Affiliation:
1Allergy and Pneumology Outpatient Clinic, 24125 Bergamo, Italy
ORCID: https://orcid.org/0000-0003-2767-6767
Affiliation:
2Departmental Unit of Allergology, Immunology & Pulmonary Diseases, Fondazione Poliambulanza, 25100 Brescia, Italy
ORCID: https://orcid.org/0000-0002-7120-5877
Affiliation:
3Department of Clinical and Experimental Medicine, Section of Pediatrics, University of Pisa, 56126 Pisa, Italy
ORCID: https://orcid.org/0000-0001-5209-9733
Affiliation:
4Department of Medical Sciences Graduate School of Allergology and Clinical Immunology, University of Turin, 10135 Turin, Italy
ORCID: https://orcid.org/0000-0001-7587-4800
Affiliation:
5Center for Medical Sciences (CISMed) and Department of Cellular, Computational and Integrative Biology (CIBIO), University of Trento, 38122 Trento, Italy
6Santa Chiara Regional Hospital, APSS, 38122 Trento, Italy
Email: alvise.berti@apss.tn.it
ORCID: https://orcid.org/0000-0002-7831-921X
Explor Asthma Allergy. 2023;1:163–173 DOI: https://doi.org/10.37349/eaa.2023.00017
Received: February 27, 2023 Accepted: October 16, 2023 Published: October 31, 2023
Academic Editor: Lawrence DuBuske, Physician George Washington University Hospital, Immunology Research Institute of New England, United States
Asthma is a respiratory disease affecting more than 300 million people around the world. Airflow obstruction and inflammation due to asthma usually involve large airways, but recently small airway involvement (internal diameter < 2 mm) has been shown to represent one of the main determinants of asthma and asthma control. In fact, compared to large airway involvement, small airway dysfunction (SAD) has been demonstrated across all the asthma severity in the majority of patients, as assessed with Global Initiative for Asthma (GINA) steps. Clinically, SAD is associated with, among other features, exercise-induced bronchoconstriction, asthma-related night awakenings, obesity/overweight, more severe airway hyperresponsiveness, worse asthma control, and more severe exacerbations. Impulse oscillometry (IOS), a forced oscillation technique (FOT) requiring less effort than spirometry from the patients, demonstrated to accurately measure SAD in children and adults. The fall in resistance from 5 Hz to 20 Hz (R5–R20), which is the most used index for the resistance of peripheral airways, is how SAD is usually identified by IOS. Other crucial parameters measured by IOS are the reactance at 5 Hz (X5), reflecting elastic recoil of the peripheral airways, the resonant frequency (Fres), which is the frequency at which the inertial properties of the airway and the capacitance of the lung periphery are equal, and the reactance area (AX), reflecting the elastic properties of the lung periphery. In this mini review, the latest findings on the utility of IOS to identify SAD and the associations between SAD and clinical features in adult asthmatic patients were addressed.
Asthma is a chronic disease across different countries, affecting more than 300 million people around the world [1, 2]. Over 260 million people had poorly controlled asthma (defined as asthma with wheezing diagnosed within the past 12 months) [2], with a high number of disabilities and, in low- and middle-income countries, several premature deaths [1, 3]. In Europe, asthma affects almost 6% of the population, resulting in a high socioeconomic burden [2, 4].
Airflow obstruction and inflammation due to asthma usually involve large airways [5], but recently the involvement of airways of small caliber (those with a diameter < 2 mm) has been shown to represent one of the main determinants of asthma and asthma control and to play a very prominent role in the pathogenesis of asthma and chronic obstructive pulmonary disease (COPD) [6, 7]. Small airway obstruction could be the consequence of smooth muscle contraction due to inflammation leading to remodeling and stiffness of the small airway walls affecting their distensibility [8]. In asthmatic patients, airway remodeling mostly involves small airways rather than large airways. This has been shown in severe asthma [9–11]. This small airway dysfunction (SAD) is associated with, among other features, obesity/overweight, exercise-induced bronchoconstriction, more severe airway hyperresponsiveness, worse asthma control, and more severe exacerbations [12–16], which are all clinical features that suggest the presence of SAD in patients with asthma.
