Affiliation:
Immunoallergology Department, Centro Hospitalar Universitário Lisboa Norte, 1649-035 Lisbon, Portugal
Email: inescerca92@gmail.com
ORCID: https://orcid.org/0000-0002-8041-1306
Affiliation:
Immunoallergology Department, Centro Hospitalar Universitário Lisboa Norte, 1649-035 Lisbon, Portugal
ORCID: https://orcid.org/0000-0002-2568-3333
Affiliation:
Immunoallergology Department, Centro Hospitalar Universitário Lisboa Norte, 1649-035 Lisbon, Portugal
ORCID: https://orcid.org/0000-0002-0323-9936
Affiliation:
Immunoallergology Department, Centro Hospitalar Universitário Lisboa Norte, 1649-035 Lisbon, Portugal
ORCID: https://orcid.org/0000-0002-1242-3524
Affiliation:
Immunoallergology Department, Centro Hospitalar Universitário Lisboa Norte, 1649-035 Lisbon, Portugal
ORCID: https://orcid.org/0000-0001-8313-1505
Explor Asthma Allergy. 2023;1:142–152 DOI: https://doi.org/10.37349/eaa.2023.00015
Received: December 29, 2022 Accepted: March 15, 2023 Published: October 31, 2023
Academic Editor: Umit Murat Sahiner, Hacettepe University Faculty of Medicine, Turkey
Aim: Allergy to lipid transfer proteins (LTPs) clinically manifests from oral allergy syndrome (OAS) to anaphylaxis. The risk of systemic symptoms and cross-reactivity make it an important target for allergen immunotherapy. Sublingual immunotherapy (SLIT) with Pru p 3 is effective and safe, but the induction phase (IP) of standard protocol (SP) is time consuming. Rush protocols (RPs) are described without serious adverse effects. The aim was to compare the safety of RP with SP and assess the existence of predictive factors for adverse reactions (ARs).
Methods: Retrospective study of patients with LTP syndrome followed at the Food Allergy Unit undergoing SLIT with Pru p 3 between 2012 and 2021. SP has an IP of 4 days and an RP of 2 days. The safety of the IP was assessed by recording the AR.
Results: Fifty-one patients: 41 (73.2% women) in SP group (SPG) and 10 (80% women) in RP group (RPG). Anaphylaxis as a presentation of LTP syndrome was overlapping in both groups (SPG 34.1%, RPG 33.3%). There were 5 (12.2%) ARs in SPG: 3 (60%) OAS, 1 (20%) oropharyngeal tightness, and 1 (20%) uvula edema; and 5 (50%) ARs in RPG: 4 (80%) OAS and 1 (20%) palmar pruritus and cough. All patients completed IP. Mean Pru p 3 specific immunoglobulin E (sIgE) value (kUA/L) of patients with ARs in IP: 6.7 kUA/L in SPG and 5.7 kUA/L in RPG. No group showed significant differences (P > 0.05) between Pru p 3 sIgE value, presence of atopy or greater severity in LTP syndrome presentation, and greater probability of AR/more severe ARs in IP.
Conclusions: ARs in IP were similar in both groups. No association was found between Pru p 3 value, atopy and higher probability of ARs in IP. RP appears to be a safe and less expensive option.
Food allergy affects more than 200 million people worldwide and its prevalence is increasing in high-income countries, mainly due to environmental and socioeconomic changes, dietary habits, and patterns of exposure to aeroallergens, especially pollens [1–6].
Lipid transfer proteins (LTPs) are panallergens present in fruits, vegetables, seeds, latex, tree and grass pollens, which are part of a superfamily of proteins, the prolamin family, containing more than 3,000 proteins. Their sensitization in the Mediterranean basin is quite frequent, mainly involving fresh fruits of the Rosaceae family (peach, apple), nuts (hazelnuts), and peanuts [1, 2, 5–14].
LTP allergy is the most common food allergy in adulthood in Italy, Spain, and Portugal, with Pru p 3 (peach LTP) being the most representative LTP (4.6–100%) of this group of allergens, probably related to its extensive production and consumption in these countries [1–4, 7, 8, 14]. Sensitization to LTPs can occur by oral ingestion, inhalation, or skin contact, and the possibility of cross-reactivity between different plant-based foods and between these and pollens is called LTP syndrome and is due to shared structural characteristics [2, 7–10, 15].
