Summary of mentioned lipopeptide vaccine adjuvants
| Structure | Name (abbreviation) | Source | Application | |
|---|---|---|---|---|
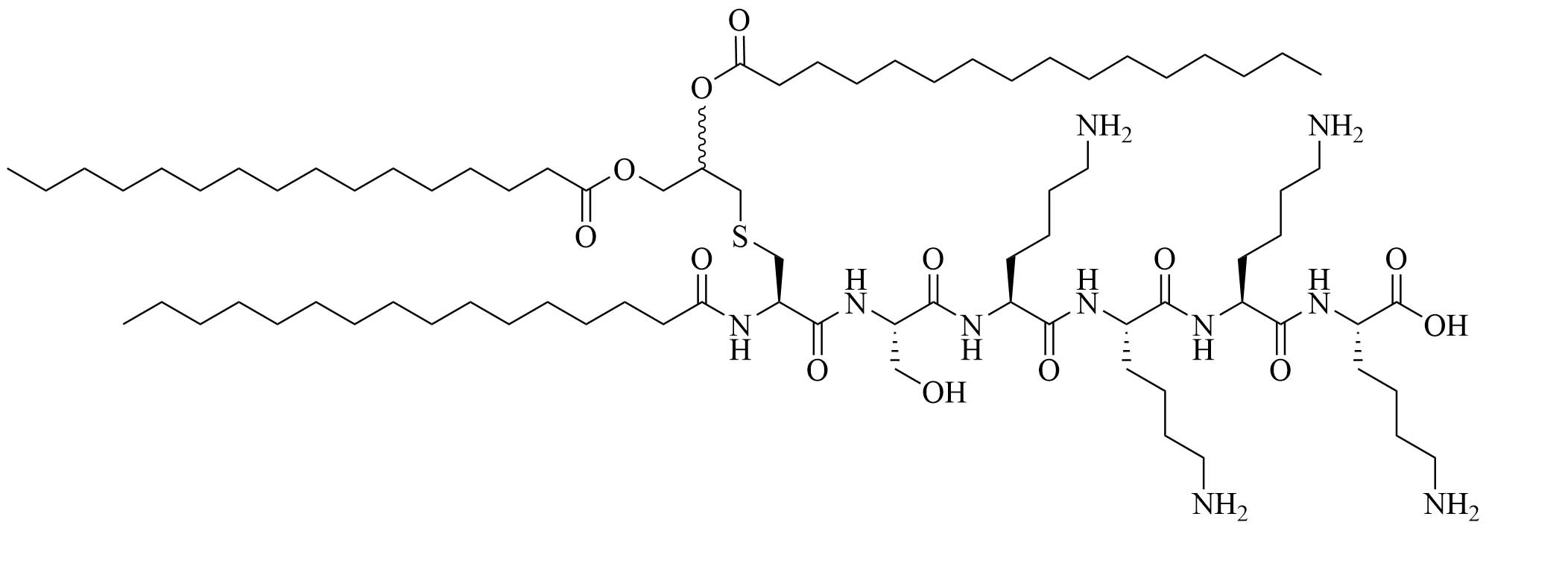 | Pam3CSK4 | Modification of MALP-2 (Mycoplasma fermentas) | Vaccine adjuvant, TLR1/TLR2 heterodimer agonist; tumor cytotoxicity [52]; used in various vaccines [53–60] | |
