Affiliation:
1Unit of Rheumatology, Immunology, Allergy and Rare Disease, San Raffaele Hospital, 20132 Milan, Italy
2Postgraduate School of Allergy and Clinical Immunology, Università Vita-Salute San Raffaele, 20132 Milan, Italy
ORCID: https://orcid.org/0000-0002-2963-9882
Affiliation:
3Allergy and Clinical Immunology, Division of Respiratory Science, National Heart and Lung Institute, Imperial College London, SW3 6LY London, UK
ORCID: https://orcid.org/0000-0002-9488-194X
Affiliation:
3Allergy and Clinical Immunology, Division of Respiratory Science, National Heart and Lung Institute, Imperial College London, SW3 6LY London, UK
Email: s.durham@imperial.ac.uk
ORCID: https://orcid.org/0000-0001-5264-6207
Explor Asthma Allergy. 2024;2:76–84 DOI: https://doi.org/10.37349/eaa.2024.00030
Received: August 08, 2023 Accepted: November 14, 2023 Published: February 29, 2024
Academic Editor: Mario Di Gioacchino, Italian Society of Allergy and Clinical Immunology, G. d’Annunzio University, Italy
The article belongs to the special issue Precision Medicine in Allergy and Rhinology
Allergen-specific immunotherapy for inhalant allergies, using allergen extracts of proven value, is highly effective in selected patients with allergic rhinoconjunctivitis and allergic asthma. Both subcutaneous and sublingual immunotherapy (SLIT) have been shown to modify the underlying cause of the disease, with long-term clinical benefits that persist for years after their discontinuation. Real-world studies have confirmed the long-term efficacy of allergen immunotherapy in allergic rhinitis (AR) and asthma and shown a reduction in the incidence of lower respiratory tract infections. Sublingual house dust mite (HDM) immunotherapy has been suggested to improve innate antiviral immunity—a likely explanation for this finding. Based on robust randomized controlled trials, the Global Initiative for Asthma (GINA) guideline has incorporated the use of SILT for the treatment of adults with HDM-driven asthma and concomitant AR, with sub-optimal control, regardless of the use of low-to-high doses of inhaled corticosteroids, as long as the patient’s forced expiratory volume in 1 second (FEV1) is > 70%.
Asthma is a chronic respiratory disease that affects 5–10% of the population (5.7% in the UK) and contributes to approximately 0.4 million deaths annually worldwide [1–4]. Even though some countries have seen a reduction in hospitalizations and deaths due to asthma, this disease still represents an important burden for patients and their families and a challenge for healthcare systems across the globe [1]. Among the different phenotypes of asthma, allergic asthma is the most common, affecting about 60% of asthmatic patients [1, 5].
Allergic asthma is characterized by a T helper type 2 (Th2)-type inflammation resulting from exposure to aeroallergens [e.g., grass, weed and tree pollens, house dust mite (HDM), animal dander, cockroach, and moulds] [6]. According to the Global Initiative for Asthma (GINA), asthma control has two domains: symptom control and risk reduction [1]. New therapies like biologics have been incorporated into the treatment of asthma, especially in those patients with severe disease, in addition to non-pharmacological strategies and standard therapies, namely inhaled corticosteroids (ICS) [1]. On the other hand, the results of recently published randomized controlled trials (RCTs) of specific allergen immunotherapy for asthma have demonstrated the efficacy and safety of sublingual immunotherapy (SLIT), particularly in subjects with HDM allergy [1, 7–9]. The aim of this brief review is to evaluate the most recent evidence assessing the efficacy of allergen-specific immunotherapy (AIT) in the treatment of allergic asthma and the changes that international guidelines have implemented on the basis of these studies.
Subcutaneous immunotherapy (SCIT) and SLIT are recommended for the treatment of seasonal and perennial allergic rhinoconjunctivitis (ARC) in subjects with bothersome symptoms despite standard treatment or in those with intolerance to anti-allergic drugs due to side effects [10–14]. Both SCIT and SLIT have been shown to have disease-modifying effects with clinical benefits that can persist for years after their discontinuation of therapy [15–20].
Two RCTs published in 2016 showed a beneficial effect of one year of a daily standardized quality (SQ)-HDM tablet in the treatment of moderate-to-severe allergic rhinitis (AR) [21, 22].
In a phase III trial, 992 adult subjects were randomized to receive a placebo, or two different doses of SQ-HDM SLIT (6 μg or 12 μg tablets) [21]. Participants receiving SLIT showed absolute reductions in total combined rhinitis score (TCRS) of 1.18 (P = 0.002) and 1.22 (P = 0.001) and relative reductions of 18% to 22% compared with placebo for 6 SQ-HDM and 12 SQ-HDM, respectively [21].
