Affiliation:
1Swami Vivekanand Subharti University, Meerut 250005, India
Email: amarprakashgarg@yahoo.com
ORCID: https://orcid.org/0000-0003-0613-9495
Affiliation:
2School of Biological Engineering & Life Sciences, Shobhit Institute of Engineering & Technology, Modipuram, Meerut 250110, India
Affiliation:
2School of Biological Engineering & Life Sciences, Shobhit Institute of Engineering & Technology, Modipuram, Meerut 250110, India
ORCID: https://orcid.org/0000-0002-6568-3469
Affiliation:
3Inpatient and Outpatient Services at CORECARE BEHAVIORAL HEALTH, Media, PA 19063, USA
4Current address: Shobhit Institute of Engineering & Technology, Deemed-to-be University, Meerut 250110, India
Explor Asthma Allergy. 2024;2:9–32 DOI: https://doi.org/10.37349/eaa.2024.00026
Received: June 20, 2023 Accepted: December 19, 2023 Published: February 20, 2024
Academic Editor: Pasquale Caponnetto, University of Catania, Italy
The article belongs to the special issue Asthma and its Relationship with Psychological and Psychopathological Factors
Asthma is one of the most common respiratory diseases in humans throughout the world. The illness continues to be the most prevalent cause of respiratory morbidity and affects both adults and children. Asthma is mainly caused by microbes, especially the species of Aspergillus. It causes continuous irritation and distracts the mental attention of the patient, leading to physical weakness and depression resulting in immune-compromised conditions. Asthmatic patients need careful attention and continuous treatment. Taking into account its major effects on patients’ quality of life, the challenging nature of the therapy, and side effects of the novel therapeutic strategies that influence the clinical course of asthma are required to be considered before finally deciding the course of treatment. Children with asthma and wheezing are frequently sustained by a type-2 immune response. In addition, people with wheezing and asthma can be identified by the presence of digestive and respiratory tract dysbiosis. Therefore, oral probiotics could be used as an additional asthmatic medication to manage asthma, but the decision should be constantly monitored by specialized persons. During the last two decades, the importance of probiotics in the treatment of various ailments has been realized and several researches are being conducted to find out the impact of healthy gut microbiome on the management of various diseases including asthma.
One of the most prevalent chronic diseases in the world is asthma. More than 339 million people worldwide are considered to have asthma, which represents a remarkable 3.6% rise in age-standardized prevalence since 2006 according to the 2016 Global Burden of Disease study [1]. It is an air-borne infection and can be characterized by the barrier of airflow. It occurs when the airway swells but in diversified presentation. It can be caused by different factors such as smoking and allergic fungi [2]. Asthma is worsened by environmental stimuli like dust, pollutants, smog, pollen, and molds [2–6]. At this stage, it becomes very difficult to treat and control asthma and the patients need at least more than one medication to manage it [4, 5]. Some omnipresent fungi are also responsible for respiratory diseases as well as invasive pulmonary mycosis, fungal balls in lung cavities, allergic bronchopulmonary mycosis (ABPM), and severe asthma with fungal sensitization (SAFS) in eczema patients [7, 8]. There is a very important role of fungi in worsened and complicated asthma globally. In 2006, the SAFS term was used in a review article and it was acknowledged that asthma has already caused illness since the seventeenth century [7]. However, the role of fungi in asthma still remains controversial and needs more research. Molecular techniques are now used to identify the causal agent of asthma where the fungi and particularly the species of Aspergillus have been shown to be associated [9, 10]. People residing in damp and dark places are more prone to asthmatic infections mainly because of higher survival rates of fungal propagules in such environments. It is, therefore, recommended that asthmatic patients to live in well-ventilated naturally lighted rooms. Sun rays are lethal and can kill the fungal spores and mycelia.
Due to its great prevalence, asthma is a significant problem for healthcare and subsequently places a significant load on the system [11]. Particularly, among kids and teenagers, asthma is the most prevalent chronic illness [12]. All of the existing treatments for asthma generally work to regulate the pathological condition because the disease cannot be totally treated. Patients and governments are under heavy financial strain [13, 14]. One of the most significant health issues, particularly in developed nations, is allergic asthma. The pathogenesis of allergic asthma involves a number of processes with trigger factors (Figure 1). Dysregulation of the immune system has a direct impact on health and contributes to the pathogenesis of a number of immunological illnesses, including allergy diseases. On the other hand, pulmonary oxidative stress and injury can result from an imbalance of inflammatory and anti-inflammatory molecules, as well as deregulation of the lung immune system via the Toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) signaling pathway [15, 16].
The most extensively researched and commercialized bacterial strains of probiotics were the research subject of a recent study by Malaysian researchers [17]. Major depressive disorder (MDD) management may involve adjunctive or primary therapy [18]. They developed a conceptual framework for probiotics using an evolutionary method in order to successfully integrate it into the precision psychiatry model, which aims to provide personalized therapy [18].