LTPs have a higher concentration in the fruits’ peel, whereas the pulp has a much lower concentration so that some patients tolerate the ingestion of these foods without the peel [3, 8, 9, 14].
Patients with LTP sensitization may be asymptomatic or have mild symptoms, such as contact urticaria and oral allergy syndrome (OAS), but frequently have systemic symptoms (25–80%), from respiratory, gastrointestinal, and cutaneous (urticaria/angioedema) to anaphylaxis, as these proteins are resistant to heat and pepsin, increasing the likelihood of systemic absorption [1, 2, 6–9, 12, 15].
There are not many studies on the economic costs of LTP allergy, and there are few on the economic costs of food allergy in general. In the USA, direct (use of emergency department, need to hospitalization, having an adrenaline auto-injector) and indirect costs (work/school absenteeism, labeling control, psychological health costs) are estimated at half a billion dollars each year. In France, a study reports a €2,000 cost for each episode of anaphylaxis related to food or medication [16].
Thus, this allergy is considered an important target for immunotherapy (IT), being the only therapeutic option that modifies the immunological mechanism underlying the allergic disease, seeking to induce desensitization and a state of tolerance for the allergens involved, allowing patients to have a better quality of life and reducing direct and indirect health costs [2, 7, 12, 17–24].
Fernández-Rivas et al. [18] used sublingual IT (SLIT) with peach peel extract enriched with Pru p 3 in 33 patients; after six months of treatment there was a significant reduction in the rate of systemic reactions in parallel with skin reactivity reduction, Pru p 3 specific immunoglobulin E (sIgE) reduction and sIgG4 increase [2, 9, 12, 20]. They used a 5-day induction protocol with mostly local adverse reactions (ARs; OAS) in about 40% of the sample [18].
Costa et al. [7] performed a study of the efficacy and tolerance of SLIT with Pru p 3 commercial extract in 10 patients with systemic reaction to peach. As recommended by the manufacturer, a standard protocol (SP) was performed with a 4-day induction phase (IP) with Pru p 3 cumulative dose of 78.44 μg, only reporting OAS with spontaneous resolution in 60% of patients.
The excellent SLIT safety profile, the fact that the SP is little time-effective, and the need to optimize patient time to reduce work/school absences, as well as optimize hospital resources, motivated the progression to shorter induction protocols. de Luque Piñana et al. [25] and Moura et al. [13] developed 2-day induction protocols with Pru p 3 cumulative doses of 102.44 μg in 12 patients and 47.92 μg in 10 patients, respectively. Both protocols proved to be safe, with the occurrence of mild ARs: in the first study, only OAS with spontaneous resolution was recorded in 100% of the patients, and in the second one there was recorded OAS with tongue paresthesia in 100% of the patients, requiring oral antihistamines in only 20% of them.
Some 1-day induction protocols (ultra-rush) have also been described with local reactions having only occurred in two of them [24, 25].
None of these studies recorded severe ARs, demonstrating Pru p 3 SLIT safety.
Other methods of IT production and administration, with the main target of increasing immunogenicity without increasing allergenicity, have been and are still being studied, some of them already with current practical application, since IT with peptides, hypoallergenic hybrid molecules, mixture of recombinant allergens, or allergens coupled to virus capsule-like particles [26–29].
Comparative studies between different induction protocols are scarce, so the aims of this work were to compare the safety of the standard SLIT induction protocol (four days) with Pru p 3 extract versus the rush induction protocol (two days), adapted from Hospital Virgen Macarena de Sevilla [25], and to assess the existence of predictive factors of AR. This is the biggest cohort of patients under SLIT with Pru p 3 described in Portugal.
Retrospective observational study in which all patients with LTP syndrome submitted to SLIT with Pru p 3 extract followed in the Food Allergy Unit were included.
Tolerance of the induction protocol was assessed by recording all ARs in detail.
Data refer to a period of 10 years, from January 2012 to July 2021, and were collected through records in the patients’ clinical files.