The trial by Nolte et al. [22], which included adults and adolescents with HDM allergic AR with/without conjunctivitis (AR/C) with or without asthma, reported comparable results. An improvement of 17% [95% confidence interval (CI), 10% to 25%] in the AIT-treated group versus placebo was reported. No treatment-related serious adverse events were reported [22]. The results are consistent with Federal Drug Administration recommendations of a minimum 15% improvement of active over placebo treatment, also bearing in mind that all participants also have access to usual efficacious anti-allergic medications as rescue medication [23]. The study by Bergmann et al. [9], randomized 509 adults with HDM-associated AR to receive placebo or HDM SLIT tablets [500 index of reactivity (IR) or 300 IR] for one year, with one year blinded follow-up after treatment discontinuation. The relative reductions of symptom scores reported after one year of treatment (reduction of 20.2% in 500 IR and of 17.9% in 300 IR versus placebo) were maintained with both doses (19.1% in 500 IR and 17% in 300 IR, P < 0.05 versus placebo) at the end of the follow-up period [9]. This study raises the possibility that HDM allergen immunotherapy for a shorter time than grass pollen immunotherapy may induce long-term tolerance, although further adequately powered controlled trials are required to test this hypothesis [9].
A recent RCT of a short course of SCIT with a grass pollen allergoid with MicroCrystalline Tyrosine and monophosphoryl lipid-A [Pollinex Quattro (PQ) Grass] demonstrated significant efficacy and a good safety profile [24]. One hundred and nineteen subjects with seasonal grass pollen AR, with or without well-controlled asthma, received either repeated pre-seasonal placebo injections at weekly intervals or 6 pre-seasonal subcutaneous injections, administered with a conventional regimen (3 up-dosing and 3 weekly maintenance injections) or an extended regimen (monthly maintenance injections). The combined symptom and medication score had a significant reduction compared with placebo both in conventional (33.1%, P = 0.03) and extended regimen (39.5%, P = 0.011). A phase III RCT is planned [24].
Several randomized, double-blind, placebo-controlled trials have been conducted in adults and children to assess the efficacy and safety of allergen immunotherapy in asthmatic subjects [25, 26]. For example, Roberts et al. [25], allocated 39 asthmatic children (3–16 years) to receive either subcutaneous Phleum pratense or placebo over 2 pollen seasons. Children in the active group presented a reduction in asthma symptom-medication score compared with that after the placebo, with a median score of 1.0 [interquartile range (IQR) 0.4–2.0] in the placebo group versus 0.5 (IQR 0.1–0.9) in AIT-treated group (P = 0.04). No serious adverse events were reported [25]. In adults, Creticos et al. [26], randomly assigned 77 asthmatic patients to receive a placebo or a SCIT with ragweed pollen immunotherapy for two years. The mean peak expiratory flow rate was higher in the SCIT group than in the placebo group in the second year, while no significant difference in medication use or asthma symptom scores was reported [26].
A large body of evidence has been summarized and analyzed in diverse systematic reviews and meta-analyses which have been conducted to assess the efficacy and safety of SCIT [27, 28], SLIT [28–33], or both [28, 34] for the treatment of allergic asthma in adults and children. The overall evidence derived from these systematic reviews is that there is a moderate to high effect of specific immunotherapy on clinical outcomes, particularly in the short-term. Nonetheless, various limitations were identified by the authors. Examples of these are the reduced number and quality of the trials analyzed in the comparisons, limitations in the standardization of the outcomes, substantial statistical heterogeneity in a significant number of analyses, potential sources of bias, and limited studies evaluating other relevant asthma outcomes [27–31, 34, 35]. This has emphasized the need to conduct high-quality and well-powered clinical trials to address these questions. There are several recent randomized clinical trials that have broadened the current knowledge of the effects of AIT in asthma [7, 8, 36].
Virchow et al. [7] conducted a double-blind, randomized, placebo-controlled clinical trial including 834 adults with HDM allergy-related asthma partially controlled/uncontrolled by ICS or combined pharmacotherapy.
The aim of the study was to evaluate the efficacy in the prevention of asthma exacerbations of daily HDM SLIT (6 SQ-HDM or 12 SQ-HDM doses) versus placebo during an ICS reduction period. During the last 6 months of the trial the ICS dose was reduced and, if possible, withdrawn. Both doses of SLIT were associated with a reduced risk of exacerbation by 31% (P = 0.03) for 6 SQ-HDM and by 34% (P = 0.02) for the 12 SQ-HDM. The adverse events were mainly mild-moderate local reactions [7].