In accordance with the main asthma guidelines and recommendations, spirometry is the milestone for the evaluation of respiratory function [17]. Nevertheless, standard spirometry cannot assess small airways with high sensitivity, which results in an alteration of the spirometry values only whether there is an obstruction of at least 75% of small airways [18]. In addition, the correlation between conventional lung function measurements [forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC, and peak expiratory flow (PEF)] and asthma control is weak [17, 19]. Evidence showing that forced expiratory flow between 25% and 75% (FEF25–75%) is associated with poor asthma control and worse asthma outcomes as compared with FEV1, is accumulating [20]. However, the FEF25–75% correlate with only weakly peripheral obstruction, being more reliable in more severe cases, and therefore have been questioned by several studies [21–24]. Hence, novel tests to assess distal airways are needed. More specialized techniques have been developed in recent years, which are to be translated from research into clinical practice [25].
In particular, impulse oscillometry (IOS), a forced oscillation technique (FOT) requiring less effort than spirometry from the patients, was shown to accurately measure SAD across all life ages. The main direct (functional) and indirect techniques to assess SAD are summarized in Table 1. IOS measurements are made during quiet breathing, and the resulting information is complementary to that from more routine tests using forced expiratory maneuvers [26]. In fact, IOS is an easy and noninvasive method that requires minimal patient cooperation. An IOS device superimposes small pressure stimuli in the form of impulses, over normal breathing while pressure and flow are measured at the mouth [26]. The interpretation of IOS is based in general on two components determining the respiratory impedance (Zrs), i.e. respiratory resistance (Rrs) and lung reactance (Xrs), both reflecting total pulmonary impedance, measured as a function of flow volume and pressure, real-time by the investigator. Respiratory resistance at 5 Hz and 20 Hz (R5 and R20, in kPa × s × L−1) are indices of total and proximal airway resistance, respectively. Therefore, the difference R5–R20 (in kPa × s × L−1) represents the contribution of the peripheral airways to airflow limitation.
Available methods to assess bronchial airways (both large and small airways)
| Assessment | Method | Large airway dysfunction | SAD |
|---|---|---|---|
| Functional assessment | Spirometry | FEV1/FVC, FEV1 | FEF25–75%, FVC, FVC/SVC |
| IOS | R20 | R5–R20, X5, AX, Fres ΔX5 in-esp | |
| MBNW or SBNW | - | Slope phase III, CV, CC, Sacin, Scond | |
| Body plethysmography | - | RV, RV/TLC | |
| Imaging assessment | HRCT | Airway wall thickness | Thickness of the airway wall, air trapping |
| Nuclear medicine (scintigraphy, SPECT, PET) | - | Regional ventilation defects | |
| 3He-MRI | - | Non-ventilated lung volume | |
| CT and computational fluid dynamics | - | Changes in airway volume and resistance | |
| Cellular/molecular assessment | Bronchoscopy | Endobronchial biopsy | Bronchoalveolar lavage, transbronchial biopsy |
| Sputum induction | Early phase sputum | Late phase sputum | |
| eNO | Bronchial eNO | Alveolar eNO |
AX: reactance area; CC: closing capacity; CT: computed tomography; CV: closing volume; eNO: exhaled nitric oxide; Fres: resonant frequency; HRCT: high-resolution computerized tomography; in-esp: in expiration; MBNW: multiple breath nitrogen washout test; MRI: magnetic resonance imaging; PET: positron emission tomography; RV: residual volume; Sacin and Scond: acinar and conductive airways ventilation heterogeneity; SBNW: single breath nitrogen washout test; SPECT: single-photon emission CT; SVC: slow vital capacity; TLC: total lung capacity; X5: reactance at 5 Hz. -: not applicable
Other parameters measured by IOS include i) X5 (in kPa × s × L−1), which reflects elastic recoil of the peripheral airways; ii) Fres (in Hz), defined as the frequency at which the inertial properties of airways and the capacitance of lung periphery are equal; iii) AX (the area under the reactance curve; in kPa/L), reflecting the elastic properties of the lung periphery and shown to be correlated with resistance at lower frequencies [26].
Compared to large airway involvement assessed with conventional spirometry, SAD has been demonstrated across all the asthma severity in the majority of patients, as assessed with Global Initiative for Asthma (GINA) steps. We and others showed that a lower proportion of SAD is found in GINA steps 2–4 by spirometry (by means of FEF25–75%) as compared to IOS (by means of R5–R20), showing that there were only nonsignificant or weak inverse correlations between R5–R20 and FEF25–75% within each GINA step [23]. Only GINA step 5 showed a stronger correlation, suggesting that SAD is found more often by IOS than spirometry, particularly in mild-to-moderate asthma. Along with the same line, several studies showed a highly significant correlation between IOS-defined SAD and complex imaging tools [27–32], defining IOS as a reliable system to detect SAD.