In addition to the demographic characteristics, the clinical evaluation included a complete clinical history with a detailed characterization of the reactions (OAS, urticaria/angioedema; respiratory and gastrointestinal symptoms; anaphylaxis) induced by the different foods containing LTPs and the presence of other atopic comorbidities (rhinitis; asthma; and/or eczema).
The laboratory evaluation included the determination of sIgE for Pru p 3 performed using ImmunoCAP™ (Phadia, Thermo Fisher, Upsala, Sweden) method, with values above 0.10 kUA/L being considered positive.
IT is composed of peach extract enriched with Pru p 3 (Bioportugal®, ALK-Abelló, S.A Madrid, Spain). It consists of an initiation or IP in the outpatient clinic that includes four concentrations of Pru p 3: 0.05 μg/mL, 0.5 μg/mL, 5 μg/mL, and 50 μg/mL, prepared according to the manufacturer’s instructions from the maximum concentration, and a maintenance phase at home, using a concentration of 50 μg/mL, in which the maintenance dose is 10 μg of Pru p 3, currently corresponding to four drops, administered, daily, sublingually and on an empty stomach, whose absorption occurs for two min after which the solution is expelled. It should be noted that until 2020 the same concentration of Pru p 3 was administered in the form of five drops daily, since then it has been four drops due to changes in the pipette diameter by the manufacturer.
Patients were divided into two groups according to the IP protocol:
SP group (SPG) with a total duration of 4 days, whose maintenance dose is reached on the 3rd day (Table 1)—performed until December 2018.
Rush protocol (RP) group (RPG) adapted from Hospital Virgen Macarena de Sevilla [25], with a total duration of two days, which maintenance dose is reached on the 2nd day (Table 2)—between January 2019 and July 2021.
Description of Pru p 3 SLIT SP IP
| Phase | Day | Concentration (μg/mL) | Drops | Cumulative dose (μg) | Time between doses |
|---|---|---|---|---|---|
| Induction | 1 | 0.05 | 1 | 0.24 | 15 min |
| 10 | |||||
| 0.5 | 1 | ||||
| 10 | |||||
| 2 | 5 | 1 | 2.2 | ||
| 10 | |||||
| 3 | 50 | 1 | 36 | ||
| 2 | |||||
| 5 | |||||
| 10 | |||||
| 4 | 50 | 20 | 40 | Single dose | |
| Maintenance | Daily | 50 | 5* | 10 | Single dose |
*: corresponding to four drops since 2020
Description of Pru p 3 SLIT rush induction protocol
| Phase | Day | Concentration (μg/mL) | Drops | Cumulative dose (μg) | Time between doses |
|---|---|---|---|---|---|
| Induction | 1 | 0.05 | 1 | 2.44 | 30 min |
| 10 | |||||
| 0.5 | 1 | ||||
| 10 | |||||
| 5 | 1 | ||||
| 10 | |||||
| 2 | 50 | 1 | 100 | ||
| 2 | |||||
| 4 | |||||
| 8 | |||||
| 15 | |||||
| 20 | |||||
| Maintenance | Daily | 50 | 4 | 10 | Single dose |
In both induction protocols, patients remained in the immunoallergology hospital day, under medical surveillance, and ARs were immediately evaluated and treated, if necessary. The ARs were then recorded in detail in the respective clinical files, which were then later consulted to gather that information.
Descriptive analysis included the frequency of positive results (in percentage) for qualitative variables compared with the χ2 test and Fisher’s test. For quantitative variables, the average ± standard deviation with 95% confidence interval (CI) was described. Normality was verified by the Shapiro-Wilk and Kolmogorov-Smirnov tests. Not normally distributed variants were expressed by median and interquartile range (IQR).
Statistical analysis was performed with GraphPad Prism software version 8.00 (Graphpad Software Inc., San Diego, USA). For the comparison between the two groups, Student’s t test and Mann-Whitney test were used. Values of P < 0.05 were considered significant.
The clinical part of the study as well as laboratory tests were carried out as part of the clinical routine evaluation. Patients gave oral informed consent to the use of their clinical data in an anonymous form.
Fifty-one patients with LTP syndrome undergoing SLIT with Pru p 3 extract were included: 41 patients in SPG, of which nine were children/adolescents, and 10 patients in RPG, of which three were children/adolescents.
Clinical and demographic characterization of the population is detailed in Table 3.
