Affiliation:
1Unit of Pediatric Allergy and Pneumology, Children’s Hospital La Fe, Health Research Institute Hospital La Fe, ZC 46026 Valencia, Spain
Email: jaume.marti.garrido@gmail.com
ORCID: https://orcid.org/0000-0003-0260-365X
Affiliation:
2Unit of Pediatric Allergy, Karolinska Hospital, ZP 17176 Stockholm, Sweden
ORCID: https://orcid.org/0000-0001-7745-8624
Affiliation:
2Unit of Pediatric Allergy, Karolinska Hospital, ZP 17176 Stockholm, Sweden
ORCID: https://orcid.org/0000-0001-7737-6506
Affiliation:
3Division of Immunology and Allergy, Department of Medicine Solna, Karolinska Institutet and University Hospital, ZP 17177 Stockholm, Sweden
ORCID: https://orcid.org/0000-0003-3091-1596
Affiliation:
1Unit of Pediatric Allergy and Pneumology, Children’s Hospital La Fe, Health Research Institute Hospital La Fe, ZC 46026 Valencia, Spain
ORCID: https://orcid.org/0000-0002-6302-6115
Affiliation:
1Unit of Pediatric Allergy and Pneumology, Children’s Hospital La Fe, Health Research Institute Hospital La Fe, ZC 46026 Valencia, Spain
ORCID: https://orcid.org/0000-0003-3968-510X
Affiliation:
1Unit of Pediatric Allergy and Pneumology, Children’s Hospital La Fe, Health Research Institute Hospital La Fe, ZC 46026 Valencia, Spain
ORCID: https://orcid.org/0000-0001-5975-7348
Affiliation:
1Unit of Pediatric Allergy and Pneumology, Children’s Hospital La Fe, Health Research Institute Hospital La Fe, ZC 46026 Valencia, Spain
ORCID: https://orcid.org/0000-0001-5639-1037
Explor Asthma Allergy. 2023;1:186–197 DOI: https://doi.org/10.37349/eaa.2023.00019
Received: December 15, 2022 Accepted: November 09, 2023 Published: November 28, 2023
Academic Editor: Lawrence DuBuske, Physician George Washington University Hospital, Immunology Research Institute of New England, United States
Aim: Asthma affects millions of people worldwide and generates a considerable economic impact. This study aims to compare the specific immunoglobulin E (sIgE) profile in sensitized children with severe asthma from two countries with great geographic and climatic differences.
Methods: A cross-sectional study was performed using serum samples analysed with a multiplex tool in 47 children from Sweden and 29 children from Spain.
Results: Patients from Spain were significantly more often sensitized to house dust mites, cockroaches, dogs, Alternaria, Cladosporium, pollen from olive trees, cypress, Platanus, and Parietaria, and to Anisakis and shrimp. Swedish patients were significantly more often sensitized to cats, pollen from birch, hazel, and Alnus, and to apple, soy, and peanut (all P < 0.05). With regard to sensitization to allergen molecules, lipid transfer proteins (LTPs), cross-reactive carbohydrate determinant (CCD)-bearing proteins and tropomyosins were more frequent in Spain, while sensitization to pathogenesis-related class 10 proteins (PR-10) molecules and to peanut storage proteins were more common in Sweden.
Conclusions: The immunoglobulin E (IgE) profile in sensitized children with severe asthma differed greatly between Sweden and Spain. The profile results were more similar to that reported in the literature for other sensitized children from the same geographic areas with non-severe disease than to that of severe asthmatics from different areas.
The prevalence of asthma has had a sustained increase in the last decades, and in parallel, there has also been a rise in the number of severe cases, which account for an estimated 50% of asthma costs [1]. According to the results of the International Study of Asthma and Allergies in Childhood (ISAAC), the prevalence of asthma at six years of age in the city of Valencia (VAL), Spain in the year 2004 was 9.3%, whilst in 2006 the global national prevalence of asthma symptoms was 9.5% in Spain and 9.7% in Sweden [2–5].
Component-resolved diagnostics with multiplex measurements can provide important information that cannot be obtained from extract-based tests [6]. For example, the microarray is particularly useful for the etiologic diagnosis of pollinosis in patients sensitized to multiple pollen species whose pollination periods overlap [7], for house dust mite sensitization [8], or hypersensitivity to furry animals [9].