The recently published technical standards for oscillometry measurements from the European Respiratory Society (ERS) [33], provided guidance on the correct approach to calibrating oscillometry systems and how to perform tests. However, there are areas where further evidence of its clinical utility is needed before its implementation in clinical practice for diagnosing or monitoring respiratory diseases [34]. For instance, an open research question is to understand if the measurements of resistance and reactance are comparable between the different devices, due to the presence of various IOS/FOT tools available [35, 36].
In this mini review, we aimed to report the latest findings on the role of IOS in identifying and measuring SAD and to update the state of the art on the associations between SAD and clinical features in adult asthmatic patients.
Fifty to sixty percent of patients with asthma have SAD, as measured by IOS, and the level of SAD tends to increase with the increase of asthma severity, particularly in more severe asthma (Table 2) [16, 23, 37]. ATLANTIS, the largest study to date evaluating the SAD contributing to asthma severity, proved this concept and defined the clinical relevance of SAD for asthma [12]. This study examined which biomarker or combination of them, instrumental tests (spirometry, MBNW, IOS, and body plethysmography), and imaging tools better identify SAD and its correlation with asthma severity. Overall, 91% of this asthma cohort were found to have SAD, defined as any abnormal physiological measure with the prevalence varying with the physiological measure used. IOS associated with spirometry allowed the best outcomes. Other studies focused on the prevalence of IOS-defined SAD, which is overall high and increases with the increasing of asthma severity [15, 16, 23, 38–42]. Our contribution to 400 patients diagnosed with asthma, anticipated and complemented the findings of the ATLANTIS, showing that the prevalence of SAD is up to 62% across GINA classes [16, 23]. Abdo et al. [15] found similar data, with SAD of 63% in 268 asthma patients, and with an increasing prevalence of SAD in more severe GINA stages, i.e. steps 4 and 5 (Table 2) [12, 15, 16, 23, 39–43].
IOS-defined SAD prevalence
| Study reference | IOS measure | Prevalence of SAD in the cohort |
|---|---|---|
| Anderson et al. [39] | R5–R20 | 65% BTS 2 64% BTS 3 70% BTS 4 |
| Postma et al. [12] | R5–R20 | 42% |
| Cottini et al. [43] | R5–R20 | 73% GINA 1 |
| Cottini et al. [16, 23] | R5–R20 | Overall 62% 58% GINA 2 61% GINA 3 63% GINA 4 78% GINA 5 |
| Abdo et al. [15] | R5–R20 | Overall 63% 53% GINA 2–3 75% GINA 4–5 |
| Alfieri et al. [40] | R5–R20 | 48% |
| Manoharan et al. [41] | R5–R20 | 42% |
| Yi et al. [42] (cough variant asthma) | R5–R20 | 73% |
BTS: British Thoracic Society
SAD prevalence also appears significant in patients with preserved pulmonary function, i.e. in subjects with normal conventional spirometry but clinically diagnosed with asthma [23, 44]. Interestingly, we recently showed a prevalence of 73% of SAD in a consecutive cohort of 60 adults with physician-diagnosed intermittent asthma treated with SABA as needed [43]. Spirometry measures were similar between patients with SAD and without SAD, and only 11.7% of patients with SAD had less well-controlled asthma as compared to 75.0% of those without SAD (P < 0. 001; Table 2). The reason for this high prevalence of SAD in this subset of patients remains unknown, but it could be speculated that the absence of inhaled corticosteroids (ICS) can lead to uncontrolled inflammation in small airways, leading to SAD and worse asthma control.
Multiple multivariable analyses indicated strong associations with several specific clinical features, which could allow to profile asthmatic patients with SAD (Figure 1) [45–62]. Overweight/obesity and type-2 inflammation [i.e. a pattern of immune response mediated by T helper cells (and innate lymphoid cells) secreting interleukin-4 (IL4), IL5, and IL13] have been recently shown to be independent predictors of SAD in patients with asthma [15, 16, 63–65]. Other features, such as active smoking status (being current or previous smokers of ≥ 10 packs/year), older age with a longer history of asthma, fixed airflow obstruction, nocturnal symptoms due to asthma, exercise-induced bronchoconstriction, and severe/uncontrolled asthma have been shown to be independently associated with SAD in asthmatic patients [14, 15, 23, 66–68].