There are two reasons for which asthma attacks happen: (a) loads of aeroallergen and grass pollen (thunderstorm asthma); (b) fungal allergen [19–22]. The fungi are the primary exogenous drivers of asthma but have been completely ignored and not explored adequately. SAFS means allergic sensitization caused by fungi but it is not justified because allergy is not responsible for moderate asthma. Fungal infection does not require an airway. The protease and other enzymes can damage tissue, disrupt the airway, and cause mucosal damage. Hence, chronic fungal bronchitis has to be acknowledged. In the environment, protease activity fungi-initiated T-helper 2 (Th2) cell-related air inflammation can cause Th2 cell chronic airway disease in asthma [23, 24]. At least one-fourth of asthma patients have symptoms of Aspergillus fumigatus sensitization and it is very difficult to treat [8]. Sensitization from fungal species can be associated with more severe asthma as well as bronchiectasis and higher eosinophil counts and immunoglobulin E (IgE) levels, and it can cause worse clinical outcomes—uncontrollable asthma. It can also cause lung infection and Intensive Care Unit (ICU) admissions increased, asthma-related lung failure and deaths were also recorded [25–28]. In previous studies, it was noticed that the Aspergillus fumigatus cultures were seen in asthma patients who were IgE-sensitized [29]. In other studies, it was stated that various fungi can cause fungal sensitization and can be caused only by some specific fungi such as Aspergillus fumigatus, Aspergillus-affected individuals are most likely to have underlying asthma or cystic fibrosis or allergic respiratory evident [30, 31]. Lung disease and weak immune system can cause phenotypic severity and it can worsen simple fungal asthma to SAFS to allergic bronchopulmonary aspergillosis (ABPA) [7, 32]. The relationship between ABPA, SAFS, and allergic fungal airway disease (AFAD) is illustrated in Figure 2.
Aspergillus is omnipresent in the environment, hence, the inhalation of Aspergillus spores is inescapable. Aspergillus fumigatus is an airborne microbe; it lives in soil and can be found in compost and waterlogged structures. The spores of Aspergillus fumigatus are 3–5 µm in size and can easily be inhaled into bronchial airways [32]. In an infected person with healthy immunological function, Aspergillus conidia can be seen as clearly associated with symptoms. Aspergillus fumigatus is a disturbing species and has pathogenic potential, so it can lead to dominant allergic and invasive fungal disease [33].
Relationship between air pollutants, asthma, and life exposure depends upon various factors [34]. They enter and take place in the host body with genetic susceptibility and many other factors yet to be defined, and these points are very important in asthma pathogenesis. Microbes like fungi can make the situation worse in asthma in children and adults [34, 35].
In previous studies, it is mentioned that those fungi which play roles in asthma belong to the genera Cladosporium, Eurotium, Penicillium, and Aspergillus. Other genera are also detected but in low abundance such as Candida, Malassezia, and Pneumocystis [36].
These fungal species are found in asthma samples. Sometimes the sample may also be contaminated with oral cavity microfungi and they need to be confirmed. Some other fungi have also been detected in sputum or fluid in respiratory diseases like asthma [23, 37] which are shown in Table 1. Fungi species like Aspergillus, Candida, Alternaria, Cladosporium, and Crytococcus species [23, 37–50] were isolated from asthmatic patients with Th2-type disease and it suggests that the infection is obvious [23]. Previous studies suggest that patients show increased fungal-specific IgE response and memory phenotype when reacting with fungal antigens and eosinophils that are involved in fungal killing [38, 51]. Fungal species contribute to asthma infection at a severe level. In this article, we discuss fungal asthma, how fungi cause asthma in healthy lungs, and how in the gut can it convert into severity and cause multiple barriers in sites [37]. Fungi such as Malassezia and Trichophyton cause skin allergy and allergic asthma through atopic march [46].
Fungal species associated with allergic asthma from various routes of sensitization
| Fungal genera | Fungal species | Allergens | Activity | Route of exposure | Type of asthma | References |
|---|---|---|---|---|---|---|
| Aspergillus spp. | A. fumigatus A. nidulans A. versicolor A. flavus A. niger A. terreus | Asp f 1 Asp f 3 Asp f 11 Asp f 22 Asp n 14 Asp t 36 | Ribotoxin Peroxisomal membrane protein Protein Enolase | Inhalation | ABPA | [28–31, 52–55] |
| Alternaria spp. | A. alternata | Alt a 4 | Trasporter, disams defense Protein initiation factor Thioredoxin | Inhalation | Bronchial asthma | [17, 32, 55–57] |
| Cladosporium spp. | C. cladosporioides C. herbarum | Clac h8 Clac c14 Clac c9 | Peroxisomal protein Transaldolase Mannitol dehydrogenase | Inhalation | Allergic asthma | [33–35, 55] |
| Malassezia spp. | M. globosa M. furfur M. sympodialis M. restricta M. japonica | MGL_1304 Mala f 2–4 Mala s 1–11 Mala Ex | Unknown Stress responses Protease activity Vesicle trafficking and innate immune activation | Skin contact Inhalation Ingestion | Allergic respiratory disease | [36, 37, 58] |
| Cryptococcus spp. | C. neoformans | Chitin Chitosan | Immune activation CNS invasion | Inhalation | Th2-type asthma | [38, 39, 58] |
| Trichophyton spp. | T. interdigitale T. tonsurans T. mentagrophytes | Trit 1 Trit 4 | Enzymatic activity Sporulation | Skin contact | SAFS | [40, 55] |
Asp f: Aspergillus fumigatus; Asp n: Aspergillus niger; Asp t: Aspergillus terreus; Alt a: Alternaria alternata; Clac h: Cladosporium herbarum; Clac c: Cladosporium cucumerinum; Trit: Trichophyton; CNS: central nervous system; spp.: species
Note. Adapted from “Environment and host-genetic determinants in early development of allergic asthma: contribution of fungi,” by Hadebe S, Brombacher F. Front Immunol. 2019;10:2696 (https://doi.org/10.3389/fimmu.2019.02696). CC-BY.
Asthma is a diverse illness supported by bronchial hyper-reactivity, reversible airflow restriction, and chronic respiratory tract inflammation [1, 7]. In addition, the severity and duration of symptoms and bronchial obstruction may change. Asthma phenotypes and endotypes are defined by clinical, functional, and pathological characteristics [1, 7]. The type-2 phenotype is more common in children in this situation, and allergic asthma predominates most of the time [1, 59].
According to Agarwal [55], a new asthma recently described is called SAFS that can cause ABPA, and it is diagnosed with severe asthma [17]. Because of the scarcity of data availability, it is doubtful how to diagnose it. More researches are required at different demographic levels to better understand such type of asthma.