Clinical-demographic characterization of the population submitted to Pru p 3 SLIT
| Feature | SPG (n = 41) | RPG (n = 10) |
|---|---|---|
| Female, n (%) | 30 (73.2) | 8 (80) |
| Mean age at onset of LTP syndrome symptoms, years | 15.6 ± 10.1 | 10.9 ± 6.1 |
| LTP syndrome clinical presentation, n (%) | Urticaria/angioedema—15 (36.6) Anaphylaxis—14 (34.1) OAS—12 (29.3) | OAS—4 (40) Anaphylaxis—4 (40) Dyspnea and oropharyngeal tightness—1 (10) Urticaria—1 (10) |
| Allergic rhinitis, n (%) | 32 (78.1) | 8 (80) |
| Asthma, n (%) | 9 (21.9) | 4 (40) |
| Eczema, n (%) | 5 (12.2) | 2 (20) |
Regarding the clinical manifestations of LTP syndrome presentation, in SPG 15 (36.6%) patients had urticaria/angioedema, 14 (34.1%) had anaphylaxis, and 12 (29.3%) had OAS.
In RPG, four (44.5%) patients had OAS, three (33.3%) had anaphylaxis, one (11.1%) had dyspnea and chest tightness, and one (11.1%) had urticaria.
All symptoms reported were immediate (less than 1 h after ingestion).
A total of 10 ARs were recorded in the IP: five (12.2%) in SPG and five (50%) in RPG (Table 4).
IP reactions in SPG and RPG
| Type of reaction [IP reaction, n = 10 (19.2%)] | SPG (n = 41) | RPG (n = 10) | ||||
|---|---|---|---|---|---|---|
| 5 (12.2%) | Cumulative dose (μg) | Treatment | 5 (50%) | Cumulative dose (μg) | Treatment | |
| OAS | 3 (60%) | 0.022 | Spontaneous resolution | 4 (80%) | 0.32 | Spontaneous resolution |
| 2.44 | 0.125 | |||||
| 0.022 | 53.05 | |||||
| 62.44 | ||||||
| Oropharingeal tightness | 1 (20%) | 8 | Oral antihistamine | - | - | - |
| Uvula edema | 1 (20%) | 38 | Intravenous antihistamine and corticosteroid | - | - | - |
| Palmar pruritus and cough | - | - | - | 1 (20%) | 102 | Intravenous antihistamine and corticosteroid |
-: no such case has occurred.
In SPG, three patients (60%) had OAS at cumulative doses of 0.022 μg, 2.44 μg, and 0.022 μg, all with spontaneous resolution; one patient (20%) reported oropharyngeal tightness, without objective edema at a cumulative dose of 8 μg, which resolved with oral antihistamine; and another patient (20%) had uvula edema at a cumulative dose of 38 μg that resolved with intravenous antihistamine and corticosteroid.
In RPG, there was recorded OAS in four patients (80%) at cumulative doses of 0.125 μg, 0.32 μg, 53.05 μg, and 62.44 μg, all with spontaneous resolution; and one patient (20%) had palmar pruritus and cough without dyspnea at a cumulative dose of 102 μg, which resolved with intravenous antihistamine and corticosteroid.
All patients completed the remaining IP of both protocols without additional ARs and need for additional therapy.
The average serum Pru p 3 sIgE value of patients who had ARs in the IP in SPG versus RPG was: 6.7 kUA/L ± 10.4 kUA/L with a minimum value of 0.34 kUA/L and maximum of 25 kUA/L versus 5.7 kUA/L ± 4.1 kUA/L with a minimum value of 1.1 kUA/L and a maximum of 8.9 kUA/L, respectively.
The patient with the highest serum sIgE value for Pru p 3 (25 kUA/L) reported anaphylaxis in LTP syndrome presentation but developed only OAS in the IP—SPG.
Patients with systemic reactions in the IP had lower serum Pru p 3 values: 0.34 kUA/L in SPG and 1.1 kUA/L in RPG.
In both the SPG and RPG, there were no statistically significant differences between Pru p 3 values and a higher risk of reaction or a more severe reaction in the IP (median 1.7 kUA/L, IQR 14.025 kUA/L and median 2.23 kUA/L, IQR 4.025 kUA/L, respectively) (Figure 1).
