Affiliation:
1Centre of Pulmonary Medicine, Hirslanden Hospital Group, Salem-Hospital, CH-3013 Bern, Switzerland
2School of Biomedical and Precision Engineering (SBPE), University of Bern, CH-3008 Bern, Switzerland
Email: richard.kraemer@hirslanden.ch
ORCID: https://orcid.org/0000-0002-1542-2420
Explor Asthma Allergy. 2024;2:85–96 DOI: https://doi.org/10.37349/eaa.2024.00031
Received: September 12, 2023 Accepted: January 22, 2024 Published: April 02, 2024
Academic Editor: Ken Ohta, Japan Anti-Tuberculosis Association Fukujuji Hospital, NHO Tokyo National Hospital, Japan
Aim: There is an increasing interest in defining the role of small airway disease (SAD) in asthma, chronic obstructive pulmonary disease (COPD), and asthma with coexisting COPD. Based on the specific pathophysiologic components of small airway dysfunction (SAdf) of these diseases, single lung function parameters characterize only fractional aspects of SAdf and that the phenotypic diagnosis of SAD, and therefore, the functional assessment must be based on more than one parameter, reflecting airway dysfunction, pulmonary hyperinflation (PHI), energy costs, trapped gases, and/or gas transfer disturbances.
Methods: The present study was undertaken to define the interactive contribution of several spirometric and plethysmographic parameters such as forced expiratory flow between 25% and 75% of vital capacity (FEF25–75), effective specific airway resistance (sReff), plethysmographic functional residual capacity (FRC; FRCpleth), the parameter defining PHI, the aerodynamic resistive work of breathing at rest (sWOB), the volume of trapped gas at FRC (VTGFRC), and the carbon monoxide diffusion capacity (DLCO) as the parameter of the gas transfer.
Results: The study clearly demonstrates that the diagnosis of SAD cannot be based on one single lung function parameter, especially not on the spirometric FEF25–75 only. Interestingly, sWOB has a high discriminatory power to define SAD in these diseases.
Conclusions: Within a future framework including functional and treatable traits, it is mandatory to define SAdf parameters diagnosing unambiguously SAD, for a successful concept of precision medicine.
Small airways are usually defined as having an internal diameter smaller than 2 mm [1] and are generally understood to include the small airway conducting zone involving the terminal bronchus, bronchioles, alveolar ducts, and alveolar sacs. Compared with the large airway, the cross-sectional surface area and airway volume of the small airway are far greater, yet the small airway contributes only 10% of the total airway resistance [2]. Consequently, the small airways are sometimes referred to as the “silent zone” and, therefore, the severity of the disease can be underestimated if only single conventional pulmonary function tests are used for the diagnosis of small airway disease (SAD). However, so far, no unanimously accepted method and/or algorithm is available for the detection of small airway abnormalities. Nevertheless, SAD contributes significantly to the phenotype of asthma, and there is evidence that the magnitude of small airway abnormalities correlates with the severity of the disease [3–6]. It has been shown to correlate with symptoms [7], and with the Asthma Control Test score even in patients with mild disease [8]. Furthermore, it has been observed that increased small airway resistance correlates with worsening health status, dyspnea [9], and asthma exacerbations [10].
SAD is not only observed in asthma but also plays an important role in the pathogenesis of chronic obstructive pulmonary disease (COPD) or both; asthma and COPD, referred to as asthma-COPD overlap (ACO) [11–17]. COPD is a common, complex, and heterogeneous disease, characterized by a few different dysfunctions due to an increased inflammatory response of the lungs [18, 19]. Complexity has been assumed to refer to multiple components and their interactions, while heterogeneity arises from the fact that not all components, especially functional ones, are present in all patients simultaneously [20, 21] throughout life [22].
Physiologically, these diseases are characterized by a combination of numerous interactive functional deficits such as airway obstruction, small airway dysfunction (SAdf), pulmonary hyperinflation (PHI), air trapping, and gas exchange disturbances [2, 23–25]. It is presumed that SAD can build up over time in peripheral zones of the lungs [26]. The characteristics of small airway obstruction include premature airway closure and airway trapping, leading to PHI. Most phenotypes of COPD are slowly progressive in most patients [27] and forced expiratory volume in 1 s (FEV1) and the ratio between FEV1 and the forced vital capacity (FVC; FEV1/FVC) lack the diagnostic sensitivity to identify early lung deterioration [28, 29]. Therefore, identifying patients with early lung damage at risk of developing COPD would enable focused efforts to prevent disease progression [30].