As we know, Aspergillus is found everywhere, indoors and outdoors. It occurs more and in severe form in dark and moist rooms on the walls and in the corners. Aspergillus is not harmful, but some species can cause serious problems and illness, especially in people with weakened and compromised immune systems. In our laboratory, we have isolated a number of species of Aspergillus from the dump of waste disposal in Meerut. The major species isolated are Aspergillus fumigatus, A. nidulans, A. versicolar, A. flavus, and A. niger; of which Aspergillus fumigatus, A. nidulans, A. versicolor, A. flavus, and A. niger are known to cause fungal asthma (Figure 3). If people with weakened immune systems inhale spores of Aspergillus species, they can be infected with lung disease or asthma. In some people, spores can trigger allergic reactions, but in others, they can be dangerous and develop mild-to-serious lung infections. Aspergillosis is one of the most serious lung diseases which is caused by fungus. It is an invasive disease, and the infection can spread to the blood vessels and all over the body. Symptoms may vary with the different types of illness patients develop.
Allergic reaction: In some patients, asthma or cystic fibrosis may develop an allergic reaction to Aspergillus. Signs and symptoms of this reaction disease called bronchopulmonary aspergillosis and the signs are fever, cough with blood or plugs, and worsening asthma.
Aspergilloma: Chronic lung disease as in pulmonary conditions like emphysema, tuberculosis, or advanced sarcoidosis which can cause cavities in lungs. When patients with these diseases get infected with Aspergillus, the fungus balls (tangled masses) grow in the lungs, and this is called aspergilloma. In this disease, mild symptoms may occur like cough bringing up blood sometimes (hemoptysis), wheezing, shortness of breath, and weight loss.
Invasive aspergillosis: Invasive aspergillosis is one of the most severe types of aspergillosis. It is a spreading disease and it occurs when infection spreads to the brain, heart, kidney, and skin. Invasive aspergillosis may be caused in those patients who have undergone chemotherapy, bone marrow transplantation, or a disease that weakened their immune system. Invasive aspergillosis sometimes may be fatal. General symptoms of invasive aspergillosis are fever and chills, cough that brings up blood (hemoptysis), shortness of breath chest pain, headache, and skin lesions.
Other types of aspergilloses: Aspergillus can spread to the patient’s body and lungs. Fungus also causes sinuses in patients and in this condition, a stuffy nose can contain blood, fever, facial pain, and headache may occur [43, 60].
ABPA was reported in 1952 by Hinson et al. [61] who described three patients who were diagnosed with pulmonary infiltrates and eosinophilia in blood and sputum. It was clinically proven that these symptoms occur when patients are suffering from severe asthma. But for ABPA, there are no specific physical or chemical examination findings at present to diagnose on the basis of symptoms like cough, wheezing, shortness of breath with fever, anorexia, and malaise. ABPA can be identified by local and IgE circulating specific Aspergillus antibodies and skin tests with reactive Aspergillus, eosinophilia, increased interleukin-2 (IL-2) level, and increased serum IgE level [51]. It includes new lung infiltrates or bronchiectasis on chest imaging. Pathologically, ABPA is characterized by one or more of the conditions like mucoid impaction of bronchi, bronchocentric granulomatosis, eosinophilic pneumonia, and exudative or obliterative bronchiolitis [43, 60].
Bronchial asthma is diagnosed using a specific skin test in IgE levels for Aspergillus antigens. If the IgE levels are below 1,000 IU/mL, high resolution computer tomography (HRCT) test is conducted to observe mild-to-severe asthma which is further diagnosed using Asthma and Allergy Foundation of America (AAFS) where a positive test suggests mild-to-moderate asthma while the SAFS-positive test indicates severe asthma. When HRCT-complete blood count (CBC) is negative then invasive bronchial-pulmonary aspergillosis (IBPA) is conducted to confirm negative asthma; if HRCT-CBC is positive than ABPA-CBC is performed for confirmation [54, 62].
Probiotics were defined by the Food and Agriculture Organization (FAO)/World Health Organization (WHO) in 2001 as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”. This definition has been widely adopted by scientific communities, businesses, organizations, and consumers. Everyone believes that before asserting the existence of a specific benefit, a probiotic strain should have been examined in well-controlled research to do so [63, 64]. The only permitted claim, in this case, would be “contains probiotics”. Studies utilizing probiotics or prebiotics have typically been exploratory in nature and have not been sufficiently structured to meet the European Food Safety Authority (EFSA)’s current standards for substantiating a health claim [65]. The therapeutic uses of probiotics and prebiotics in conditions like asthma and pneumonia have not been adequately researched. It has been demonstrated that probiotics and prebiotics have immune-modulator effects and are implicated in the pathophysiology of a number of disorders [66–68]. Probiotics (beneficial bacteria) and short- and long-chain fructo-oligosaccharides (lcFOS) prebiotics have been shown to minimize allergy sensitization by controlling immune responses. B cells, Th1, Th2, Th17, T regulatory (Treg), and other immune cells can all be modulated by probiotic bacteria [15, 66, 67].
Probiotics as a component of the commensal gut microbiota can have an impact on the TLRs, particularly TLR4 [68, 69]. There are numerous microbial illnesses that can activate TLR4 signaling. Lipopolysaccharide (LPS) is a component of bacterial structure, and bacteria use the LPS/TLR4/NF-κB pathway to activate TLR4 [16, 70, 71]. However, LPS can cause acute lung damage and inflammation, which causes an inflammatory cell buildup in the lung and airway [72, 73]. Ovabumin (OVA) can cause allergic asthma in a model, but OVA-LPS can also cause asthma and acute airway inflammation. Additionally, TLR4 and active TLR4 signaling may be involved [15]. The gut microbiome plays a significant role in the management of asthma and its quality and quantity change with the type and severity of asthma (Table 2). Whether the change of gut microbiota is the cause of asthma or the disease results in the change of gut microbiota is not yet very clear. Gastroenterologists are finding scientific causes for such a change in gut microbiota.