As allergy is one of the main drivers of childhood asthma, this study aimed to evaluate patterns of sensitization to allergen molecules in sensitized children with severe asthma from two countries with great geographical and climatic differences, and to explore whether there are similar or different hypersensitivity molecular profiles.
We performed a cross-sectional study on school children and adolescents with severe asthma and allergic sensitization. Patients with mild or moderate asthma were not included in the study as they are not usually sent to tertiary centers, where most of them are treated; and other allergic diseases were not considered. Severe asthma was defined, according to Global Initiative for Asthma (GINA) classification, as requiring treatment in steps 4 or 5 to prevent it from becoming uncontrolled or remaining uncontrolled despite this treatment [10]. Patients with severe asthma, as those evaluated, are usually followed in specialized clinics of tertiary hospitals, like the two participating centers.
Serum samples were analysed using the microarray Inmuno Solid-Phase Allergen Chip (ISAC)-CRD89 multiplex tool comprising 112 allergen molecules from 50 allergen sources (VBC-Genomics, ThermoFisher Scientific, Sweden), following the manufacturer’s recommendations [11, 12]. Serum samples from VAL were revised on March 2017. Samples from Stockholm (STO) had been already revised [13]. The cut-off for detection was 0.30 ISAC standardized units (ISU). Patients were considered sensitized to an allergen source if any of its components was positive.
The variables were compared through Chi-square or Fisher’s exact tests with the Statistical Package for the Social Sciences (SPSS) 15.0 software program (IBM, Chicago, Ill.). Values of P < 0.05 were considered significant.
Forty-seven children were enrolled in Sweden (15 different departments of paediatrics) and 29 children in Spain (Children’s Hospital La Fe, VAL). Patients from Sweden were older and more gender-balanced, but no differences were found in body mass index, forced expiratory volume in the first second, or fraction of exhaled nitric oxide (Table S1). Considering five groups of allergens (food, pollen, animals, molds, house dust mites), 9 patients (11.8%) were sensitized to only one group, 14 (18.4%) to two groups, 29 (38.2%) to three, 13 (17.1%) to four, and 11 (14.5%) to all five groups.
Patients from Spain were significantly more often sensitized to inhalant allergen sources like house dust mites, cockroaches, dogs, Alternaria, Cladosporium, and pollen from olive trees, cypress, Platanus, and Parietaria compared to the Swedish patients, who were significantly more often sensitized to cats and pollen from birch, hazel, and Alnus, as shown in Table 1.
Percentage of children with severe asthma sensitized to inhalants, foods, and other allergen sources according to ISAC® results. The most relevant results are shown comparing children enrolled in Sweden
| Allergenic source | STO (n = 47) | VAL (n = 29) | P |
|---|---|---|---|
| Component | |||
| House dust mites | 17 | 89.7 | < 0.001 |
| rDer p 1 | 8.5 | 79.3 | |
| rDer p 2 | 8.5 | 69.0 | |
| nDer p 10 | 6.4 | 41.4 | |
| rDer f 1 | 10.6 | 79.3 | |
| rDer f 2 | 8.5 | 69.0 | |
| Cladosporium | 4.3 | 20.7 | 0.048 |
| rCla h 8 | |||
| Cats | 72.3 | 55.2 | 0.003 |
| rFel d 1 | 72.3 | 37.9 | |
| nFel d 2 | 2.1 | 20.7 | |
| rFel d 4 | 29.8 | 20.7 | |
| Grass pollen | 63.8 | 41.4 | 0.056 |
| rPhl p 1 | 40.4 | 17.2 | |
| rPhl p 2 | 10.6 | 6.9 | |
| nPhl p 4 | 38.3 | 34.5 | |
| rPhl p 5 | 29.8 | 6.9 | |
| rPhl p 6 | 17.0 | 10.3 | |
| rPhl p 7 | 0 | 6.9 | |
| rPhl p 11 | 8.5 | 6.9 | |