There is, however, a principal question of how SAdf, and hence SAD can accurately be defined by functional parameters. The role of SAdf has been explored already years ago in several studies [31–33], and today there is an increasing interest in evaluating different SAdfs as potential predictors of new functional and therapeutical traits within a concept of precision medicine [34–44]. From the physiological point of view, the two contents of SAdf versus SAD must be differentiated. It is anticipated that SAdf refers only to a single functional property of a parameter, while SAD indicates that conditionally several functional components of airway destruction prove a specific pathophysiological phenotype of a clinically distinct disease.
There are several functional parameters that are thought to be surrogates of SAdf. Using spirometry, (i) the forced expiratory flow between 25% and 75% of vital capacity (FEF25–75) is the spirometric variable most commonly cited as an indicator of small airway obstruction and thought to be more accurate in detecting SAdf than FEV1 [45–47]. Regarding plethysmographic parameters, (ii) parameters of airway dynamics [48, 49], especially the effective specific airway resistance (sReff) and its inverse measuring the specific airway conductance (sGeff) were closely associated with symptoms of dyspnea [50]. Noteworthy in that context, plethysmographic parameters, such as the sReff and notably also the aerodynamic resistive work of breathing at rest (sWOB), both obtained from the resistive airway resistance loop (sRaw-loop) by the integral method [49, 51], feature SAdf, but have not yet reached enough attention in the literature concerning their potential. Other focusses are (iii) PHI assessed by increased plethysmographic functional residual capacity (FRC; FRCpleth), or the ratio between residual volume (RV) and total lung capacity (TLC; RV/TLC) [47], (iv) expiratory air trapping given by the volume of trapped gas at FRC (VTGFRC) [47], and/or (v) gas exchange disturbances evaluated by carbon monoxide diffusion capacity (DLCO) transfer measurements [52].
In the present study, we evaluated the role of several spirometric and plethysmographic parameters defining SAD within three diagnostic groups (asthma, ACO, and COPD) to elaborate potential discriminating functional and/or treatable traits in an optimized concept of precision medicine.
In the present study, we refer to retrospectively evaluated data obtained from four Swiss centers (University Children’s Hospital, Bern, Switzerland; Center of Pulmonary Diseases, Hirslanden Hospital Group, Salem-Hospital, Bern, Switzerland; Clinic of Pneumology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland; Center of Pulmonology, Hirslanden Hospital Group, Clinic Hirslanden, Zürich, Switzerland). The patients have been referred to the centers for extended pulmonary function testing and optimizing therapy. Data were exported from the database systems of each clinic and subdivided into 3 diagnostic classes: (i) bronchial asthma; (ii) COPD, including a group of patients with (iii) ACO. The anamnestic, clinical features, and the diagnosis for each patient were made by well-trained pulmonologists based on history-taking, chest radiographs, high-resolution computed tomography (CT) scans, spirometry, whole-body plethysmography, and measurement of the fraction of exhaled nitric oxide (FeNO); additional detail regarding how the clinical diagnoses have been established was previously given [48, 49, 53].
From a previous database [48, 49], measurements data of 688 patients (198 with asthma, 89 males, 109 females; 152 with ACO, 83 males, 69 females; and 338 with COPD, 186 males, 152 females) were selected for a discriminating analysis of SAdf and SAD.
In all four centers, the same type of constant-volume whole-body plethysmographs (MasterScreen Body, Jaeger Würzburg, Germany) were used by standard techniques according to the American Thoracic Society (ATS)-European Respiratory Society (ERS) criteria [52, 54–56] and revised Swiss guidelines [57], and the exported data were obtained from the same system software (JLAB version 5.2 and SentrySuite version 1.29, respectively). Inclusion criteria were reproducible baseline measurements with (i) at least 5 shift volume-tidal volume loops of comparable shapes; (ii) especially closed at zero flow points; and (iii) closed inspiratory part in the shift volume-tidal flow loops. All parameters were assessed in absolute values, as a percentage of predicted normal values, and z-scores according to normative equations recently used [58, 59].