Changes of gut microbiota in allergic asthma and allergic rhinitis (AR)
| Types of disease | Increase | Decrease |
|---|---|---|
| Allergic asthma | Streptococcus Firmicutes Candida Rhodotorula | Lachnospira Veionella Faecalibacteium Rothia Bifidobacterium Ruminococcusgnavus Alloprevotella Oscillibacter Lactobacillus |
| AR | Klebsiella Parabacteroides Butyricicoccus Proteobacteria | Actinobacteria Escherichia-Shigella Oxalobacter Clostridiales Eisnbergiella Becteroidetes Bifidobacterium |
Note. Adapted from “Effect of probiotics on respiratory tract allergic disease and gut microbiota,” by Huang J, Zhang J, Wang X, Jin Z, Zhang P, Su H, et al. Front Nutr. 2022;9:821900 (https://doi.org/10.3389/fnut.2022.821900). CC-BY.
It is thought that the gut microbiota plays a significant role in defining allergy risk and immune system development [74]. However, recent investigations have cast doubt on this. It is widely believed that the human fetus is sterile while it is developing in the womb [75]. However, newborns are exposed to germs from their mother and the environment both during and after the delivery birthing process [76]. The type of delivery has a substantial impact on the microorganisms that can colonize the gut, and kissing, sucking, and cuddling the newborn shortly after birth can also transmit microbes from the mother to the child [76]. The colonization of the gut starts on the 6th day of the birth of the child and gets stabilized at the 450–600 days. Sociological contacts and psychological factors greatly influence the gut microbiota. Infants are exposed to new microorganisms after birth through diet (such as breastfeeding) and environmental exposure.
There are numerous different probiotic species, but the most prevalent ones are Lactobacilli and Bifidobacteria [77]. Each has been thoroughly investigated in the context of allergies and has been linked to decreased incidence of asthma, AR rhinoconjunctivitis (ARC), food allergy, and atopic dermatitis (AD) [78]. Through the production of cytokines that support the Th1 pathway while suppressing the Th2 pathway, which is linked to the development of allergies, these bacteria have been demonstrated to alter the host’s Th1/Th2 balance [79]. Probiotics have been associated with the primary prevention of allergies and allergy disorders in a number of studies, particularly when given to youngsters [80, 81].
Intestinal microbiota that is “healthy gut” promotes the rate of immunity [17, 31]. Gut-associated lymphoid tissue (GALT), which includes Peyer’s patches, has been shown in other investigations to be underdeveloped or missing in germ-free mice [82, 83]. It was revealed that the redevelopment of GALT and the induction of tolerance may result from the neonatal administration of Bacteroides fragilis to germ-free mice’s lower gut [84]. It is also established that the host’s risk of contracting allergy and inflammatory disorders increases if an effective immunological tolerance cannot be developed early in life [85]. For instance, mice raised in sterile environments have fewer Treg cells that produce IL-10 and IgA, and they are unable to acquire oral antigenic tolerance [17, 85–87]. The several factors that influence the advantages of probiotic supplementation include a system for action. Maldonado Galdeano et al. [88] recently examined probiotics’ advantages for people. Oral probiotics interact with the body after administration via TLRs, intestinal epithelial cells (IECs), and immune-competent cells [89]. The creation of mediators, cytokines, and chemokines is encouraged by this interaction. The activation of mucosal immunity is caused, in part, by macrophage chemo-attractant protein 1, which is secreted by IECs and is sustained by an increase in mucosal tissues’ IgA-secreting cells [90, 91].
Filamentous bacteria with segments, specifically clusters IV and XIVa, support the growth of Treg cells and IL-17-producing T cells, respectively [90–92]. Additionally, food’s gut microbiome, when put into germ-free mice and allergic mice but not tolerant ones, transmitted susceptibility to food allergy [69]. The examination of dysbiosis in people with food allergies is complicated by a number of circumstances. Because of the variety in study design, sample size, age at fecal collection, methods of gut microbiome analysis, and geographical location, comparisons between studies are challenging [93]. Nevertheless, with time and the increasing accessibility of new tools, the evidence of gut dysbiosis in food allergy is growing. Babies with cow’s milk allergies had higher total bacterial and anaerobic counts as revealed in bacterial cultures [94], numerous hypothesized mechanisms have been proposed to explain how the commensal microbiota affects the course of the allergic reaction [95]. Through their TLRs, intestinal bacteria can directly affect Tregs, modulating innate lymphoid cells. By means of a Treg intrinsic, TLR and myeloid differentiation primary response gene 88 (MyD88) reliant mechanisms and commensal microbiota support the differentiation of induced Tregs (iTreg) from naive clusters of differentiation-4 (CD4+) T-cells [96, 97].
There are a number of Lactobacillus species that are helpful in the treatment of respiratory allergies such as L. reuteri, L. casei, L. rhamnosus, L. acidophilus, L. plantarum, B. lactis, and Bifidobacterium longum. L. reuteri modification caused pro-inflammatory cytokines like IL-5 and IL-13 to be produced at lower levels and the outcome is a reduction in the influx of inflammatory cells to the lungs (Figure 4). L. casei helps in dendritic cell (DC)-specific intercellular adhesion molecule 3-grabbing non-integrin modulates DC activity in vitro after the induction of Tregs that produce IL-10 in vitro. Increased L. rhamnosus and B. lactis transforming growth factor-β (TGF-β) in vivo expression is connected with the induction of Tregs that prevent allergic sensitization and airway illness in mouse models of asthma [62]. L. rhamnosus and Bifidobacterium decrease persistent chronic allergic inflammation through upregulation of TLR4 and messenger RNA (mRNA) expression, which helps in airway remodeling, mast cell degranulation inhibition, and suppression of pulmonary airway inflammation. L. acidophillus stimulates DCs in vitro and this increases TGF-β production. L. plantarum regulates high concentrations of specific IgG2a, inhibits the specific IgE response, and enhances interferon-gamma (IFN-γ) production [98].
