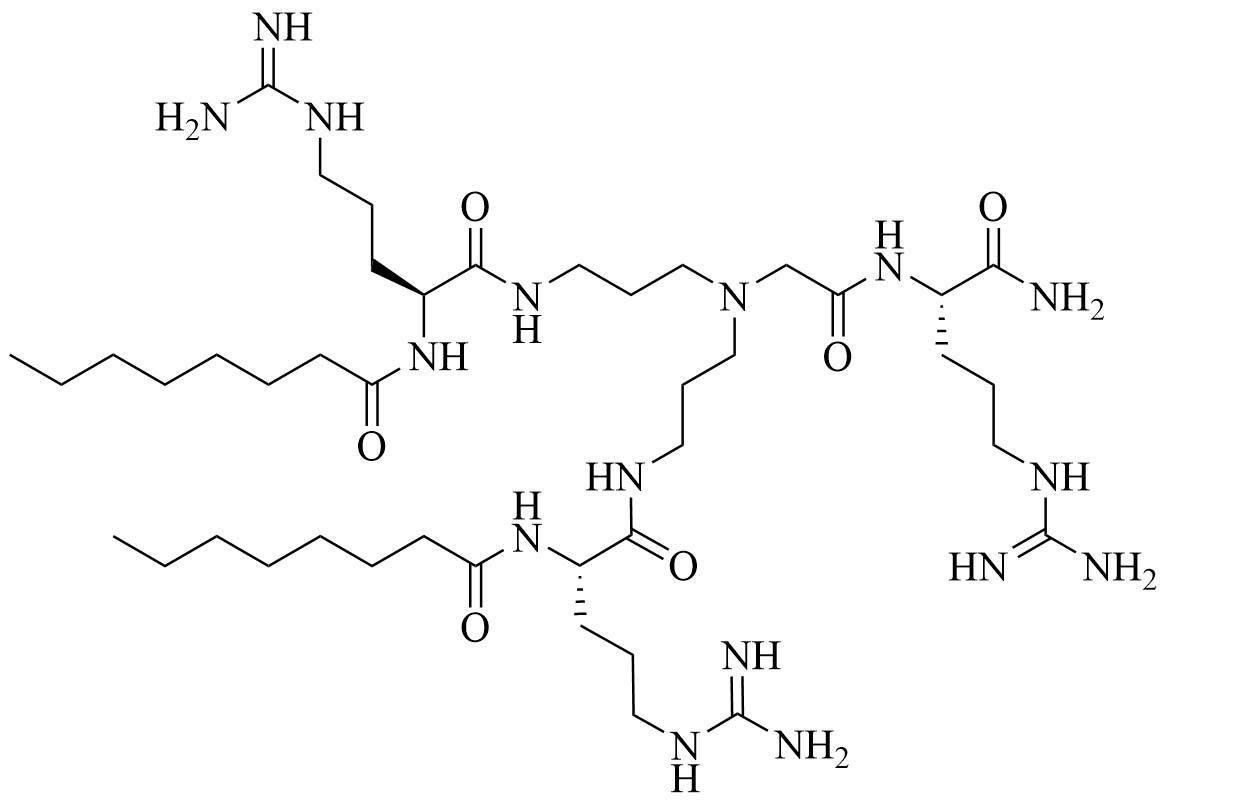 | Dilipid ultrashort tetrabasic peptidomimetic (dUSTBP) di(C8-Arg)-Nbap-Arg-NH2 (8) | Peptidomimetics [39] | Antibiotic adjuvant, synergy with novobiocin and rifampicin against P. aeruginosa, A. baumannii, and Enterobacteriaceae [39] |
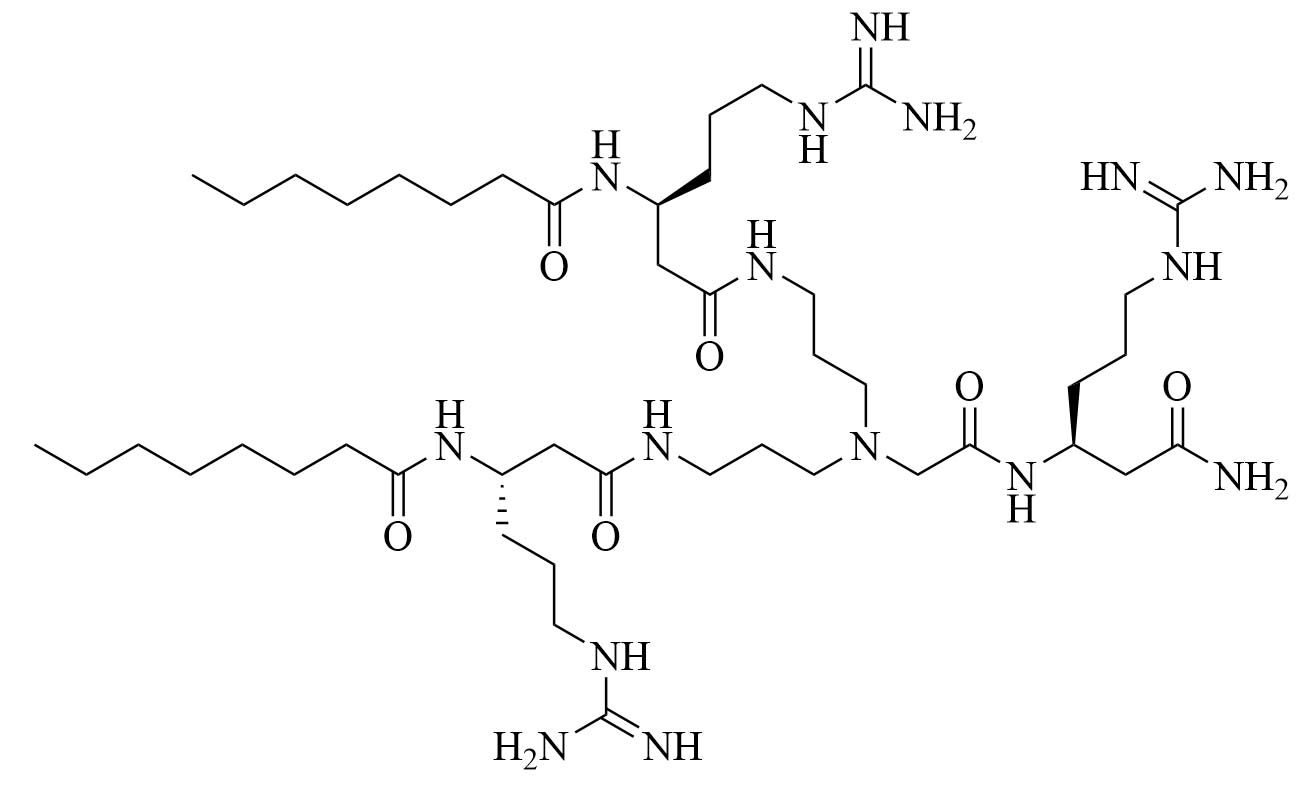 | dUSTBβP 3 | Modification of 8 to incorporate beta-amino acid (β-AA) instead of α-AA [40] | Antibiotic