The database on which the present study was completed was a merge sort from previously used databases [48, 49, 60] with the condition that the following parameters must be available: FEF25–75, sWOB, sReff, FRCpleth, FRC obtained by helium dilution (FRCHe) VTGFRC at FRC (computed as FRCpleth-FRCHe), and DLCO, all expressed as z-scores. For the following parameters, SAdf was calculated if the z-scores were < –1.645 for SAdf-FEF25–75 and SAdf-DLCO, or > 1.645 for SAdf-sReff, SAdf-sWOB, SAdf-FRCpleth, and SAdf-VTGFRC. The diagnosis of SAD was established only if 3 functional parameters were out of the upper limit of normal (ULN) or lower limit of normal (LLN). All analyses were done using the IBM Statistical Package for Social Science (SPSS) software (version 29.0; SPSS Inc., Chicago, IL, USA), and the R statistical software (version 4.1.2; R Statistics, Vienna, Austria), together with the extension packages MASS, FactoMineR, caret, rpart, and ade4.
Demographics of the patients within the 3 diagnostic groups are given in Table 1. Gender, age, and body mass index (BMI) distributions were similar in the 3 diagnostic groups. The highest baseline z-scores are found for sReff (10.9 ± 6.9), followed by sWOB (9.5 ± 7.3); lowest for FEF25–75 (–1.5 ± 0.9), followed by DLCO (–1.6 ± 1.3). Multi-comparisons between the diagnostic groups revealed (with exception of FEV1 % predicted) significant z-score mean differences between asthma and ACO, highest for sWOB (3.2), followed by sReff (3.0); lowest for FEF25–75 (0.4). Such z-score mean differences were significantly higher between asthma and COPD (sReff: 7.8; sWOB: 7.6).
Demographics of the patients within the 3 diagnostic groups
| Parameters | Asthma | ACO | COPD | All |
|---|---|---|---|---|
| Measurements (% total) | 198 (28.8) | 152 (22.1) | 338 (49.1) | 688 (100) |
| Gender (male/female, n) | 89/109 | 83/69 | 186/152 | 358/330 |
| Age (mean, years) | 50.9 ± 18.1 | 63.1 ± 13.4 | 67.9 ± 8.6 | 61.9 ± 14.9 |
| BMI (mean, kg/m2) | 27.1 ± 5.6 | 27.0 ± 5.4 | 26.6 ± 6.7 | 26.8 ± 6.1 |
| Spirometry | ||||
| FEV1 z-score ± SD | –1.3 ± 1.3 | –2.1 ± 1.5 | –3.3 ± 1.4 | –2.5 ± 1.6 |
| FEV1 % predicted ± SD | 92.3 ± 12.50 | 85.6 ± 18.65 | 54.9 ± 16.10 | 79.2 ± 22.45 |
| FEF25–75 z-score ± SD | –1.1 ± 1.2 | –1.4 ± 1.0 | –1.9 ± 0.7 | –1.5 ± 0.9 |
| End-expiratory level | ||||
| FRCpleth z-score ± SD | 0.65 ± 1.29 | 1.73 ± 1.60 | 2.56 ± 2.06 | 1.83 ± 1.95 |
| Airway dynamics | ||||
| sReff z-score ± SD | 6.43 ± 5.17 | 9.38 ± 5.54 | 14.23 ± 6.52 | 10.91 ± 6.86 |
| sWOB z-score ± SD | 4.98 ± 5.69 | 8.23 ± 6.09 | 12.61 ± 7.06 | 9.45 ± 7.27 |
| Gas exchange | ||||
| DLCO z-score ± SD | –0.6 ± 0.9 | –1.5 ± 1.1 | –2.25 ± 1.2 | –1.6 ± 1.3 |
SD: standard deviation
The assessment of SAD, based on SAdf of the different lung function parameters is given in Table 2. The three-parameter-criterion for the diagnosis of SAD was reached for all measurements in 72.5% of all measurements (row a: asthma 10.9%; ACO 74.3%; COPD 90.2%). Noteworthy is that the observation for asthma, in 35.4% (row b), one single parameter (mainly sReff) presented with SAdf, however not proving SAD in asthma, and in 20.2% (row c), even 2 single parameters (mainly sReff and VTGFRC) presented with SAdf, however not proving SAD in asthma. In both subgroups, FEF25–75 was in the normal range.