T cells are also triggered by probiotics. Additionally, probiotics encourage the release of IL-10, the primary regulatory and anti-inflammatory cytokine from Treg cells [99]. Probiotics also strengthen the intestinal barrier by boosting mucins, tight junction molecules, goblet and Paneth cells. Probiotics regulate the gut microbiota by maintaining homeostasis and preventing the spread of infections, among other things [100]. Additionally, probiotics’ viability is essential to guarantee their impact on innate immunity [101]. Probiotics may therefore be an intriguing natural method for both prevention and treatment. By decreasing IgE production and boosting the immune response to combat infections, they could increase type-1 response [102, 103]. SCFAs, which are produced by bacterial fermentation of food fibers, are another way in which the commensal flora encourages tolerance. By increasing colonic Treg cells, SCFA protects mice from intestinal inflammation by acting on T cells through the G-protein-coupled receptor 43 (GPR43). Intestinal Treg cells can be produced from naive CD4+ T cells by T-cell intrinsic epigenetic pathways, which is another benefit of SCFAs [104]. With increased forkhead box protein 3 (Foxp3) protein acetylation, butyrate, an SCFA recognized as a histone deacetylase inhibitor, confers higher stability and enhanced suppressive action on de novo-produced intestinal iTreg cells [105]. A high-fiber diet reduces allergic airway inflammation by changing the flora’s composition, which increases Bacteroidetes and decreases Firmicutes while raising the amounts of SCFAs in the bloodstream [106].
In general, preventive strategies are [107]:
Prevention of exposure to tobacco smoking during pregnancy and after delivery is a general health education message.
Primary prevention for babies who are more vulnerable. If one or both parents have allergies or have had allergies themselves, there is a clearly elevated risk of allergy symptoms.
Secondary preventive techniques for kids who already have allergic sensitization or the early signs of allergic disorders; these techniques work to lower the frequency of clinical manifestations like rhinitis, food allergies, or asthma.
Pre-clinical research has demonstrated that altering the microbiota can influence the host’s overall immune response, hence lowering allergy inflammation and sensitization shown in Figure 5 [108, 109]. Numerous researches have raised the possibility that pre- and probiotics could provide protection against asthma [65, 67, 74, 79, 89].
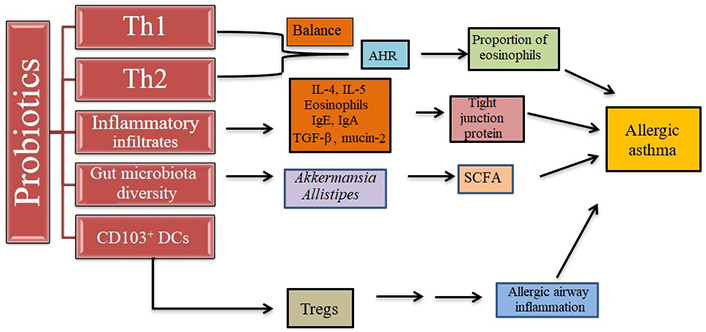
Effect of probiotics on allergic asthma. Probiotics regulate the Th1 and Th2 cell balance for the protection of the immune system, increase the number of Tregs, reduce inflammatory response, and regulate gut microbiota. AHR: airway hyperresponsiveness
Note. Adapted from “Effect of probiotics on respiratory tract allergic disease and gut microbiota,” by Huang J, Zhang J, Wang X, Jin Z, Zhang P, Su H, et al. Front Nutr. 2022;9:821900 (https://doi.org/10.3389/fnut.2022.821900). CC-BY.
When allergens are inhaled, the innate immune system is stimulated to release cytokines that help CD4+ T cells express and recognize antigens and activate T cells and antigen-presenting cells to induce Th2 responses [110, 111]. Th2 cytokines (IL-4, IL-5, IL-9, and IL-13) cause eosinophilia in the airways, pulmonary lymphocytosis, mastocytosis, alternative macrophage activation, and epithelial cell proliferation with goblet cell hyperplasia, which are alterations that resemble asthma in the airways and lung parenchyma. A number of inflammatory disorders have been linked to matrix metalloproteinases (MMPs), a family of enzymes that cleaves extracellular matrix proteins [84]. MMP9 levels are specifically markedly elevated in asthma [112]. It has also been demonstrated that administration of Lactobacillus rhamnosus GG (LGG) reduces MMP9 expression in lung tissue and prevents inflammatory cell infiltration. In addition, in OVA-sensitized mice, LGG decreased blood levels of OVA-specific IgE, inhibited methacholine-induced AHR, and decreased the quantity of infiltrating inflammatory cells and Th2 cytokines in both serum and bronchoalveolar lavage fluid [113]. Other probiotics have yielded comparable outcomes [114]. Particularly in children with paediatric asthma, LGG was found to lower the level of exhaled nitric oxide in 4- to 7-year-olds [115], however, it was unable to duplicate these findings [116]. Early administration of Lactobacillus reuteri, Lactobacillus rhamnosus HN001, or Lactobacillus paracasei species paracasei F19 did not reduce asthma [respiratory rate (RR) 1.16; 95% confidence interval (CI) 0.33–4.10], and neither did Lactobacillus paracasei species paracasei F19 (RR 1.05; 95% CI 0.39–2.81) [117–119].