adjuvant, synergy with novobiocin and rifampicin against P. aeruginosa, A. baumannii, K. pneumoniae, and Enterobacter cloacae (E. cloacae) [40]; increased resistance against proteolysis but slower bactericidal activity than 8 |
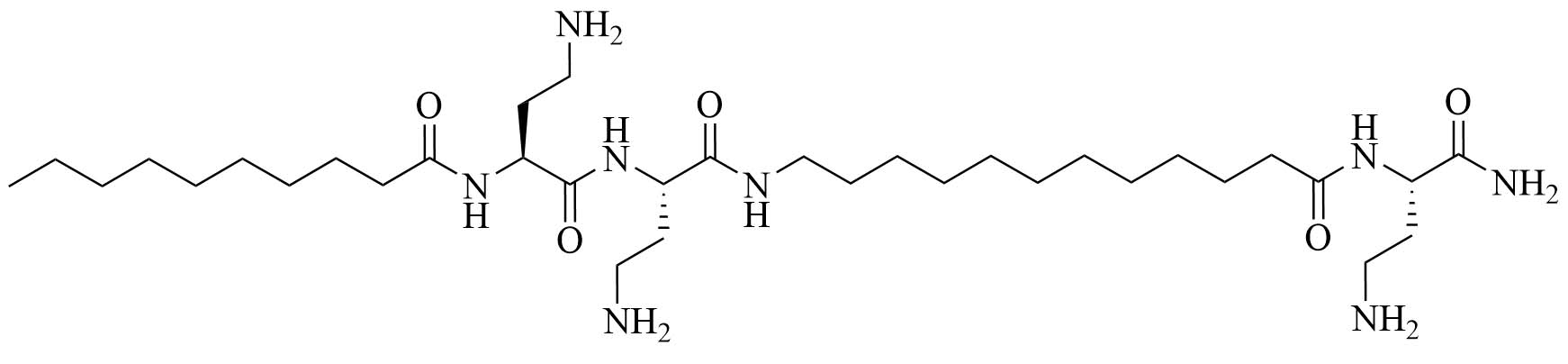 | C10BBc12B | Peptidomimetics | Antibiotic adjuvant, synergy with rifampin against P. aeruginosa, A. baumannii, K. pneumoniae, and E. coli; synergy with erythromycin