Assessment of SAD, based on SAdf by the different lung function parameters
| Parameters | Asthma (n = 198) | ACO (n = 152) | COPD (n = 338) | All (n = 688) | |
|---|---|---|---|---|---|
| SAD [n (%)] | a | 81 (40.9) | 113 (74.3) | 305 (90.2) | 499 (72.5) |
| No SAD [n (%)] | 7 (3.5) | 1 (0.7) | 0 (0) | 8 (1.2) | |
| One SAdf-parameter [no SAD; n (%)] | b | 70 (35.4) | 8 (5.3) | 2 (0.6) | 80 (11.6) |
| Two SAdf-parameters [no SAD; n (%)] | c | 40 (20.2) | 30 (19.7) | 31 (9.2) | 101 (14.7) |
Sensitivity and specificity were best for VTGFRC (row d: 93.8%, 52.4%, respectively) and lowest for FEF25–75 (row d: 28.0%, 3.8%, respectively) (Table 3). It follows that also the accuracy, i.e., the closeness to the diagnosis of SAD within all measurements was best for VTGFRC (row e: asthma: 70.7%; ACO: 82.2%; COPD: 89.9%) followed by sWOB (row e: asthma: 59.1%; ACO: 71.7%; COPD: 91.4%) and sReff (row e: asthma: 59.6%; ACO: 74.3%; COPD: 92.6%) (Table 3). Finally, we have been interested in evaluating by which combination of parameters SAD can be predicted within the 3 diagnostic classes. For asthma in 50 of 81 (row g: 61.7%), measurements by 4 parameters, for ACO in 39 of 113 (row g: 34.5%), measurements by also 4 parameters, and for COPD in 137 of 305 (row i: 44.9%), measurements by 6 parameters are needed for the diagnosis SAD (Table 4).
Sensitivity, specificity, and accuracy of lung function parameters within the 3 diagnostic groups
| Parameters | Reliability | Asthma (n = 198) | ACO (n = 152) | COPD (n = 338) | All (n = 688) | |
|---|---|---|---|---|---|---|
| VTGFRC | d | Sensitivity (%) | 77.1 | 93.8 | 98.4 | 93.8 |
| Specificity (%) | 66.1 | 48.7 | 6.5 | 52.4 | ||
| e | Accuracy (%) | 70.7 | 82.2 | 89.9 | 82.7 | |
| sWOB | d | Sensitivity (%) | 84.3 | 88.5 | 96.4 | 92.6 |
| Specificity (%) | 40.9 | 23.1 | 41.9 | 37.3 | ||
| e | Accuracy (%) | 59.1 | 71.7 | 91.4 | 80.5 | |
| sReff | d | Sensitivity (%) | 100 | 94.7 | 98.7 | 98.0 |
| Specificity (%) | 30.4 | 15.4 | 32.3 | 27.6 | ||
| e | Accuracy (%) | 59.6 | 74.3 | 92.6 | 79.1 | |
| FRCpleth | d | Sensitivity (%) | 50.6 | 68.1 | 69.1 | 65.8 |
| Specificity (%) | 95.7 | 100 | 96.8 | 96.8 | ||
| e | Accuracy (%) | 76.8 | 74.3 | 71.6 | 74.1 | |
| DLCO | d | Sensitivity (%) | 84.3 | 49.6 | 26.4 | 41.2 |
| Specificity (%) | 10.4 | 30.8 | 12.9 | 15.1 | ||
| e | Accuracy (%) | 41.4 | 44.7 | 25.1 | 34.2 | |
| FEF25–75 | d | Sensitivity (%) | 33.7 | 30.1 | 25.7 | 28.0 |
| Specificity (%) | 1.7 | 0 | 16.1 | 3.8 | ||
| e | Accuracy (%) | 15.2 | 22.4 | 24.9 | 21.5 | |
Assessment of SAD by combining 3 or more parameters within the 3 diagnostic groups (based on at least 3 SAdf > 1.645 SDS)
| Number of parameters [n (%)] | Asthma [n = 81 (16.2%)] | ACO [n = 113 (22.6%)] | COPD [n = 305 (61.1%)] | All SAD [n = 499 (100%)] | |
|---|---|---|---|---|---|
| 3 | f | 12 (14.8) | 14 (12.4) | 10 (3.3) | 36 (7.2) |
| 4 | g | 50 (61.7) | 39 (34.5) | 80 (26.2) | 169 (33.9) |
| 5 | h | 17 (21.0) | 31 (27.4) | 78 (25.6) | 126 (25.3) |
| 6 | i | 2 (2.5) | 29 (25.7) | 137 (44.9) | 168 (33.7) |
SDS: SD score
By which age the onset of the parameters with a z-score over the ULN and under the LLN respectively within the 3 diagnostic groups can be expected, is presented in Figure 1. Most early onset in asthmatic was observed for sReff and sWOB (in 10% at age 22.4 years), in ACO for sReff (in 10% at age 41.5 years), and in COPD for FEF25–75, sReff, and sWOB (in 10% at age 57.3 years).