Based on the in vitro manipulation of cytokine production, probiotic bacteria have produced better effects. It has been demonstrated that Bifidobacterium bifidum, B. lactis, and Lc. lactis can effectively induce IL-10 and significantly suppress the Th2-related cytokines IL-5 and IL-13 [120]. Administered perinatally in a selected combination, they reduced the development of eczema up to the age of 2 years. Their beneficial effect does not reach the age of 6 years and does not lead to primary prevention of asthma. A systematic review of randomized trials assessing the effects of any probiotic administered to pregnant women, breastfeeding mothers, or infants demonstrated that probiotics could reduce the risk of eczema in infants [121]. Due to the possibility of bias, the inconsistent and imprecise nature of the results, and the indirect nature of the available research, the certainty of the evidence is low or extremely low. Replication of the encouraging findings in collaborative, well-coordinated multicenter harmonized studies with multidisciplinary expertise in paediatrics, immunology, and microbiology would, therefore, be of utmost significance to enable future evidence-based implementation, as emphasized in two recent reviews [122]. A longer-term effect from gut microbiota control is possible [123, 124].
Probiotics’ positive benefits are significantly influenced by a number of factors, including strain type, duration, and method of administration. Probiotics have been the subject of numerous studies looking into the impact on allergic airway disorders in mouse models. These probiotics are commonly administered orally, through food or drink [125, 126], or intra-gastric intubation [127, 128]. In many mouse investigations, intranasal probiotic bacteria delivery has recently received more attention [125, 129]. In the study by Spacova et al. [130], the researchers showed that giving mice LGG intravenously reduced the birch pollen-cause asthma in mice. Treatment of asthma using probiotics has been summarized by Huang et al. [98] where various combinations are currently being used and prescribed by medical doctors throughout the world including in developing countries like India (Table 3).
Treatment of asthma with the help of probiotics
| Types of diseases | Single probiotics | Combination of probiotics |
|---|---|---|
| Allergic asthma | Lactobacillus paracasei L9 Lactobacilli Bifidobacterium breve M-16V Bifidobacterium 7952 Bifidobacterium longum 51A Feacalibacterium prausnitzii L. salivarius L. gasseri L. acidophilus L. paracasei L. rhamnosus Bulgarius | Lactobacillus plantarum GREEN CROSS Wellbeing 1001, Lctobacillus rhamnosus GCWB1085, and Lctobacillus rhamnosus GCWB1156 Lactobacillus gasseri LK001, Lactobacillus salivarius LK002, Lactobacillus johnsonii LK003, Lactobacillus paracasei LK004, Lactobacillus reuteri LK005, and Bifidobacterium animalis LK011 |
| AR | Lactobacillus rhamnosus LGG Lactobacillus Bifidobacterium shortum Lactobacillus plantarum Lactobacillus plantarum CJLP133 or CJLP243 Clostridium butyricum CGMCC0313-1 Bifidobacterium longum IM55 Lactobacillus plantarum IM55 Enterococcus faecalis | Bifidobacterium longum BB536, Bifidobacterium infantis M-63 Bifidobacterium shortum M-16V Lactobacillus garciae KS-13, Bifidobacterium bifidum G9-1 and Bifidobacterium longum MM-2 |
Note. Adapted from “Effect of probiotics on respiratory tract allergic disease and gut microbiota,” by Huang J, Zhang J, Wang X, Jin Z, Zhang P, Su H, et al. Front Nutr. 2022;9:821900 (https://doi.org/10.3389/fnut.2022.821900). CC-BY.
In the study by Pellaton et al. [125], the efficacy of intranasal administration was also demonstrated. Their research showed that, as compared with oral therapy, intranasal administration of L. paracasei NCC2461 can alter both the systemic and local immune response [131]. Additionally, human clinical trials to examine the impacts of probiotics on the airways have just been taken up [132]. Probiotics are typically given orally during clinical trials, such as when added to dairy products or taken as capsule supplements, and they primarily work through modulating the immune system. On the other hand, we believe that local administration spray can cause more niche-specific effects both in the upper and the lower airways, leading to a more successful treatment, based on the data obtained in several mouse models [125, 129, 132].
There have been numerous randomized placebo-controlled trials to look at the possibilities of oral probiotic administration in conditions affecting the upper respiratory system [132, 133]. In a meta-analysis by Peng et al. [134], probiotic effectiveness for the prevention and/or treatment of AR was examined in 11 randomized controlled trials. Results indicated that probiotic medication improved the patients’ quality of life and nasal symptoms in these 11 trials. However, none of the probiotic approaches examined could lessen the inflammatory markers in these people or stop AR [135]. Most recently, in a meta-analysis by Güvenç et al. [133], 22 double-blind, randomized, and placebo-controlled studies were included, and 17 of them showed improvements in the clinical symptoms of AR patients, such as nasal obstruction, rhinorrhea, and nasal itching. In 8 of the included trials, these favorable outcomes were related to a drop in inflammatory markers [133, 135]. Patients with AR have also been investigated when multiple strains are combined. For instance, AR patients were evaluated by Hartsock and Nelson [136] on a probiotic yoghurt containing a combination of L. rhamnosus GR1 and Bifidobacterium adolescentis. Although they did not find any appreciable benefits in the participants’ clinical outcomes, they did notice elevated serum levels of IL-10 and IL-12 during the grass pollen season and elevated TGF-β during the ragweed season in the probiotic group in comparison to the placebo group 83. Therefore, after receiving probiotics, these patients’ essential regulatory indicators rose even if no changes in their symptoms were seen. The use of probiotics in patients with cytokine release syndrome (CRS) has received much less research than that for AR. In brush samples of the mucosal surfaces of the lateral, central, and medial sections of the maxillary sinuses, it was discovered that patients with CRS had less bacterial diversity and a particular depletion of helpful lactobacilli [135, 137]. Interestingly, after intranasal inoculating animals, these same scientists were able to demonstrate that Lactobacillus sakei ATCC 15521 was adequate to protect against Corynebacterium tuberculostearicum-induced sinusitis [135]. The species L. sakei was the focus of the authors’ attention since it was one of the taxa that was severely decreased in CRS patients and was present in higher abundances in the mucosa of healthy people [135]. Another multicenter, double-blind, placebo-controlled trial showed that taking Enterococcus faecalis (Symbioflor 1) for six months helped lessen the frequency of acute CRS exacerbations [137]. Furthermore, these positive outcomes persisted for 8 months following the conclusion of the therapy [135]. In agreement with these findings, Kitz et al. [138] have observed that recurrent acute sinusitis has decreased in incidence and duration. It’s interesting that these same scientists were later able to demonstrate that Lactobacillus sakei ATCC 15521 was adequate to prevent the recurrence of acute sinusitis in children who had been given oral E. faecalis Symbioflor 1 for 8 weeks [135, 138]. Additionally, previous studies investigating the use of a probiotic nasal spray for various upper respiratory tract (URT) infections, like otitis media, have already noted the safety of a probiotic spray in the nasal cavity [135, 139]. Additionally, we think that better strain selection (particularly using human isolates) from the URT may be required to detect meaningful effects on patients’ clinical symptoms [135].