| rPhl p 12 | 8.5 | 3.5 | |
| nCyn d 1 | 29.8 | 24.1 | |
| Hazel pollen | 46.8 | 3.5 | < 0.001 |
| rCor a 1.0101 | 36.2 | 0 | |
| rCor a 1.0401 | 44.7 | 3.5 | |
| Cockroaches | 8.5 | 41.4 | 0.002 |
| rBla g 1 | 2.1 | 0 | |
| rBla g 2 | 2.1 | 10.3 | |
| rBla g 5 | 2.1 | 3.5 | |
| nBla g 7 | 4.3 | 41.4 | |
| Alternaria | 19.1 | 44.8 | 0.016 |
| rAlt a 1 | 14.9 | 37.9 | |
| rAlt a 6 | 8.5 | 31.0 | |
| Dogs | 29.8 | 48.3 | 0.105 |
| rCan f 1 | 25.5 | 48.3 | |
| rCan f 2 | 17.0 | 13.8 | |
| nCan f 3 | 6.4 | 6.9 | |
| Birch pollen | 61.7 | 6.9 | < 0.001 |
| rBet v 1 | 57.5 | 3.5 | |
| rBet v 2 | 10.6 | 3.5 | |
| rBet v 4 | 0 | 3.5 | |
| Alnus pollen | 40.4 | 3.5 | < 0.001 |
| rAln g 1 | |||
| Olive tree pollen | 2.1 | 65.5 | < 0.001 |
| nOle e 1 | |||
| Platanus pollen | 8.5 | 34.5 | 0.007 |
| rPla a 1 | 0 | 3.5 | |
| nPla a 2 | 8.5 | 34.5 | |
| Cypress pollen | 10.6 | 45.0 | 0.002 |
| nCup a 1 | |||
| Parietaria pollen | 2.1 | 27.6 | 0.002 |
| rPar j 2 | |||
| Peanuts | 51.1 | 10.3 | < 0.001 |
| nAra h 1 | 23.3 | 6.9 | |
| nAra h 2 | 27.7 | 6.9 | |
| nAra h 3 | 25.5 | 3.5 | |
| rAra h 8 | 23.4 | 0 | |
| Soy | 36.2 | 3.5 | 0.013 |
| rGly m 4 | 19.2 | 3.5 | |
| nGly m 5 | 10.6 | 3.5 | |
| nGly m 6 | 17.0 | 3.5 | |
| Apples | 40.4 | 3.5 | < 0.001 |
| rMal d 1 | |||
| Celery | 12.8 | 0 | 0.077 |
| rApi g 1 | |||
| Peaches | 44.7 | 27.6 | 0.14 |
| rPru p 1 | 44.7 | 3.5 | |
| nPru p 3 | 6.4 | 27.6 | |
| Hen’s egg | 12.8 | 31.0 | 0.075 |
| nGal d 1 | 10.6 | 24.1 | |
| nGal d 2 | 6.4 | 27.6 | |
| nGal d 3 | 6.4 | 20.7 | |
| nGal d 5 | 0 | 10.3 | |
| Kiwi | 21.3 | 37.9 | 0.19 |
| nAct d 1 | 14.9 | 10.3 | |
| nAct d 2 | 8.5 | 31.0 | |
| nAct d 5 | 2.1 | 0 | |
| Fish | 0 | 10.3 | 0.052 |
| rGad c 1 | |||
| Shrimp | 4.3 | 41.4 | < 0.001 |
| nPen m 1 | |||
| Anisakis | 4.3 | 41.4 | < 0.001 |
| rAni s 1 | 2.1 | 0 | |
| rAni s 3 | 4.3 | 41.4 |
nAct d 1: Actinidia deliciosa allergen 1; nAra h 1: Arachis hypogaea allergen 1; nBla g 7: Blatella germanica allergen 7; nCup a 1: Cupressus arizonica allergen 1; nCyn d 1: Cynodon dactylon allergen 1; nGal d 1: Gallus domesticus allergen 1; nGly m 5: Glycine max allergen 5 ; nOle e 1: Olea europaea allergen 1; nPen m 1: Penaeus monodon allergen 1; nPla a 2: Platanus acerifolina allergen 2; rAln g 1: Alnus glutinosa allergen 1; rAlt a 1: Alternaria alternata allergen 1; rAni s 1: Anisakis simplex allergen 1; rApi g 1: Apium graveolens allergen 1; rBet v 1: Betula verrucosa allergen 1; rCan f 1: Canis familiaris allergen 1; rCla h 8: Cladosporium herbarum allergen 8; rCor a 1.0101: Corylus avellana allergen 1.0101; rDer f 1: Dermatophagoides farinae allergen 1; rDer p 1: Dermatophagoides pteronyssinus allergen 1; rFel d 1: Felis domesticus allergen 1; rGad c 1: Gadus callarias allergen 1; rMal d 1: Malus domestica allergen 1; rPar j 2: Parietaria judaica allergen 2; rPhl p 1: Phleum pratense allergen 1; rPla a 1: Platanus acerifolia allergen 1; rPru p 1: Prunus persica allergen 1
With respect to food allergens, the Swedish patients had significantly more often immunoglobulin E (IgE) reactivity to apples, soy, and peanuts, and Spanish patients to shrimp (Table 1). There were trends for higher sensitization frequencies to fish and egg in the Spain patients and to celery in the Swedish patients. There were no significant differences for peach as an allergen source, a very common food allergen in Spain; however, patients in STO were all sensitized to rPru p 1 while those in VAL to nPru p 3. In addition, IgE reactivity to Anisakis was significantly more common among children from Spain. No differences were found for other foods, inhalants, latex, and bee venom (Table S2).