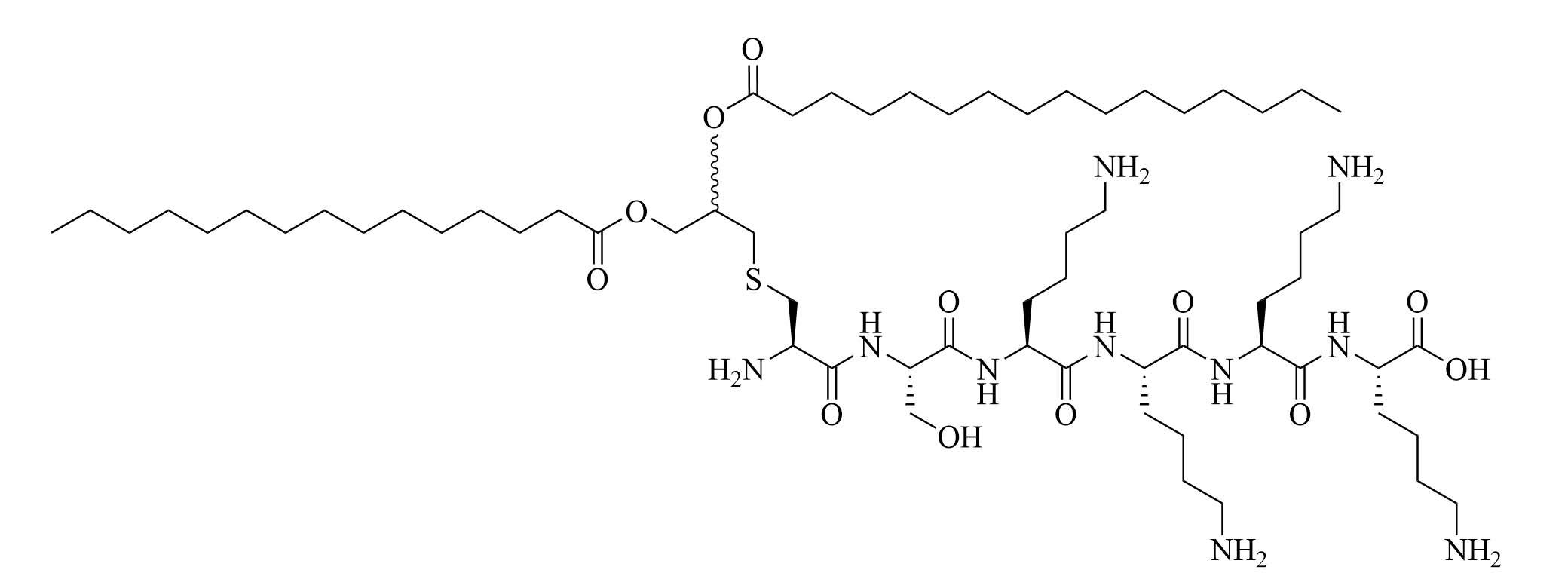 | Pam2CSK4 | Modification of MALP-2 (Mycoplasma fermentas) | Vaccine adjuvant, TLR2/TLR6 heterodimer agonist; used in various vaccines [53, 58, 61–63]; clinical trials: phase 1/2a in PUL-042, a prophylactic and therapeutic drug used against bacterial and viral pulmonary infections [64] | |
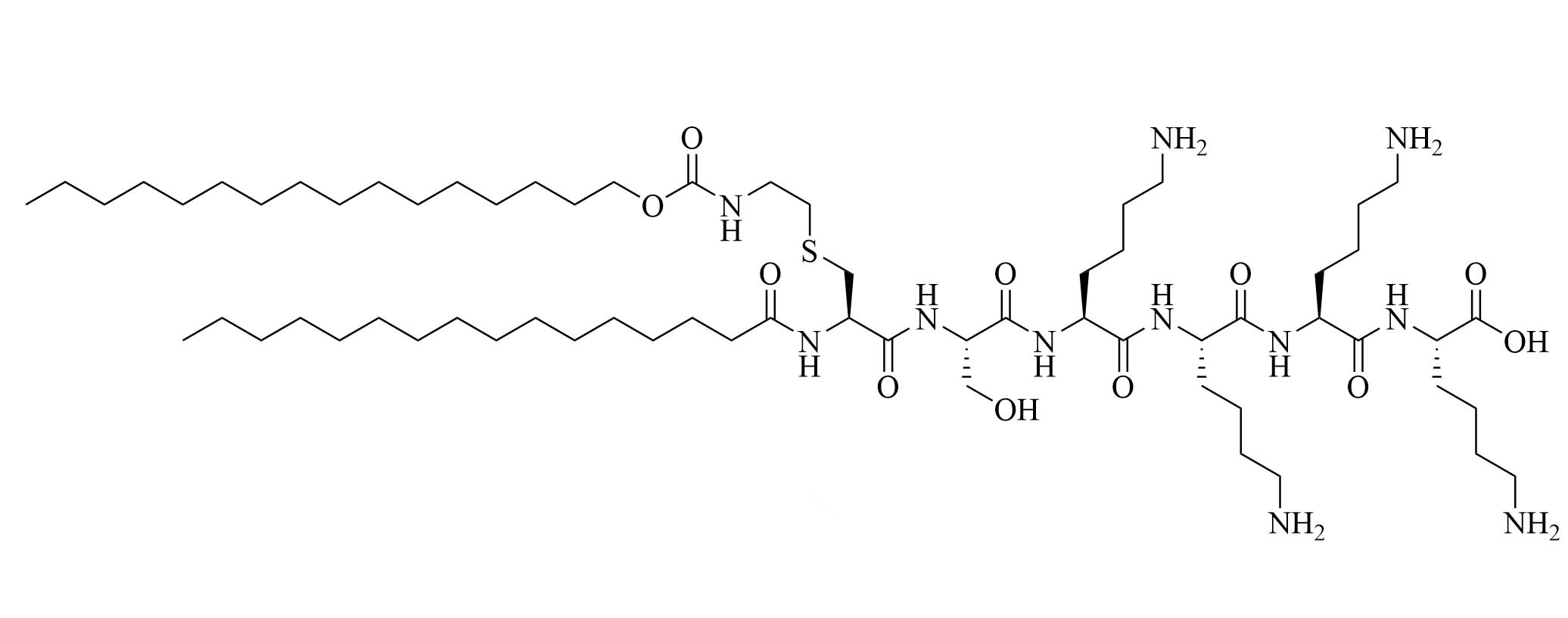 | 35d | Modifications of Pam2CSK4 | TLR2 agonist [65] | |