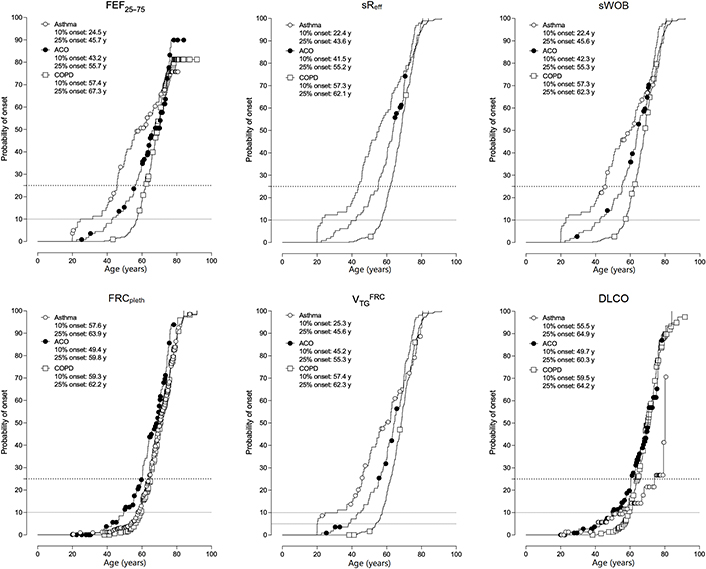
Onset of the functional parameters with a z-score over the ULN and under the LLN respectively, within the 3 diagnostic groups. y: years
The diagnosis of “SAD” in patients with obstructive pulmonary disease is not easy to assess. This study demonstrates that in the present dataset, this diagnosis could not be established based only on one single lung function parameter, especially not only on the spirometric FEF25–75. In contrast to other parameters, the accuracy of FEF25–75 was by the mean only 21.5% compared with VTGFRC with 82.7% accuracy (Table 3, row e). This may be because of different well-known factors: (i) Although easily available, FEF25–75 is hampered by physiological variability and measurement inconsistency issues [54, 61, 62]. (ii) FEF25–75 can be interpreted only when FVC stands within normal value limits [47]. (iii) The main drawback of use of FEF25–75 is that it tends to be less reproducible than other parameters because it is a volume-dependent measurement, afflicted with a high heterogeneity of airflow, even in moderately sized airway, and is altered by the presence of severe obstruction and/or PHI [63]. (iv) Finally, although it has been clearly proposed by both ATS and ERS, to express the degree of flow limitation based on the FEV1, FVC, and FEV1/FVC-ratios by z-scores, several author groups continue to present their data of flow limitation as percent predicted [54, 64, 65]. The advantage of presenting lung function data as z-scores is, that not only parameter mean values are provided, but individual data are corrected for gender, age, height, and ethnicity [48, 49, 64–67]. Moreover, according to the findings from the Severe Asthma Research Program of the National Heart Lung and Blood Institute and others, which showed a lack of correlation between FEF25–75 and indices of air trapping [61], the FEF25–75 appears of limited reliability in assessing distal airway function [61, 68–70]. To elaborate on the different confounding functional aspects of small airway function, Lipworth et al. [63] recommended that no single outcome should be considered the gold standard. An interpretation based on single SAdf parameters might merely be sensitive indicators of early disease across a range of phenotypes in patients with obstructive airway diseases [63].