Results on sensitization to the different protein families and allergen molecules are shown in Table 2 and Table S3. Swedish children were more often sensitized to pathogenesis-related class 10 proteins (PR-10) molecules, and those from Spain to lipid transfer proteins (LTPs) and cross-reactive carbohydrate determinant (CCD)-bearing proteins. Tropomyosin sensitization was more frequent in Spain whereas sensitization to peanut storage proteins was more common in the Swedish patients. No differences for other storage proteins were noted.
Percentage of patients with severe asthma sensitized according to protein families
| Protein family | STO (n = 47) | VAL (n = 29) | P |
|---|---|---|---|
| Component | |||
| LTPs | |||
| nPru p 3 | 6.4 | 27.6 | 0.02 |
| rPar j 2 | 2.1 | 27.6 | 0.002 |
| nArt v 3 | 8.5 | 17.2 | 0.29 |
| rCor a 8 | 2.1 | 10.3 | 0.15 |
| Tropomyosins | |||
| nPen m 1 | 4.3 | 41.4 | < 0.001 |
| rDer p 10 | 6.4 | 41.4 | < 0.001 |
| nBla g 7 | 4.3 | 41.4 | < 0.001 |
| rAni s 3 | 4.3 | 41.4 | < 0.001 |
| Prophyllines | |||
| rBet v 2 | 10.0 | 3.5 | 0.40 |
| rPhl p 12 | 8.5 | 3.5 | 0.64 |
| rHev b 8 | 10.6 | 3.5 | 0.40 |
| CCDs | |||
| nCup a 1 | 10.6 | 44.8 | 0.002 |
| nCry j 1 | 4.3 | 31.0 | 0.002 |
| nPla a 2 | 8.5 | 34.5 | 0.007 |
| nPhl p 4 | 38.3 | 34.5 | 0.81 |
| Uteroglobin | |||
| rFel d 1 | 72.3 | 37.9 | 0.004 |
| Lipocalins | |||
| rFel d 4 | 29.8 | 20.7 | 0.43 |
| rCan f 1 | 25.6 | 48.3 | 0.05 |
| rCan f 2 | 17 | 13.8 | 1 |
| Serum albumins | |||
| nFel d 2 | 2.1 | 20.7 | 0.01 |
| nCan f 3 | 6.4 | 6.9 | 1 |
| nEqu c 3 | 4.3 | 10.3 | 0.36 |
| Storage proteins | |||
| rAna o 2 | 4.3 | 0 | 0.52 |
| nAra h 1 | 38.3 | 6.9 | 0.003 |
| nAra h 2 | 27.7 | 6.9 | 0.04 |
| nAra h 3 | 25.5 | 6.9 | 0.01 |
| rBer e 1 | 4.3 | 0 | 0.52 |
| nCor a 9 | 14.9 | 10.3 | 0.73 |
| nGly m 5 | 10.6 | 3.5 | 0.40 |
| nGly m 6 | 17 | 6.9 | 0.14 |
| nSes i 1 | 8.5 | 0 | 0.29 |
| PR-10 proteins | |||
| rBet v 1 | 57.5 | 3.5 | < 0.001 |
| Pru p 1 | 44.7 | 3.5 | < 0.001 |
| rGly m 4 | 19.2 | 3.5 | 0.08 |
| rAra h 8 | 23.4 | 0 | 0.005 |
| rApi g 1 | 12.8 | 0 | 0.08 |
| rCor a 1.0101 | 36.2 | 0 | < 0.001 |
| rCor a 1.0401 | 44.7 | 3.5 | < 0.001 |
| rMal d 1 | 33.3 | 3.5 | < 0.001 |
| Cysteine protease | |||
| nAct d 1 | 14.9 | 10.3 | 0.73 |
| Thaumatin-like | |||
| nAct d 2 | 8.5 | 31.0 | 0.025 |
| Kiwellin | |||
| nAct d 5 | 2.1 | 0 | 1 |
| Alt a 1-related | |||
| rAlt a 1 | 14.9 | 37.9 | 0.028 |
| Enolase | |||
| rAlt a 6 | 8.5 | 31.0 | 0.025 |
nArt v 3: Artemisia vulgaris allergen 3; nCry j 1: Cryptomeria japonica allergen 1; nEqu c 3: Equus caballus allergen 3; nSes i 1: Sesamum indicum allergen 1; rAna o 2: Anacardium occidentale allergen 2; rBer e 1: Bertholletia excelsa allergen 1; rHev b 8: Hevea brasiliensis allergen 8
The most relevant sensitization patterns are presented in the heat map (Figure 1).