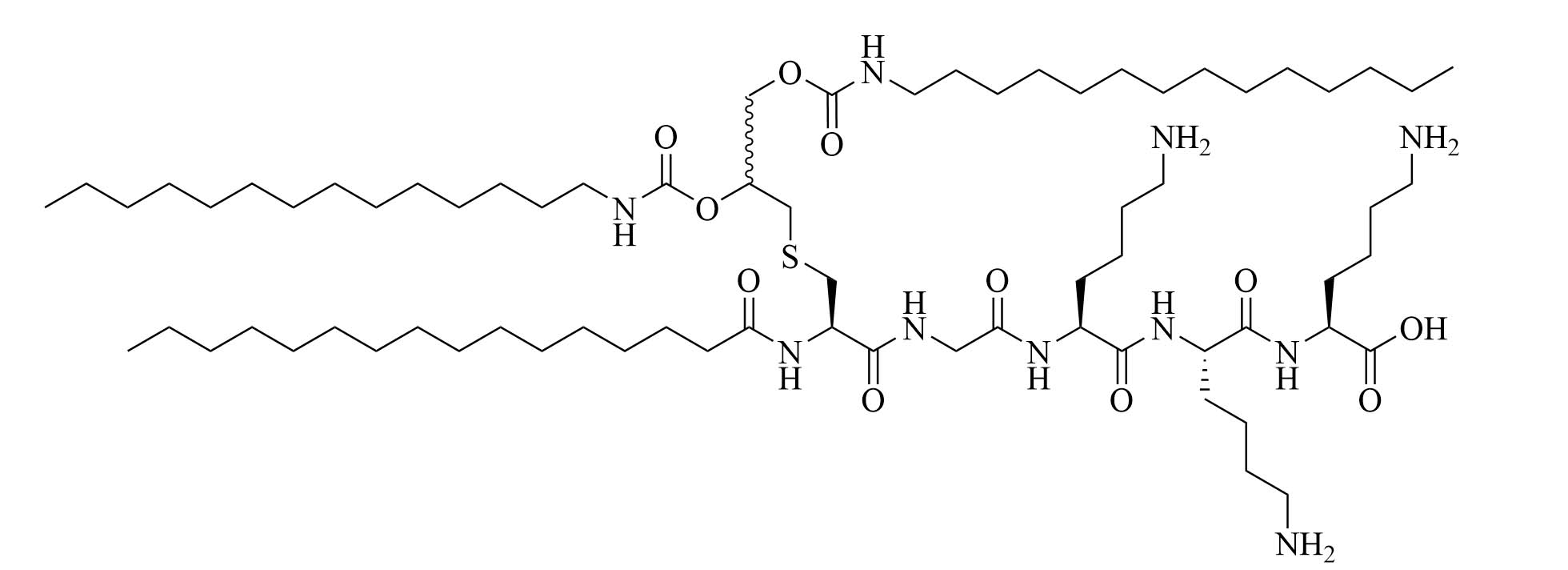 | (2S,5S,8S,14R,18R)-2,5,8-tris(4-aminobutyl)-4,7,10,13,21-pentaoxo-14-palmitamido-18-[(tetradecylcarbamoyl)oxy]-20-oxa-16-thia-3,6,9,12,22-pentaazahexatriacontan-1-oic acid (SUP3) | Modifications of Pam3CSK4 | Vaccine adjuvant (tumor model); TLR2 agonist [66] | |