There is a general understanding that SAD plays an important role in asthma, ACO, and COPD [5, 7, 47, 71]. However, there are open questions about how SAD can be assessed by sound, discriminating lung function parameters reflecting the interactive derangements regarding bronchial obstruction, PHI, gas trapping, and gas exchange disturbances in the lungs. The main findings of the present study are, that the diagnosis of SAD can only be claimed if at least 3 specific lung function parameters (mainly sReff, VTGFRC, and DLCO) are interactively assessed. According to our data, the prevalence of SAD in asthma is much lower than reported by others [5, 6, 70, 72, 73]. This may be due to the fact, that in other studies the prevalence of FEF25–75 is erroneously hyper-estimated. Another reason may be that the prevalence of so-called SAD assessed by impulse oscillometry is very high [6, 70, 72–74]. We do not dispose of oscillometric data, and to our knowledge, there are no studies comparing the prevalence of plethysmographic and oscillometric parameters defining SAD, at least not based on z-scores. FEF25–75 is thought to be a precursor for early small airway involvement. However, the 10% and 25% onset over age for FEF25–75 is not earlier compared to the other parameters. In fact, the onset of the target parameters (sWOB, sReff, FEF25–75, and VTGFRC) is significantly earlier in asthma than in ACO and COPD (Figure 1).
Precision medicine features a new personalized approach, to “treatable traits”, suggested to address the limitations of the existing treatment strategies. Assessing SAD by interactive spirometric and plethysmographic lung function parameters may be of great help to better characterize SAD as treatable traits, leading to more targeted asthma management and more individualized patient care [35]. The importance of the peripheral airway in the pathophysiology and clinical manifestations of obstructive lung diseases makes them the intuitive target for long-term pharmacological approaches [75, 76]. In clinical routine practice, the identification of SAD during the diagnostic work-up should guide clinicians towards the treatment choice. Therefore, extended lung function assessment may be of great help to better characterize SAD as a “treatable trait,” leading to more targeted asthma management and individualized patient care.
In conclusion, our study shows that in a population of clinically stable moderate-to-severe asthma, ACO, or COPD, SAD is quite variably (asthma: 10.9%; ACO: 74.3%; COPD: 90.2%) predictable, and only if at least 3 spirometric or plethysmographic lung function parameters are used. Whether the presence of SAD may influence the long-term outcome of asthma requires prospective studies comparing patients with or without persistent SAD based on sound discriminating criteria. Only on pathophysiological basic principles, such criteria can be embedded into concepts of precision medicine tailoring disease treatment and prevention.
ACO: asthma-chronic obstructive pulmonary disease overlap
COPD: chronic obstructive pulmonary disease
DLCO: carbon monoxide diffusion capacity
FEF25–75: forced expiratory flow between 25% and 75% of vital capacity
FEV1: forced expiratory volume in 1 s
FRC: functional residual capacity
FRCpleth: plethysmographic functional residual capacity
FVC: forced vital capacity
LLN: lower limit of normal
PHI: pulmonary hyperinflation
SAD: small airway disease
SAdf: small airway dysfunction
sReff: effective specific airway resistance
sWOB: aerodynamic resistive work of breathing at rest
ULN: upper limit of normal
VTGFRC: volume of trapped gas at functional residual capacity
The authors are grateful to the staff of all study centers, especially to the study nurses for their excellent and enduring work in data collection, and the authors thank Prof. Sabina Gallati from Human Genetics of Hirslanden Precise, Zürich, for the critical reviews of the manuscript.
RK: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. HM: Writing—review & editing. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was planned according to the Federal Law of Human Research, conceptualized according to the Swiss Ethics Committees on research involving humans, and was conducted in accordance with the tenets of the Declaration of Helsinki. The study is part of the framework of the project entitled “Functional diversification of the Asthma-ACO-COPD multi-center study” (ID 2017-00259), approved by the Governmental Ethics Committees of the State of Bern, St. Gallen, Solothurn, and Zürich (project KEK-BE PB_2017-00104).
All 4 Ethics Committees exempted the informed consent to participate since the data were retrospectively evaluated and anonymous.
Not applicable.
Master-files have been stored and secured in the Clinical Trial Unit (CTU), Hirslanden, Corporate Office, CH-8152 Glattpark, Switzerland, and are available upon request from the corresponding author (richard.kraemer@hirslanden.ch) or the office manager, Mr. Daniel Tschopp, Head Clinical Trial Unit, Hirslanden (ClinicalTrialUnit.Hirslanden@hirslanden.ch).
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