In a more recent trial with a similar design 826 subjects with allergic asthma were treated with SQ-HDM SLIT or placebo [36]. Participants were in treatment maintenance for 7–13 months and the period of ICS (fluticasone propionate) reduction was 6 months. The primary endpoint was the time of the first asthma exacerbation during the phase of ICS reduction/withdrawal. In this study, there were no statistically significant differences between the active groups and the placebo group for the primary endpoint. This difference may be explained by the strict definition of “well-controlled asthma” of the local guidelines compared to GINA 2023 and by the higher baseline ICS dose of patients in this trial [36]. Importantly, in a large subgroup of the participants whose asthma diagnosis at baseline was supported by a finding of bronchodilator reversibility to a short-acting β2 agonist (SABA), a reduction in asthma exacerbations by 30% (P = 0.049) was observed [36] comparable to that observed in the trial by Virchow et al. [7], with a reduction by 34% in 12 SQ-HDM group.
Mosbech et al. [8], investigated the efficacy and safety of a standardized HDM SLIT tablet in 604 subjects with HDM AR and mild-to-moderate asthma. Participants received a placebo (n = 143) or 3 active doses of sublingual tablets: 1 SQ-HDM (n = 146), 3 SQ-HDM (n = 159), or 6 SQ-HDM (n = 156). The primary outcome was a reduction in ICS dose after one year of AIT. The active group with the highest dose of 6 SQ-HDM showed a reduction in daily ICS dose of 81 μg compared to the placebo (P = 0.004). At the end of treatment, there was a relative mean reduction of ICS of 42% for the highest SLIT dose versus 15% for placebo [8].
Recent real-life studies provide supportive evidence that allergen immunotherapy may reduce asthma exacerbations [37]. The REAl-world effeCtiveness in allergy immunoTherapy (REACT) study evaluated retrospectively 10 years of follow-up (2007–2017) of a cohort of 46,024 subjects affected by AR with or without asthma treated for 10 years [37]. Subjects were propensity-matched 1:1 with a control group of 46,024 patients with AR with or without asthma who had not received immunotherapy. At baseline, 14,614 participants had mild or moderate asthma, while only 4% were at GINA step 4 treatment. AIT-treated patients had a significantly greater reduction of prescriptions for rhinitis and asthma (both controllers and relievers), which remained sustained during the 9 years of follow-up. Patients in the pre-existing asthma cohort had a reduction in severe asthma exacerbations (P < 0.05), an improvement in asthma treatment steps (P < 0.0001), and a reduction in hospitalizations. An unexpected finding was a reduction in the number of diagnoses of pneumonia, throughout the 9 years follow-up (Figure 1) [37].
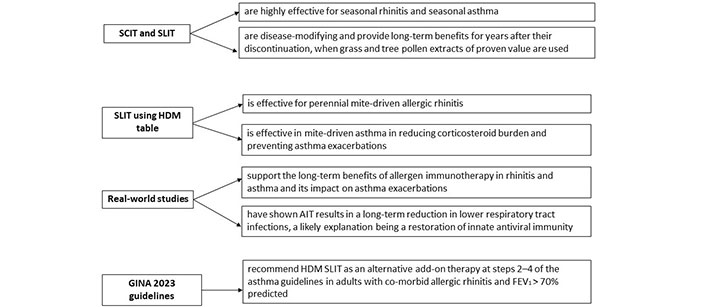
Key points of the use of AIT in the treatment of AR and asthma. FEV1: forced expiratory volume in 1 second
Viral infections are known to be a risk factor for asthmatic exacerbations, possibly due to altered interferon (IFN) production. A link with bacterial infections is also described, i.e. a positive association between asthma and pneumococcal infections has been reported [38] and subjects with allergic asthma seem to have a greater risk of lower respiratory tract infections than those with non-allergic asthma [39]. A possible mechanism to explain bacterial infection susceptibility is a deficiency of interleukin (IL)-12 and IFN-γ [40].
A preventive effect on asthma exacerbations and lower respiratory tract infections has also been reported in a retrospective register-based nationwide study of 2,688 AIT-treated patients [41]. In this population, an average decrease in lower respiratory tract infections requiring antibiotics was found in both perennial (20%) and seasonal allergic asthmatics (17%) during 3 years of follow-up after discontinuation of AIT [41]. A prolonged reduction in asthma exacerbations was also described for both groups.