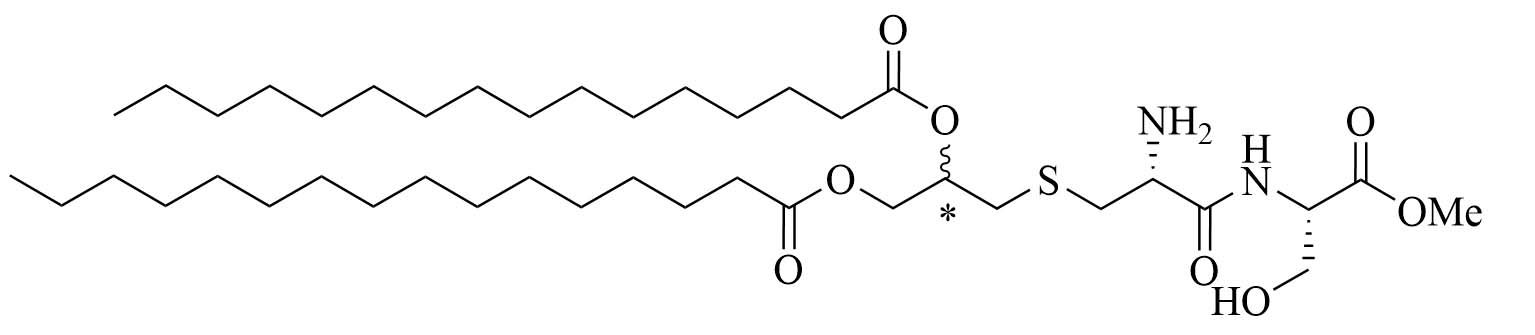 | Pam2CS(OMe)R&S: 8aR: 8bS: 8c | Structure optimization of Pam2CSK4/Pam3CSK4 [67, 68] | Vaccine adjuvant (influenza) [68]; TLR2/6 agonist | |
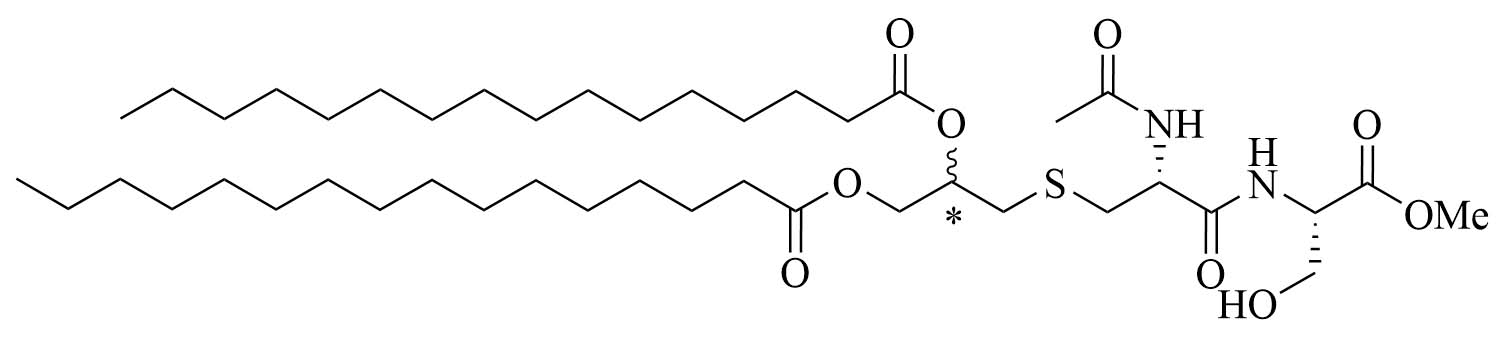 | NAc-Pam2CS(OMe)R&S: 9aR: 9bS: 9c | TLR2/6 agonist | ||
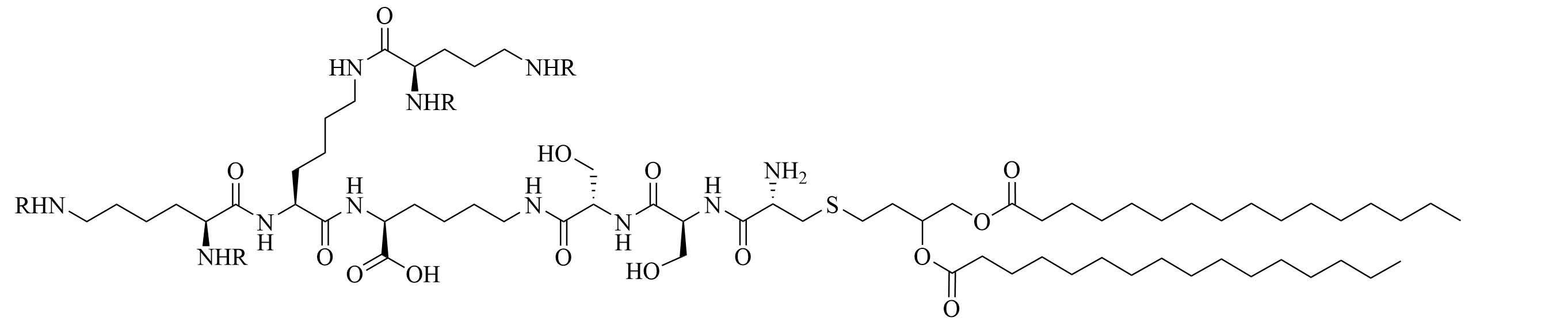 | R4Pam2CysB | Structure optimization of Pam2CSK4/Pam3CSK4 [69]R: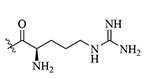 | Vaccine adjuvant [ovalbumin (OVA)]; TLR2 agonist [69] | |
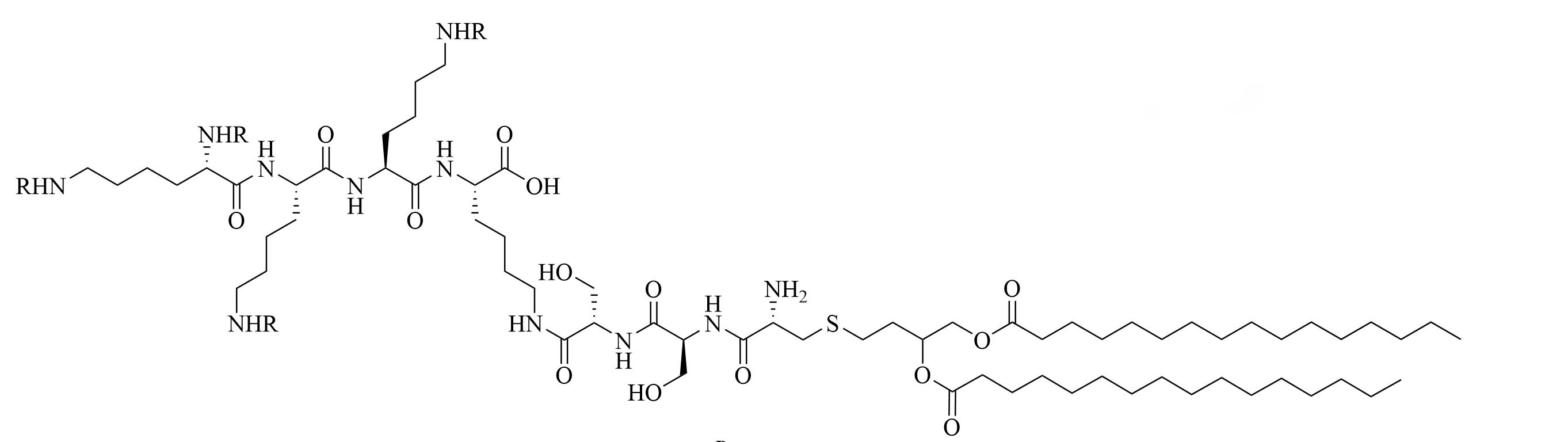 | R4Pam2CysP | |||
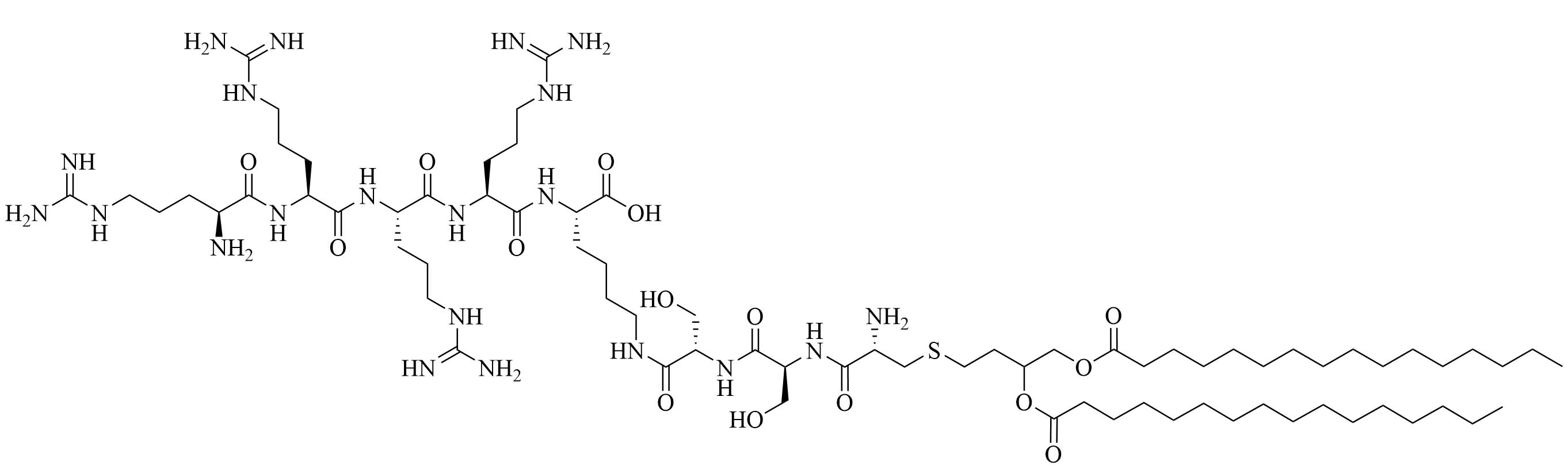 | R4Pam2CysL | |||
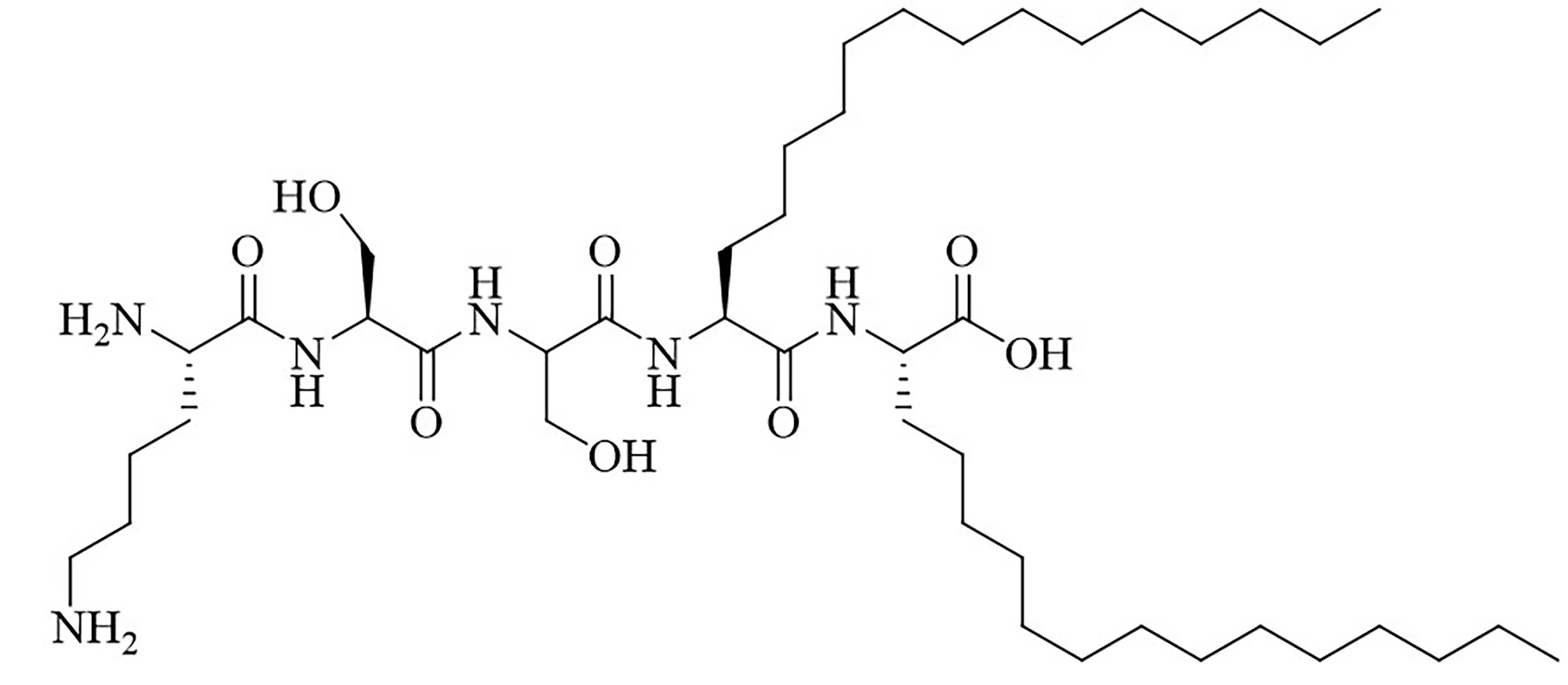 | Lipid core peptide (LCP) | N/A | TLR2, self-adjuvanting antigen carrier used against group A Streptococcus (GAS) infections [70–72] | |