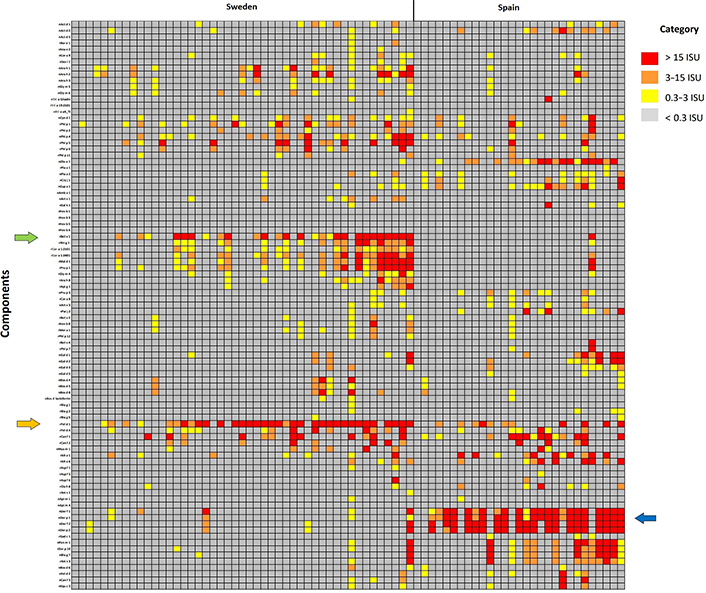
Heat map with the most relevant sensitization patterns in Sweden and Spain. Different clusters appear, where Spanish patients have the highest IgE levels against mite allergen components whereas Swedish patients are against Bet v 1, the major birch pollen allergen, PR-10 proteins, and Fel d 1, the major cat allergen
The mild and humid climate of VAL favours the growth of house dust mites (Dermatophagoides species), molds, and a varied array of plants. Children from Spain showed more frequent sensitization to the pollen of several taxa compared to Swedish patients. Conversely, as expected, sensitization to pollen from birch and Alnus was frequent in Sweden and nearly non-existent in VAL, where these trees hardly grow. However, sensitization to pollen of widespread grasses was not significantly different, reflecting similar exposures.
Sensitization to house dust mites was found to be much more prevalent in Spanish children. A study from South Europe (France) found Der p 2 and Der f 2 sensitization is more common in patients with severe asthma, compared to intermittent, mild, and moderate asthma [8], while sensitization to Der f 2 was associated with wheezing in a cohort of Swedish children [14]. We are not able to confirm these findings as in both of the patients’ cohorts with severe asthma, the sensitization rates were similar for Dermatophagoides group 1 and 2 antigens (Table 1).
Sensitization to cats was significantly higher in Sweden, even though there are no major differences in cat ownership between Sweden and Spain (28% vs. 23%) [15]. However, cat allergens are ubiquitous in society and have been found in classrooms in Sweden [16], while there are no data for Spain. The higher IgE reactivity to dogs noted in Spain probably depends on the fact that household dog ownership in Spain is nearly double that of Sweden (37% vs. 22%).