against E. coli and K. pneumoniae; additive effect with erythromycin against P. aeruginosa and A. baumannii [41, 42] |
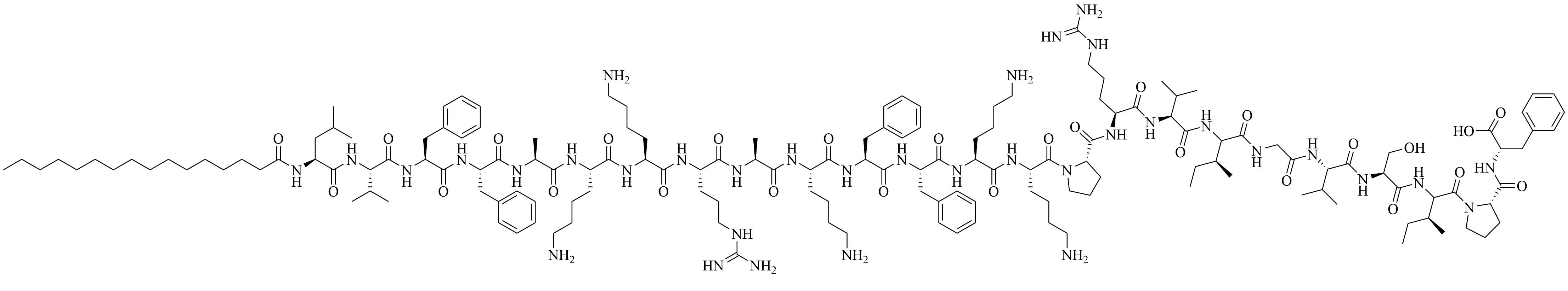 | Lipo S | N/A | Used together: antibiotic adjuvant, encapsulation of ciprofloxacin with synergistic effects against K. pneumoniae [43]; Lipo 20 alone: antibiotic activity against K. pneumoniae |
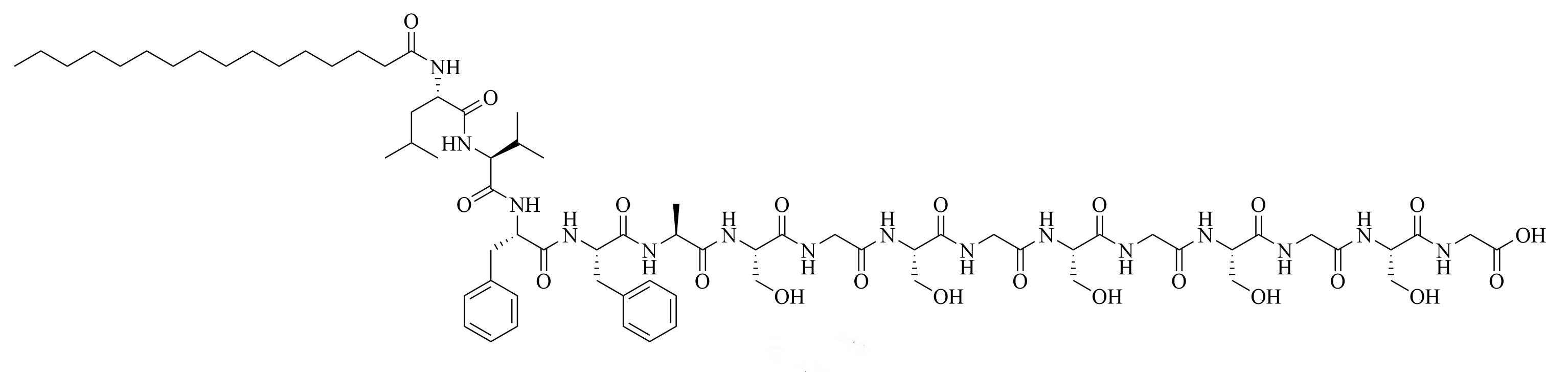 | Lipo 20 |
N/A: not applicable