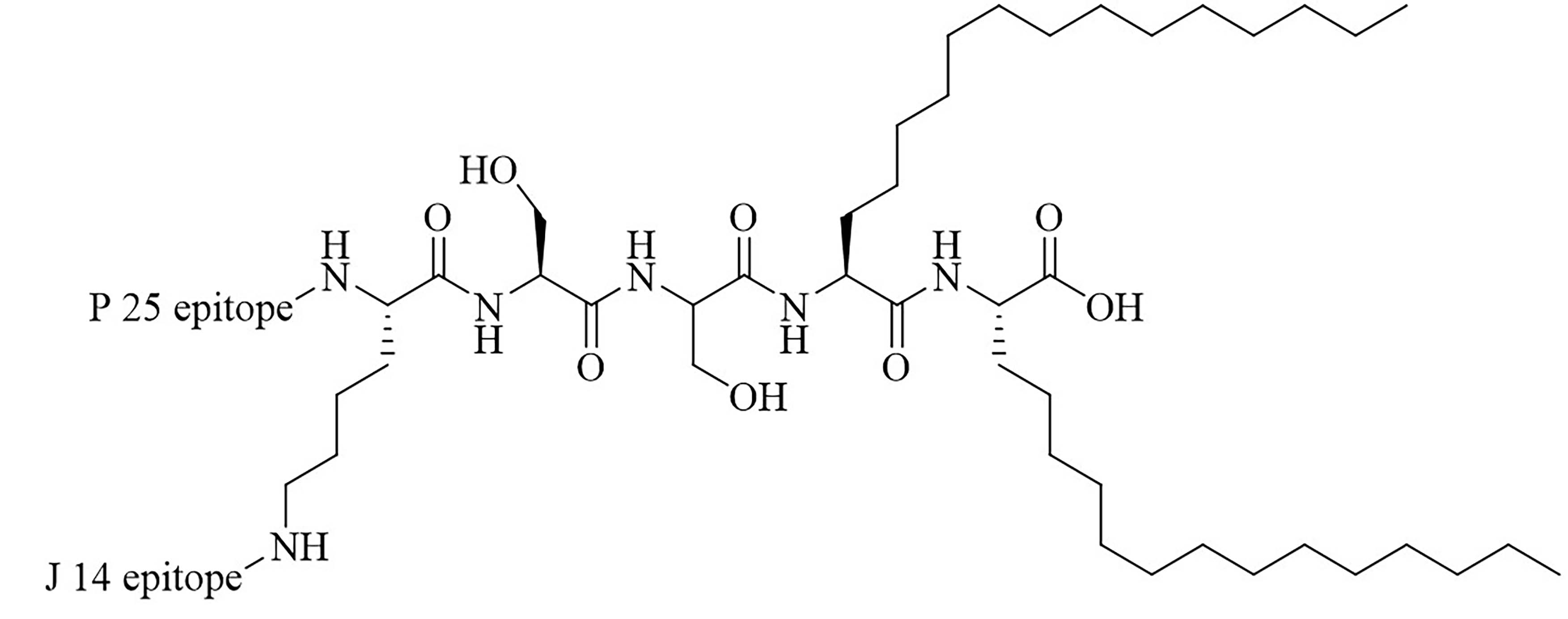 | L1 | Conjugation of LCP to universal Th cell epitope P25 and GAS epitope J14 | Self-adjuvanting vaccine [70, 71] | |
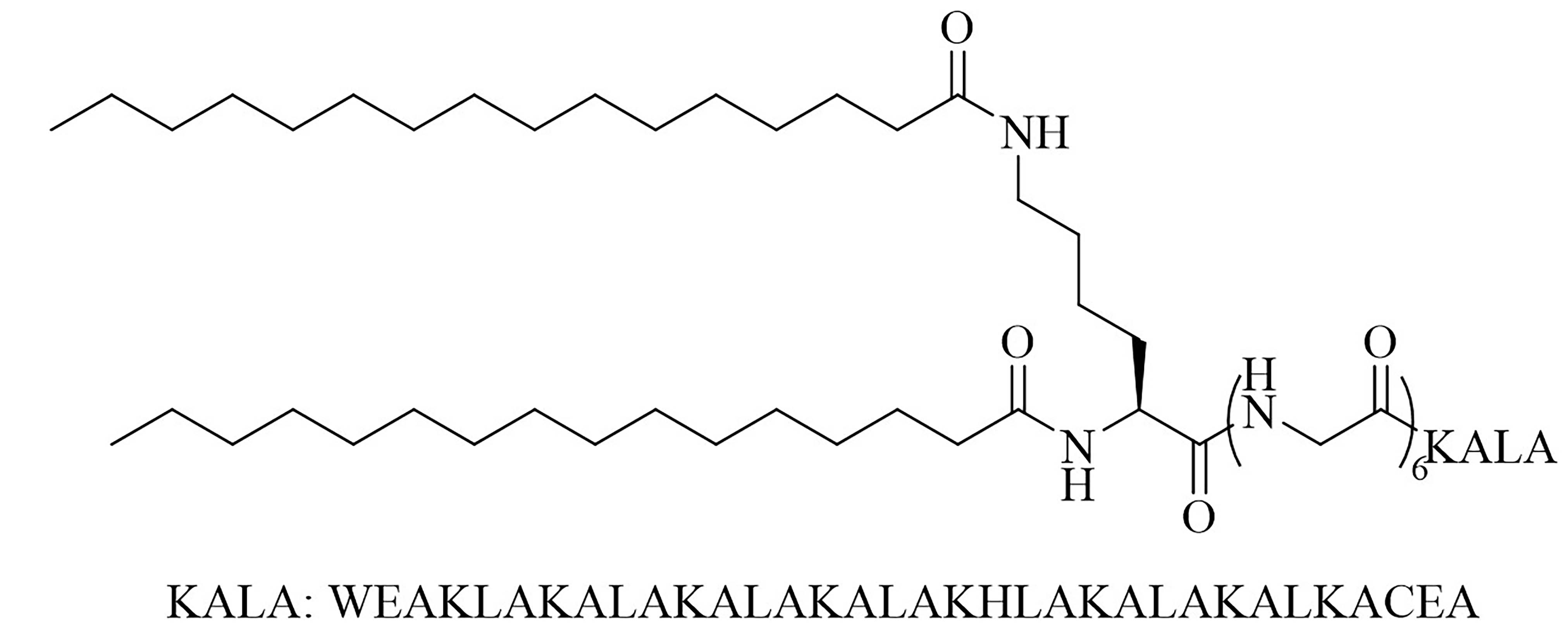 | lipoKALA | N/A | Vaccine adjuvant used in combination with L1 to obtain J8-specific opsonizing antibodies upon vaccination [72, 73] | |
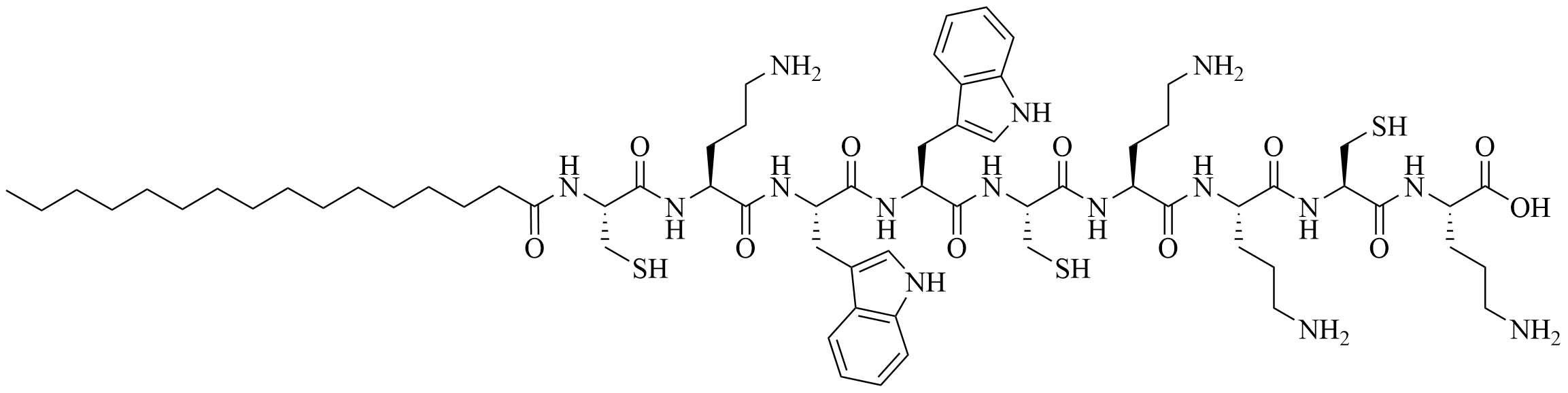 | AG2-C16 | N/A | Self-adjuvanting vaccine carrier [bovine serum albumin (BSA)] [74] | |
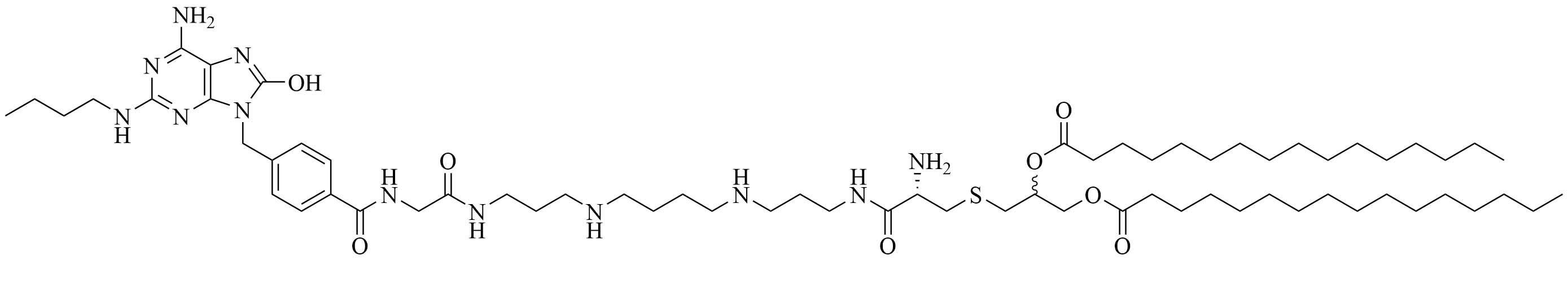 | PamadiFectin | Modification of Pam2CSK4 | TLR2/TLR7 agonist [75] | |
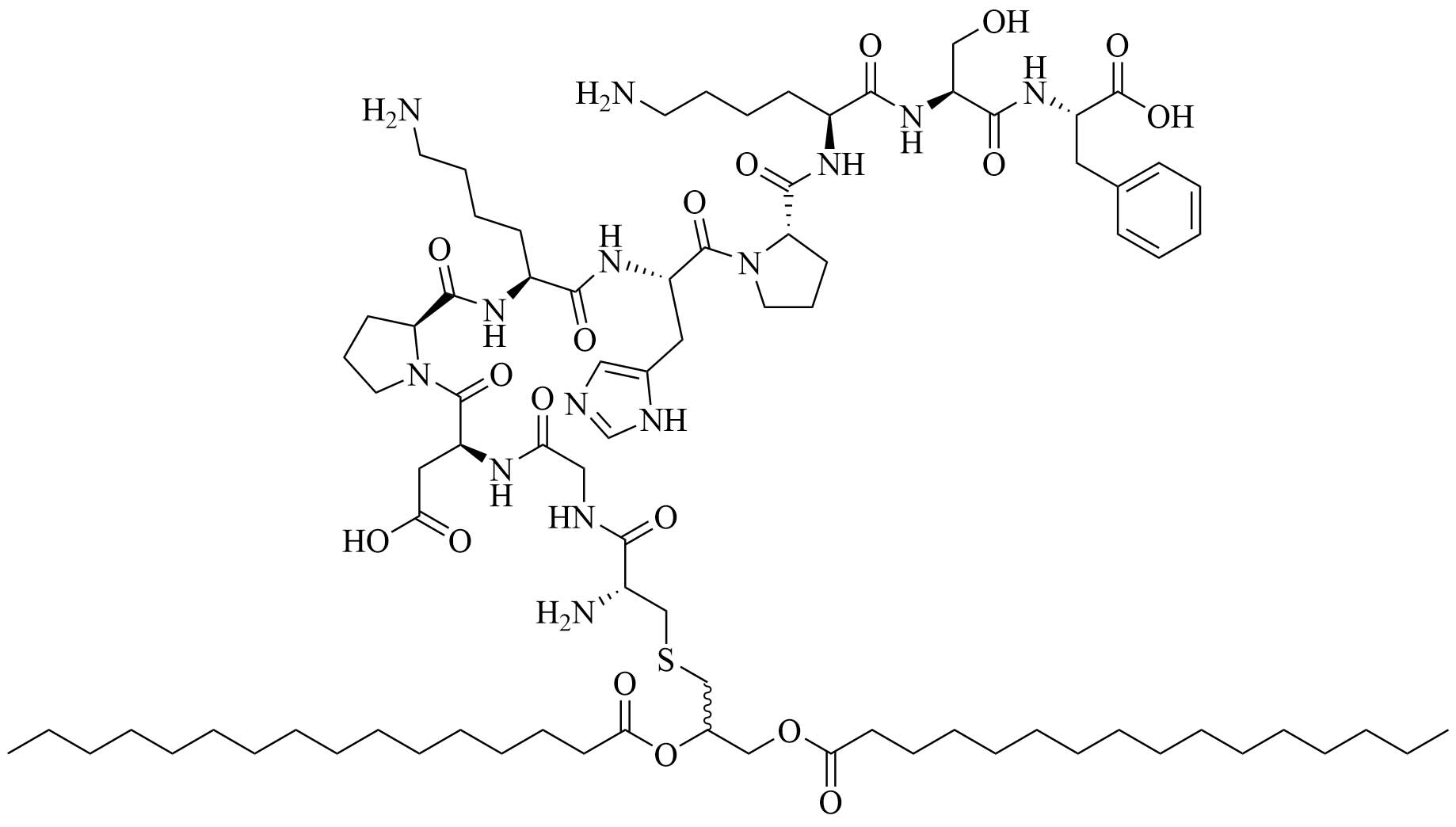 | Fibroblast stimulating lipopeptide 1 (FSL-1) | Streptococcus pyogenes (S. pyogenes) | TLR2 agonist, vaccine adjuvant used against GAS [76] | |