No definite pattern of cross-reactivity was found among the lipocalins Fel d 4, Can f 1, and Can f 2 probably due to their low sequence homology (23–26%). The importance of lipocalins Can f 4 and Can f 6 has been assessed through their association with positive results in nasal provocation tests [17], opposite to results with prostatic kallikrein Can f 5, but none of them were included in the panel of allergens when the study was made. The prevalence of sensitization to cats and dogs was very high in our patients; previous studies have described that IgE antibodies to lipocalins tend to associate with airway hyperresponsiveness [18], and multisensitization towards them is a marker of extensive exposure leading to that multisensitization and severe disease [9].
Sensitization to birch pollen, by the major birch pollen allergen Bet v 1, a PR-10, explains the possible cross-sensitization to apple, celery, soy, or peanut, all of which contain PR-10 proteins (Table 2), which are associated with mild or even no reaction on ingestion of the food. Sensitization to peach was not significantly different, but component analysis has a clinical impact, as it showed that in Sweden it was due to sensitization to Pru p 1, another PR-10 protein, and in Spain to Pru p 3, an LTP, associated with severe and systemic reactions.
Sensitization to molds was more frequent in VAL than in STO. A higher prevalence of dampness and molds at home has been described in Europe for regions with temperate and warm climates compared to regions with cold climates [19]. Sensitization to kiwi and Alternaria often coexist; spores of Alternaria present on the surface of kiwi can penetrate into the pulp, where they interact and bind to kiwi proteins, leading to positive IgE results for both allergen sources. This interaction has been specifically described for the pair Alt a 1/Act d 2 [20]. The prevalence of sensitization to that pair of allergens was quite similar, as shown in Table 2, but the absolute levels of specific immunoglobulin E (sIgE) were higher for Alt a 1 (Table S3), which points to Alternaria as the primary sensitizer.
Some studies have revealed that sensitization to dust mites on the Mediterranean coast and molds in an inland dry area was associated with the presence of asthma in those populations, sensitization to weeds/pollen did not show the same correlation [21, 22]; these differences in specific allergens between areas is attributed to differences in climate. Our study adds more data to that valuable previous information but focuses on molecular allergen diagnosis and only in severe asthmatic children.
There were no significant differences in sensitization to storage proteins, except for the peanut allergens Ara h 1, 2, and 3, which were more frequent in Sweden. This pattern is associated with clinical food allergy to peanuts, which is less common in Spain than in the Nordic countries [23], where it can either be driven by PR-10, giving rise to oral allergy syndrome (OAS), or by storage proteins, resulting in often severe peanut allergy.
Molecular allergology permits the interpretation of sensitization beyond allergenic sources [9]. Sensitization to tropomyosins, found in mites, can account for cross-sensitization to crustaceans, cockroaches, and Anisakis in Spanish patients, as the four tropomyosin molecules (with > 70% homology) showed the same prevalence. The 12 patients with sensitization to tropomyosins were sensitized to all four tested tropomyosins, and with very similar values as shown in Table S3.
Regarding CCD-bearing proteins, in Sweden, the predominant one was Phl p 4. There were six patients who were sensitized to Phl p 4 and also to some of the others, while twelve patients were sensitized only to Phl p 4. In Spain there were 15 patients sensitized to this type of protein (mostly from Platanus and cypress pollen); seven to all four proteins, two to three proteins, three to two proteins, and three to only one protein. This pattern, so different from tropomyosins, is due to the low homology (14–19%) of these CCD-bearing proteins.
Nearly 90% of the studied patients showed positive results to more than one group of allergens, supporting the idea that, as described in the literature, a multimorbid polysensitized phenotype exists, associated with the severity of asthma and rhinitis [24], and may also reflect the fact that interactions between pairs of sIgE components are associated with increased risk of asthma [25].
Limitations of the study are that only children with severe asthma, as defined by GINA, were included and only if they were sensitized and had ISAC results available for a uniform comparison between countries. Non-sensitized children and less severe degrees of asthma were out of the scope of the study and extrapolation of our results cannot be made to patients not meeting our criteria of inclusion. Furthermore, positive IgE results prove sensitization, but not necessarily allergy with impact on the disease, especially when considering sensitization to foods. There are important climatic differences between Sweden and Spain, but also across regions within these countries, as it happens in almost every country in the world; results could vary accordingly just like other aspects of diseases with great environmental influence. No information about exposure to molds or dampness in children’s houses was collected despite knowing that its exposition during infancy increases the risk of asthma and rhinitis asthma through 16 years of age, and may be associated with persistent asthma up to adolescence [26]. Food allergy has been associated with the development of asthma and acute episodes of bronchospasm [27]. Its role in chronic severe asthma remains to be elucidated. As we aimed to compare severe asthma from two distinct populations, we did not collect data on symptoms, like OAS or others elicited by food allergens, which is another limitation of our study.