The same group went on to study ex vivo the effect of HDM SLIT tablets on the bronchial epithelium during a simulated antiviral response. In a randomized double-blind controlled trial of HDM SLIT tablets over 6 months, bronchial epithelial brushings were obtained by fibreoptic bronchoscopy before and after treatment from asthma patients receiving HDM SLIT or placebo [42]. Cultured epithelial cells were stimulated with a Toll-like receptor 3 (TLR3) agonist. Cultured bronchial epithelial cells of AIT-treated subjects demonstrated increased expression of IFN-β at gene and protein levels, and an increase in IFN-λ gene expression. The epithelial-derived cytokine alarmin, IL-33, was decreased, while no difference was detected in thymic stromal lymphopoietin (TSLP) expression [42]. This study highlights a putative role for HDM SLIT in restoring antiviral innate immunity, a likely explanation for the observed decreases in lower respiratory tract infections [37, 41]. It remains to be tested whether this mechanism operates following AIT for seasonal pollinosis. Key points are summarized in Figure 1.
According to GINA 2023 guidelines, asthma control must be assessed both in symptoms and risk of adverse outcomes, namely exacerbations [1]. Regarding the role of AIT, the need for more robust evidence is underlined and is currently under review. SCIT is associated with a reduction in symptom and medication scores, but the level of evidence of its efficacy is low due to the paucity of well-powered studies [1]. However, the effects of sublingual HDM tablet immunotherapy on ICS reduction [8] and a reduction in asthma exacerbations [7] are reported.
The GINA 2023 guideline states that HDM SLIT can be considered in steps 2 to 4 for HDM-allergic asthmatics with associated AR and an FEV1 > 70%, and asthma symptoms despite inhaled ICS (Figure 2) [1].
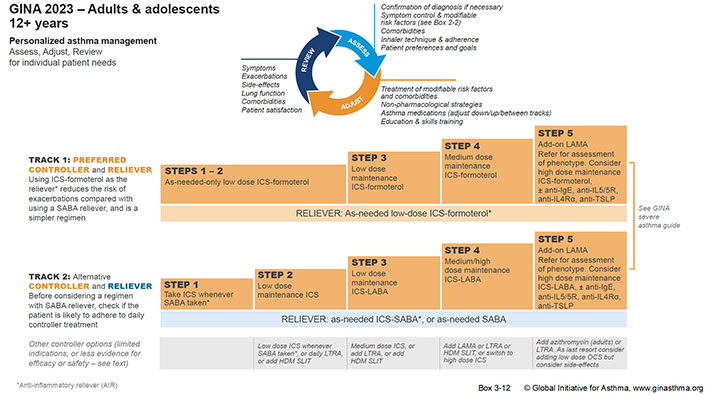
Personalized management for adults and adolescents to control symptoms and minimize future risk. LAMA: long-acting muscarinic antagonist; IgE: immunoglobulin E; LTRA: leukotriene receptor antagonist; OCS: oral corticosteroids; LABA: long-acting-β2 agonist; IL5R: IL-5 receptor
Note. Reprinted with permission from “Global strategy for asthma management and prevention [Internet]”. Global Initiative for Asthma; 2023 [cited 2023 Aug 8]. Available from: https://ginasthma.org/wp-content/uploads/2023/07/GINA-2023-Full-report-23_07_06-WMS.pdf
Allergen immunotherapy is known to be effective for seasonal rhinitis and asthma in adults and children. Recent RCTs support the use of HDM SLIT for perennial rhinitis and for perennial mite-driven asthma with a reduction in asthma exacerbations and a decrease in corticosteroid burden [7, 8]. Real-world evidence supports that these findings are generalizable to asthmatics in the community, and further, that allergen immunotherapy may reduce lower respiratory infections, a likely contributory factor for the observed reduction in asthma exacerbations [37, 41, 42].
AIT: allergen-specific immunotherapy
AR: allergic rhinitis
GINA: Global Initiative for Asthma
HDM: house dust mite
ICS: inhaled corticosteroids
IFN: interferon
IL: interleukin
IR: index of reactivity
RCTs: randomized controlled trials
SABA: short-acting β2 agonist
SCIT: subcutaneous immunotherapy
SLIT: sublingual immunotherapy
SQ: standardized quality
TSLP: thymic stromal lymphopoietin
CA and MP: Conceptualization, Writing—original draft, Writing—review & editing. SRD: Conceptualization, Writing—original draft, Writing—review & editing, Supervision.
C Asperti declares no conflict of interest. M Penagos has received personal fees from Stallergenes and ALK. SR Durham has received lecture fees from Abbott Laboratories, ALK, and Stallergenes Greer.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Sérgio Duarte Dortas ... Solange Oliveira Rodrigues Valle
Klaus Vogt, Sebastian Roesch
Dichapong Kanjanawasee ... Pongsakorn Tantilipikorn