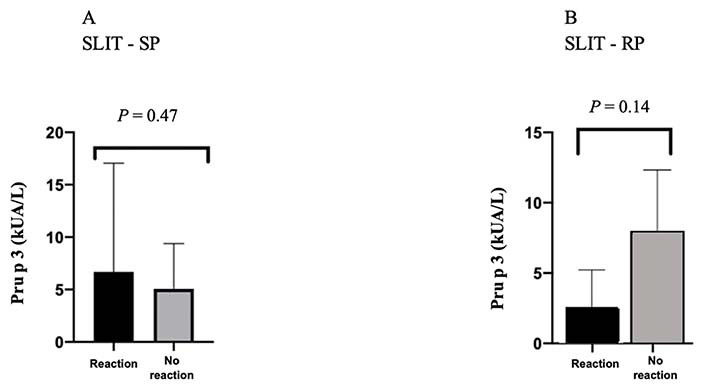
sIgE values (kUA/L) for Pru p 3 in patients with ARs in the IP versus in patients without ARs in the IP, in both protocols
Regarding other allergic comorbidities, such as rhinitis, asthma, or eczema, 34 patients (82.3%) in SPG had at least one of these and only three patients (8.8%) developed ARs in the IP (two OAS and one uvula edema), with no statistical significance between the presence of atopy and a higher probability of ARs in the IP [odds ratio = 0.24 (CI 95%); P = 0.20] (Table 4 and Figure 2).
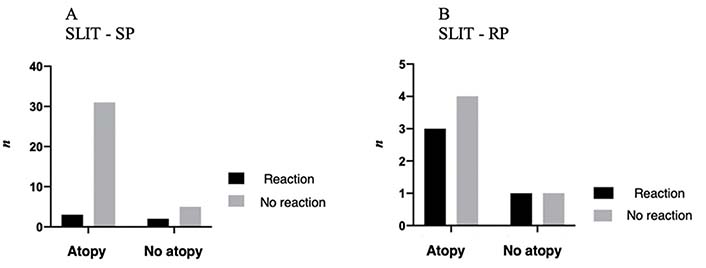
Presence of atopy in patients with AR in the IP versus in patients without AR in the IP, in both protocols
In RPG, seven (70%) patients had a history of atopy, of which three (42.9%) developed an AR in the IP (OAS), with no statistical significance between the presence of atopy and greater probability of reaction in the IP [odds ratio = 0.75 (CI 95%); P > 0.99] (Table 4 and Figure 2).
An evaluation of the relationship between the greater severity of LTP syndrome presentation and the greater probability of reaction in the IP was also performed, with no statistical significance (P = 0.51).
Food allergy is the first cause of anaphylaxis in the world. Allergy to plant foods is one of the most common, especially in southern Europe (> 60%), and is rising in other continents [1–4, 30].
In recent years, allergy to fresh fruits has been the subject of increasing investigation, given its high prevalence (> 15%), potential severity and persistence. In the Mediterranean area, peach allergy is representative of food allergies to fresh fruit related to LTPs [7–9, 14].
As a potentially severe and persistent allergy, the main therapeutic measure is allergen avoidance (together with emergency therapy in case of accidental exposure), which is not always easy due to the wide variety of foods containing LTPs and the possibility of cross-contamination, with countless familiar, social, and economic impact [9]. For all these reasons, there has been a search for an effective treatment, such as IT [8, 9, 11–13, 15, 18, 21, 22, 24].
As in the general population, where the presence of food-derived anaphylaxis LTPs is between 20% to 40%, also in this study, systemic reactions were found to be the most frequent manifestations of LTP syndrome, namely urticaria/angioedema, with anaphylaxis being present in about one third of the cases in both groups, and the symptoms were entirely immediate, in agreement with other published studies [2, 7, 11, 12].
In the RPG, compared to the SPG, there was a proportionally greater number of ARs in the IP (50% versus 12.2%). This would be expected not only because the SPG sample was larger, but mainly because the cumulative dose of Pru p 3 in RPG was significantly higher (78.44 μg versus 100 μg). However, the ARs recorded in both groups were mostly local with spontaneous resolution and, when systemic, were always mild. These results agree with the available literature on rush induction protocols, where there was a record, in 100% of patients, of OAS with spontaneous resolution/with oral antihistamines, with no record of severe ARs, proving Pru p 3 SLIT safety profile [7, 13, 18, 25, 31].