In summary, the relative sensitization profile in our children with severe asthma was more similar to that reported in the literature for other sensitized children from the same geographic areas with non-severe disease than to that of severe asthmatics from different areas [14, 28]. According to the analysis of the applied ISAC chip, there is not a specific sensitization profile shared by children with severe asthma across countries, supporting the concept that sensitization is linked to the exposome of the area. Nevertheless, a polysensitized phenotype was found in both Swedish and Spanish children’s severe asthma cohorts.
It is described that the Mediterranean diet is characterised by high consumption of plant-based foods and olive oil, moderate consumption of alcohol, and limited consumption of meat; and Swedish diet encourages the intake of fruits, vegetables, fish, and fibre, while discouraging the intake of sugar and saturated fat [29, 30]. We might acknowledge that children from both areas should probably follow these general patterns, but the lack of data doesn’t allow us to assume that with more severe asthma.
Other factors, such as degree of exposure, environment, genetic or epigenetic characteristics, defects of skin barrier facilitating sensitizations, other comorbidities, and maybe sensitization to other allergen molecules not explored in the current study, could account for asthma severity in sensitized children [31].
CCD: cross-reactive carbohydrate determinant
IgE: immunoglobulin E
ISAC: Inmuno Solid-Phase Allergen Chip
LTPs: lipid transfer proteins
nAct d 1: Actinidia deliciosa allergen 1
nAra h 1: Arachis hypogaea allergen 1
PR-10: pathogenesis-related class 10 proteins
rAlt a 1: Alternaria alternata allergen 1
rBet v 1: Betula verrucosa allergen 1
rCan f 1: Canis familiaris allergen 1
rDer f 1: Dermatophagoides farinae allergen 1
rDer p 1: Dermatophagoides pteronyssinus allergen 1
rFel d 1: Felis domesticus allergen 1
rPhl p 1: Phleum pratense allergen 1
rPru p 1: Prunus persica allergen 1
STO: Stockholm
VAL: Valencia
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100919_sup_1.pdf.
We gratefully thank the collaboration of ThermoFisher Scientific. This work was also supported by the Freemason Child House Foundation in Stockholm, the Consul Th. C. Bergh’s Foundation, the Swedish Asthma and Allergy Association’s Research Foundation, the Centre for Allergy Research at Karolinska Institutet, the Swedish Heart‐Lung Foundation, the Swedish Research Council, Region Stockholm (ALF-project), and the Swedish Cancer and Allergy Foundation.
JMG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Visualization, Writing—original draft, Writing—review & editing. JRK: Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing—review & editing. GH: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing—review & editing. MvH: Formal analysis, Investigation, Methodology, Supervision, Validation, Writing—review & editing. ANG: Conceptualization, Methodology, Resources, Supervision, Writing—review & editing. MNC and SU: Data Curation, Resources, Supervision, Writing—review & editing. AM: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Supervision, Visualization, Writing—original draft, Writing—review & editing.
Dr. van Hage reports personal fees from Thermo Fisher Scientific outside the submitted work. Dr. Konradsen has received research support from Thermo Fisher Scientific outside the submitted work.
The study was approved by the Ethics Committee from Hospital La Fe-Comité de Ética de la Investigación con medicamentos (Registry number 2020-005-1) and sheltered by the regional board of ethics at Karolinska Institutet (Registry number Dnr 2006/1324-31/1 and 2008/378) and was conducted in accordance with the Declaration of Helsinki.
The informed consent to participate was waived by the Ethics Committee from Hospital La Fe-Comité de Ética de la Investigación con medicamentos since the data collected for the study was retrospective.
Not applicable.
All data generated or analysed during this study are included in this article or its Supplementary materials. Further enquiries can be directed to the corresponding author (jaume.marti.garrido@gmail.com).
This project has been granted awards from Sociedad Española de Alergología e Inmunología Clínica (SEAIC), Asociación Valenciana de Alergia e Inmunología Clínica (AVAIC) and Colegio Oficial de Médicos de Valencia (COMV). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1964
Download: 15
Times Cited: 0