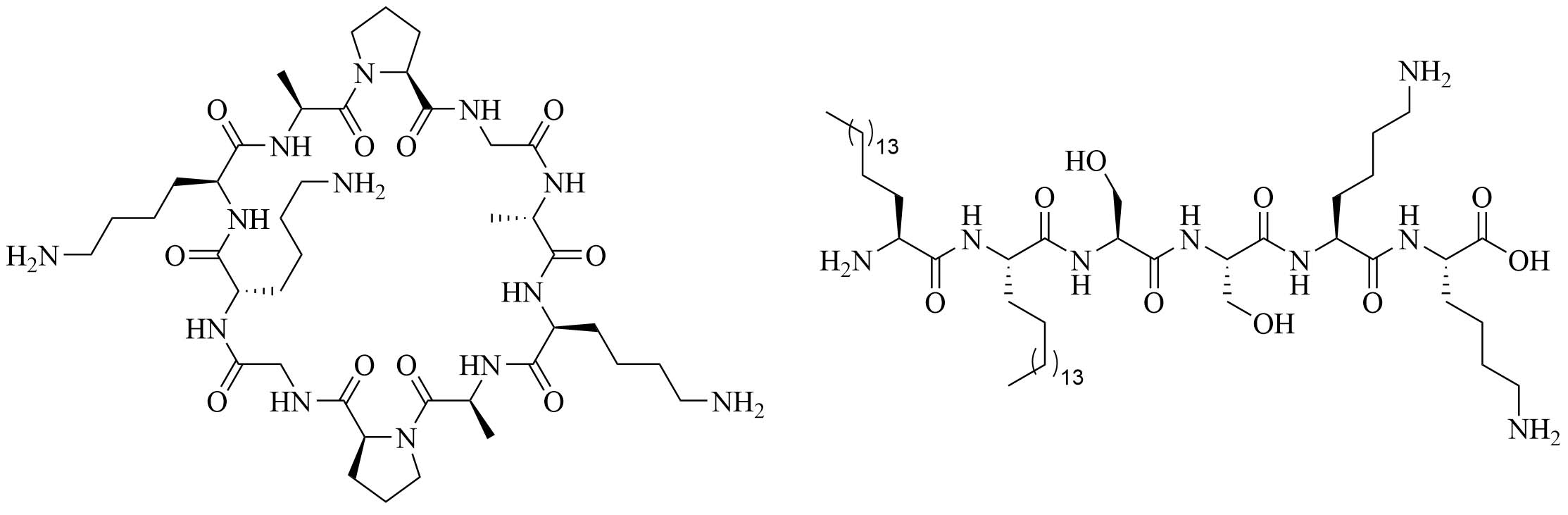 | Cyclic decapeptide carrier (CDC) and lipopeptide adjuvant KKSS-C16-C16-NH2 | N/A | Vaccine adjuvant system used against GAS [77–79] | |
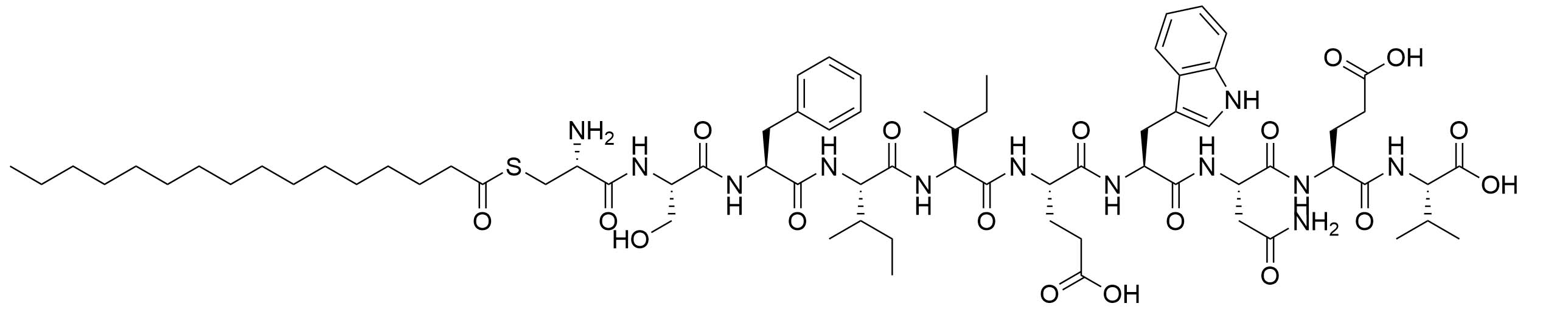 | LP1 | Helicobacter pylori (H. pylori) adhesin A | TLR2 agonist, vaccine adjuvant against H. pylori [80, 81] | |
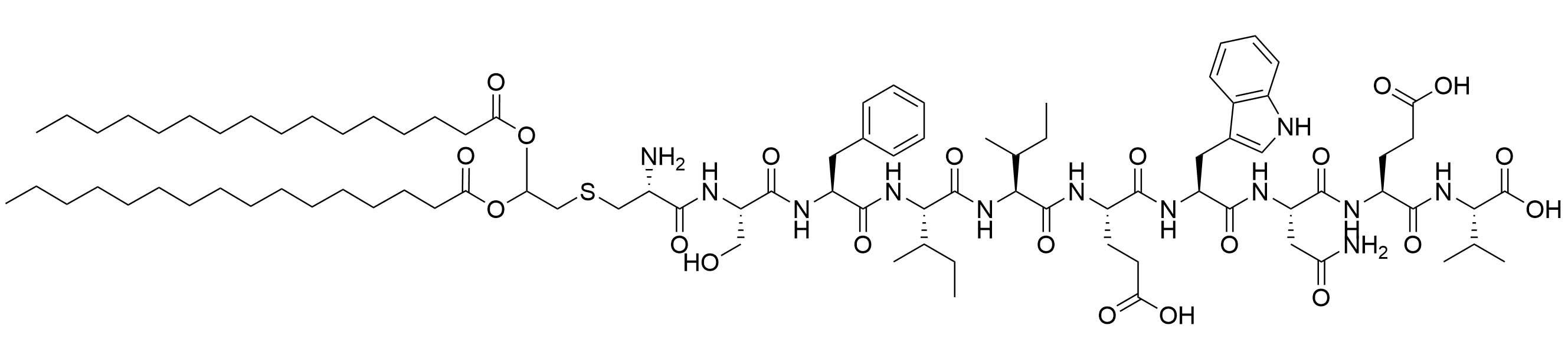 | LP2 | |||