Probiotics have the potential to cure chronic inflammatory URT disorders like AR and CRS, but it’s vital to keep in mind that these conditions are frequently accompanied by lower airway comorbidities, most notably, asthma [135]. Although preliminary, clinical research into the use of probiotics to treat asthma patients has produced some encouraging findings [137]. For example, Chen et al. [140] conducted a randomized, double-blind, placebo-controlled trial to examine the therapeutic efficacy of Lactobacillus gasseri PM-A0005 in asthmatic children (6–12 years old). They observed an improvement in asthma symptom scores and an improvement in airway functions, namely peak expiratory flow rates, after two months of daily treatment with 2 × 109 colony forming unit (CFU)/capsule of L. gasseri PM-A0005 [135, 140].
Moreover, they saw a considerable decline in the pro-inflammatory cytokine concentrations in the patients’ blood, including tumor necrosis factor (TNF-α, IFN-c, IL-12, and IL-13 [140]. According to these outcomes, Gutkowski et al. [141] demonstrated that giving children with mild-to-moderate atopic asthma a 12-week oral course of Trilac, a blend of Lactobacillus acidophilus, Lactobacillus delbruecki species bulgaricus, and Bifidobacterium bifidum, improved lung function and decreased asthma exacerbations. In a different trial, van de Pol et al. [142] looked at the impact of synbiotic therapy in people with asthma and dust mite allergies. In the study by Rose et al. [143], the scientists studied the effects of LGG meal supplementation over a six-month period in newborns with atopic susceptibility and recurrent wheeze. Results showed no change in clinical outcomes as determined by AD severity grading and asthma symptom score [142]. However, oral administration of LGG led to a slight reduction in aeroallergen sensitivity as a bronchial asthma predictor [143]. There has been a thorough examination of more research addressing the role of airway microbial dysbiosis in asthma and the potential of probiotics to treat these individuals [135]. These trials are encouraging, but additional study is required to determine whether or not modulating the lung microbiota by targeting the URT with nasal and/or oral probiotic therapy is effective enough.
There are insufficient pieces of evidence to recommend the use of prebiotics, probiotics, or other dietary supplements based on specific nutrients to prevent food allergy in at-risk groups and the general population, as per the European Academy of Allergy and Clinical Immunology (EAACI) food allergy and anaphylaxis guidelines on primary prevention of allergy (grade of recommendation B) [144].
After conducting a systematic literature review in 2011 on the impact of infant formula supplemented with prebiotics or probiotics on the preventive effect on allergy, the Nutrition Committee of the European Society of Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) came to the conclusion that “There is too much uncertainty to draw reliable conclusions from the available data.” [145].
The World Allergy Organization (WAO) advised considering the use of probiotics in 2015 on their guidelines for the prevention of allergy in (a) pregnant women with children who have a high risk of allergy; (b) women who breastfeed infants who have a high risk of developing allergy; and (c) infants who are at risk of developing allergies because there is a net benefit that results in primary prevention of eczema [146].
Probiotics may affect the gut microbiota and control the inflammatory response according to certain research, albeit their precise method of action in allergic illnesses is yet unknown. Given that atopic illnesses like asthma are characterized by polarized Th2 responses, it is possible to administer treatments that restore the unstable Th1/Th2 balance [147]. According to research, immune-modulatory substances, such as probiotics, can change the immune response by increasing levels of IFN-γ, a cytokine unique to the Th1 response, and lowering IL-4, a cytokine specific to the Th2 response. A few meta-analyses on the utility of probiotics in the treatment of asthma have been done recently [147]. The goal of the meta-analysis was to assess the effectiveness of probiotic supplements during pregnancy or the first few months of a child’s life for preventing AD disorders [148]. There were a total of 4,755 kids across 17 studies. Asthma and wheezing were an issue in eight investigations. Regarding asthma prevention, there was no discernible change (RR 0.99; 95% CI 0.77–1.27, P = 0.95). Additionally, comparable findings were seen in data on the advantages of probiotic usage in wheezing (RR 1.02; 95% CI 0.89–1.17, P = 0.76; fixed-effect analysis) [148, 149]. The results of the meta-analysis include the written word. Prenatal and/or early infant probiotic supplementation decreases the likelihood of atopic sensitization but may not decrease the risk of allergic reactions, according to a significant meta-analysis by Wei et al. [150]. Tang et al. [151] also discovered that there is little evidence to suggest probiotics throughout pregnancy or the first few months of a child’s life to prevent asthma in kids. The meta-analysis demonstrating the advantages of probiotic supplementation in asthmatic children was provided by Lin et al. [149]. Nine hundred and ten children from eleven studies were included in the analysis. According to the findings, kids who received probiotics had fewer asthma attacks than kids who received a placebo (RR 1.3; 95% CI 1.06–1.59). Additionally, it was shown that IFN-γ levels increased (mean differences 2.5; 95% CI 1.23–3.76) and IL-4 levels decreased (mean differences 2.34; 95% CI 3.38–1.29) [147]. The childhood asthma control test, the number of symptom-free days, the forced expiratory volume, and the peak expiratory flow did not show any statistically significant differences. The analysis’s authors acknowledge that poor enrollment in trials may have led to unsatisfactory outcomes [149].