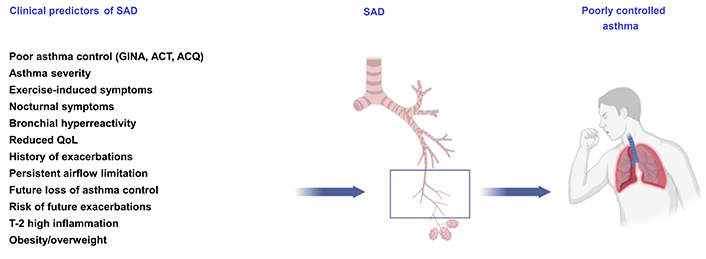
Association of IOS-defined SAD with clinical features and the impact of asthma control. ACQ: asthma control questionnaire; ACT: asthma control test; QoL: quality of life
Ultimately, disease control achievement is the long-term goal in asthma [17], which is not always reached and this is reflected in an impairment in the QoL of these patients [44]. Several studies showed that SAD negatively impacts asthma control as defined by GINA [67]. Recently, we showed in a “real life” study [16] that peripheral airway resistance (the fall in resistance measured by R5–R20) and other IOS measurements, namely, R5, X5, AX, and Fres, progressively worsened with increasing GINA steps and was significantly higher in stages 4 and 5 (P < 0.05). These findings confirmed the increase of resistance in the small airways goes along with the worsening of asthma [16]. In addition, a longitudinal analysis of the ATLANTIS study showed that small airway disease predicted asthma control and the rate of exacerbations experienced [13]. In particular, the function of the small airways measured with the IOS parameters R5–R20, AX, and X5 appeared significantly correlated with exacerbations and other measures of small airway function, including FEV1, FEF50%, and FEF25–75%. On the other hand, CT parameters did not correlate significantly with exacerbations, asthma control, and QoL. This led the author to suggest that small airway function should be assessed along with large airway function and biomarkers as routine clinical practice for optimal care of patients with asthma, adding SAD to the risk factors associated with worse asthma outcomes [17].
In recent years, some studies have evaluated the frequency of SAD in patients with reduced FEV1 or persistent airflow limitation (PAL); PAL (i.e. FEV1/FVC less than the lower limit of normal) occurs in a subgroup of patients with asthma, especially with moderate-to-severe disease, probably due to central airway remodeling. For example, recent evidence showed that having an impairment in both IOS (X5) and FEV1 is associated with poorer asthma control and severe exacerbations [69]. In addition, patients with moderate-to-severe asthma with FEV1/FVC < 0.7 were associated with worse SAD impairment by IOS and higher levels of type 2 markers [70]. In this study, bronchial wall thickness and PAL correlated with the presence of nasal polyposis, severe exacerbations, peripheral airway resistance, and reactance in persistent asthma [70]. In addition, an ATLANTIS post hoc analysis demonstrated that persistent airway obstruction correlated with SAD and bronchial eosinophils. PAL correlated with a higher exacerbation risk in moderate-to-severe asthma [71].
Precision medicine approach to treating and preventing disease emerged also in asthma, considering the individual variability of individual genetic background, environment, and lifestyle [72]. The use of IOS can be of use in identifying SAD as a “treatable trait”, leading to individualized asthma management and patient care. Given the clear association with worse asthma control, for the clinician and patient, active vigilance for any SAD and a more targeted treatment would be advocated, e.g., using extra-fine formulations of inhaled bronchodilators, glucocorticoids, and biologics [73–79]. This could result in better asthma control and a lower exacerbation rate.
Clinicians should be actively looking for SAD as part of the routine management of asthmatic patients. As it has been widely demonstrated that asthma control is related to SAD and that SAD can be better assessed by IOS than conventional spirometry, IOS should be complementary to conventional spirometry as part of the routine diagnostic management of asthma patients in a routine clinical setting. The degree of bronchoconstriction assessed by IOS together with routinely collected clinical information can ultimately lead the clinician to predict which patient is likely to have a worse outcome, potentially helping him to choose the best treatment for the single patient, leading to more individualized asthma management and patient care.
AX: reactance area
FEF25–75%: forced expiratory flow between 25% and 75%
FEV1: forced expiratory volume in 1 s
Fres: resonant frequency
FVC: forced vital capacity
GINA: Global Initiative for Asthma
IL4: interleukin-4
IOS: impulse oscillometry
PAL: persistent airflow limitation
QoL: quality of life
R5: resistance at 5 Hz
SAD: small airway dysfunction
X5: reactance at 5 Hz
MC, PC, AB, ML, and CL: Conceptualization, Investigation, Writing—review & editing. MC: Writing—original draft. AB: Validation, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