Almost all patients (93.3%) have a history of atopy, with allergic rhinitis being the most frequent allergic comorbidity (more than 75% of patients), agreeing with the literature [4, 7, 9, 14].
There was no direct correlation in either group between the presence of atopy and a higher probability of AR or more severe AR in the IP.
This was also not proved with a higher sIgE Pru p 3 value or with more severe reactions presenting LTP syndrome. These results are similar to those in the available literature, including another study by the same authors [7, 12, 15, 32].
A limitation of this study is the fact that the size of RPG (n = 10) is much smaller than SPG (n = 41). However, ARs recorded in RPG were mostly local, with only a mild systemic one, according to the results shared by other authors, proving Pru p 3 SLIT safety [13, 17, 18, 24, 25, 31].
The available literature on RPs in SLIT with Pru p 3 is still quite scarce, and this is the largest series of national patients and one of the largest international, so it could be a valuable asset for the entire scientific community.
ARs in the IP were similar in both groups, demonstrating the RP to be considered safe, even in patients with LTP syndrome with severe systemic manifestations such as anaphylaxis, as described in the literature [17, 18, 25].
It was not possible to find an association between the serum value of Pru p 3 sIgE, nor the presence of atopy, with greater probability of AR in the IP or greater severity of the reaction.
While the sample of RPG was smaller, rush induction protocol seems to be a safe option, directly and indirectly less expensive, both for the patient and for health services.
More and wider studies will be important to validate the safety of Pru p 3 SLIT rush induction protocols, considering the progression to 1-day ultra-rush induction protocols [24, 25, 31], and to document direct and indirect cost reduction, with fewer patient visits to the hospital and therefore less absence from work/school, as well as greater logistical availability of the hospital to treat more patients.
Because the cumulative dose of the IP was more than ten times the maintenance dose, the authors readapted the current RP, maintaining the two days, but reducing the number of steps and the final cumulative dose, and reaching the concentration of the maintenance dose on the first day (Table 5). This readaptation took effect at the Food Allergy Unit in July 2021, and so far, five patients already successfully performed this new RP. These results will be presented later.
Description of the IP of the Pru p 3 SLIT rush desensitization protocol, in use since July 2021
| Phase | Day | Concentration (μg/mL) | Drops | Cumulative doses (μg) | Time between doses |
|---|---|---|---|---|---|
| Induction | 1 | 0.05 | 1 | 22 | 15 min |
| 5 | |||||
| 0.5 | 1 | ||||
| 5 | |||||
| 5 | 1 | ||||
| 3 | |||||
| 5 | |||||
| 50 | 1 | 30 min | |||
| 2 | |||||
| 3 | |||||
| 4 | |||||
| 2 | 50 | 3 | 22 | 60 min | |
| 4 | |||||
| 4 | |||||
| Maintenance | Daily | 50 | 4 | 10 | Single dose |
Future trends will eventually be to adopt these shorter induction protocols described (1-day) and possibly develop studies with other routes of IT administration, such as in milk and peanut—oral and epicutaneous.
ARs: adverse reactions
CI: confidence interval
IP: induction phase
IQR: interquartile range
IT: immunotherapy
LTPs: lipid transfer proteins
OAS: oral allergy syndrome
RP: rush protocol
RPG: rush protocol group
sIgE: specific immunoglobulin E
SLIT: sublingual immunotherapy
SP: standard protocol
SPG: standard protocol group
MITS, FCD, and CC: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. MP: Conceptualization, Data curation, Formal analysis, Validation, Visualization, Writing—review & editing. EP: Conceptualization, Methodology, Validation, Writing—review & editing.
The authors declare that they have no conflicts of interest.
This study was approved by the Ethical Committee of Centro Académico de Medicina de Lisboa (approval number 83/23) and complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The data that support the findings of our study are not publicly available because they contain information that could compromise the privacy of its participants, but are available from the corresponding author upon reasonable request for the de-personalized sections.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2942
Download: 29
Times Cited: 0