Inactivated bacterial extracts from the most prevalent respiratory tract pathogens make up bacterial lysates. Based on the techniques employed to create the combination, they are separated into two groups: polyvalent chemical bacterial lysate (PCBL) and polyvalent mechanical bacterial lysate (PMBL). Although the precise method of blood-letting (BL) work is unclear, evidence indicates that they may enhance the immune response by shifting the Th1/Th2 axis, which lowers the risk of allergen-induced AHR during asthma attacks [152]. In addition, the therapy lowers the frequency of viral infections (a triggering factor) by enlisting neutrophil recruitment, activating macrophages, and producing antiviral cytokines [153]. A meta-analysis on the use of the most well-liked PCBL-OM-85 in recurrent respiratory tract infections (RTIs) was published in 2018 [154]. A total of 4,851 pediatric participants from 53 trials were included in the study. Eight of them (702 patients) demonstrated the OM-85’s influence on a decrease in wheeze episodes. The duration of wheezing decreased as a result of the therapy (mean difference 3.37; 95% CI –4.25, –2.22). Additionally, the treatment’s adverse effects were minimal. The most frequent side effects were rash and mild gastrointestinal problems, neither of which prevented patients from receiving therapy (RR 1.39; 95% CI 1.02, 1.88, P = 0.04) [154]. de Boer et al. [155] conducted a meta-analysis in 2020 about the positive impact of PMBL and PCBL treatment in the prevention of wheeze episodes and asthma exacerbations. The results of the study demonstrated that BL therapy reduced wheeze episodes in children as well as asthma exacerbations (mean difference –0.90; 95% CI –1.23–0.57, P ≤ 0.001). The medication also decreased the duration of wheezing episodes and the need for antibiotics. No notable negative effects were noted [155].
A synthetic dipeptide called pidotimod influences both innate and adaptive immunity. It activates TLR2 and TLR4, two pattern recognition receptors (PRRs). It is widely recognized that the severity of atopic disorders correlates with PRR nucleotide polymorphisms that result in lower expression of them [147]. Pidotimod increases the release of antimicrobial peptides and enhances mucociliary transport by stimulating PRRs [153]. The use of pidotimod in treating RTIs and the related disorder obstructive syndrome (OS) has been the subject of two investigations in recent years [154–156]. Even though RTIs and OS are associated with the condition, there are actually no high-quality analyses on the effectiveness of pidotimod in treating asthma.
Similar research was done by Namazova-Baranova et al. [157] regarding pidotimod usage in kids with RTIs. Both the pidotimod-treated and placebo groups of patients were assigned. Children underwent therapy for 30 days before being monitored for 6 months.
Three time points were used to count the RTIs. The study found that the therapy program improved the rate of RTI recurrence after three months [131, 157]. Additionally, a rapid disappearance of symptoms and a quicker recovery were noted. Furthermore, pidotimod’s immune-modulatory action was demonstrated by maintaining the levels of IL-8 [131]. There is, however, a lot of hope that this medicine would improve the course of asthma because the treatment decreased the frequency of RTIs, which is closely related to asthma episodes [148].
ABPA: allergic bronchopulmonary aspergillosis
AD: atopic dermatitis
AFAD: allergic fungal airway disease
AHR: airway hyperresponsiveness
AR: allergic rhinitis
CBC: complete blood count
CD4+: clusters of differentiation-4
CI: confidence interval
CRS: cytokine release syndrome
DC: dendritic cell
DNA: deoxyribonucleic acid
HRCT: high resolution computer tomography
IFN-γ: interferon-gamma
IgE: immunoglobulin E
IL-2: interleukin-2
LGG: Lactobacillus rhamnosus GG
LPS: lipopolysaccharide
MMPs: matrix metalloproteinases
OVA: ovabumin
PCBL: polyvalent chemical bacterial lysate
PRRs: pattern recognition receptors
RR: respiratory rate
RTIs: respiratory tract infections
SAFS: severe asthma with fungal sensitization
SCFAs: short-chain fatty acids
TGF-β: transforming growth factor-beta
Th2: T-helper 2
TLR4: Toll-like receptor 4
Treg: T regulatory
URT: upper respiratory tract
APG: Conceptualization, Writing—original draft, Writing—review & editing. AA: Methodology, Data curation, Software, Investigation. NB: Formal analysis, Visualization. BP: Resources, Data curation.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
All data related to the species of Aspergillus, and their cultures are from the Department of Biotechnology, Shobhit Institute of Engineering & Technology, Deemed-to-be University, Meerut, and the Research and Development Cell of Swami Vivekanand Subharti University, Meerut (India) and are available upon request from the corresponding author (amarprakashgarg@yahoo.com).
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Graziella Chiara Prezzavento
Jim E. Banta ... James M. Banta
Nassiba Bahra ... Samira El Fakir
Silvina Monica Alvarez ... Nidia Noemi Gomez
