Affiliation:
1Department of Medicine, National and Kapodistrian University of Athens, 115 27 Athens, Greece
ORCID: https://orcid.org/0009-0006-6374-7859
Affiliation:
1Department of Medicine, National and Kapodistrian University of Athens, 115 27 Athens, Greece
ORCID: https://orcid.org/0000-0002-2989-4541
Affiliation:
21st Department of Respiratory and Critical Care Medicine, National and Kapodistrian University of Athens, “Sotiria” Hospital, 115 27 Athens, Greece
ORCID: https://orcid.org/0000-0001-8933-6800
Affiliation:
3Allergy Department, “Sotiria” Hospital, 115 27 Athens, Greece
ORCID: https://orcid.org/0009-0001-6213-6128
Affiliation:
21st Department of Respiratory and Critical Care Medicine, National and Kapodistrian University of Athens, “Sotiria” Hospital, 115 27 Athens, Greece
Email: nikrovina@med.uoa.gr
ORCID: https://orcid.org/0000-0003-0138-5582
Explor Asthma Allergy. 2024;2:205–218 DOI: https://doi.org/10.37349/eaa.2024.00041
Received: November 17, 2023 Accepted: March 13, 2024 Published: June 07, 2024
Academic Editor: Mario Di Gioacchino, Italian Society of Allergy and Clinical Immunology, G. d’Annunzio University, Italy
Eosinophilic gastrointestinal diseases (EGIDs) are a group of chronic conditions, characterized by an excessive accumulation of eosinophils in various areas of the mucosal of the gastrointestinal (GI) tract. EGIDs encompass a spectrum of diseases, including eosinophilic esophagitis (EoE), eosinophilic gastritis (EoG), eosinophilic enteritis (EoN), and eosinophilic colitis (EoC), each affecting different segments of the GI tract. The pathogenesis of EGIDs is multifaceted and involves an intricate interplay between genetic predisposition, environmental triggers, and dysregulated immune responses. Although the exact etiology behind EGIDs is not fully understood, it is clear that they are immune-mediated, with eosinophils having a central role in inflammation and tissue damage of GI mucosal. Clinical manifestations depend on the organ that is affected by the disease and on the depth of the eosinophil infiltration of the bowel wall. They range from mild discomfort to severe dysphagia, abdominal pain, malnutrition, and growth failure, particularly in pediatric cases. Regarding EGID management, it is a challenging issue to achieve clinical and histologic remission using pharmacotherapy and dietary elimination. Corticosteroids and proton pump inhibitors can be selected as an effective first-line treatment for certain patients and six-food elimination diet (6-FED) has been proven effective in inducing remission. Furthermore, biologic therapies have emerged as essential tools in controlling eosinophilic-driven inflammation. This review focuses on the complex pathogenesis and treatment of these inflammatory diseases, especially EoE.
Eosinophilic gastrointestinal diseases (EGIDs) are a group of chronic disorders, characterized by eosinophil infiltration of segments of the GI tract, in the absence of known causes for eosinophilia (e.g., medications, parasitic infection, malignancy) leading to inflammation, organ dysfunction, and clinical GI symptoms [1, 2]. The specific affected areas of the GI tract include the esophagus, stomach, small intestine, and large intestine either simultaneously or sequentially [3]. Eosinophilic esophagitis (EoE) is the best characterized EGID, but lately, there has been a growing interest in pathophysiology, epidemiology, diagnosis, and therapeutic options of the other EGIDs as well.
A recently published nomenclature for EGIDs provides a standardized and comprehensive approach in order to better characterize and diagnose these conditions [1]. According to this publication, EGID is an umbrella term whose initial branchpoint divides into EoE and non-EoE EGIDs. Non-EoE EGIDs are further divided based on the anatomical site of inflammation; stomach [eosinophilic gastritis (EoG)], small bowel [eosinophilic enteritis (EoN)], and colon [eosinophilic colitis (EoC)] [1, 3]. Additionally, EoN is subdivided into eosinophilic duodenitis (EoD), jejunitis, and/or ileitis (Figure 1). This review aims to present the suggested pathophysiologic pathways for the different EGIDs, focusing on EoE, and to consider the latest data for the management of these conditions.
The prevalence of EoE is higher in first degree family members of patients with EoE than in the general population. Genome-wide association studies (GWAS) have detected numerous loci with genome-wide significance and specific genes including thymic stromal lymphopoietin (TSLP)/WD repeat domain 36 (WDR36), calpain 14 (CAPN14), leucine rich repeat containing 32 (LRRC32)/EMSY BRCA2 interacting transcriptional repressor (EMSY), and C-type lectin domain family 16 member A (CLEC16A)/dynein expressed 1 (DEX1) [4–8]. These genes are implicated in type-2 helper T (Th2) cell response and barrier dysregulation [5].
Unknown epigenetic factors, especially in early life, seem to have an important impact on the development of EoE. In twin family studies, the concordance of EoE in dizygotic twins was higher than in nontwin siblings, even if both groups have a 50% similarity in their genome, which implies that epigenetic factors influence the occurrence of EoE [9]. Early life exposures like preterm labor and maternal infections, formula feeding, caesarian section, and antibiotic use during infancy are associated with high frequency of EoE [10]. The role of esophageal microbiota and the change of gut colonization during antibiotic exposure may have an influence on EoE occurrence [11], but more studies have to be conducted in this field, in order to elucidate possible related epigenetic mechanisms.
EoE is a chronic immune mediated inflammatory disease of the esophagus [4], associated with symptoms of esophageal dysfunction of varying intensity, and histologically with at least 15 eos/hpf (≥ 15 eos/0.3 mm2 or > 60 eos/mm2) in esophageal biopsies provided that other causes of esophageal eosinophilia are excluded [12, 13]. Common clinical manifestations of EoE are abdominal pain, dysphagia, nausea and vomiting, and failure to thrive in children. An overall estimate of its prevalence is 0.5–1 persons/1000 persons [14], followed by a male predominance (3:1) in pediatric patients and a strong correlation with atopic diseases as comorbidities [15, 16]. Although it has been considered as a rare disease, nowadays is one of the most common diagnoses in pediatric patients with feeding problems [17] and in the assessment of dysphagia in adults [17].
The pathophysiology behind EoE involves a complex interplay between genetic, immunologic, and environmental factors which results in the accumulation of eosinophils in the esophagus [5]. EoE is a type 2 driven inflammatory response to various stimuli, such as food allergens, aeroallergens, toxins, and microorganisms that affect the esophageal epithelium in genetically predisposed individuals, leading to varying degrees of tissue remodeling, fibrosis, and organ dysfunction (Figure 2) [5]. Due to an impairment of proteins associated with barrier function and of adhesion molecules, like desmoglein-1 and filaggrin [17, 18], a dysfunctional epithelium is created, which can easily be penetrated by antigens. Penetrating the epithelium, these exogenous factors activate the dendritic cells and also epithelium-delivered alarmin cytokines like TSLP and interleukin 33 (IL-33) [5, 19] which stimulate Th2 cells to release IL-4, IL-5, and IL-13. Chemokine C-C motif chemokine ligand 6 (CCL6, eotaxin-6) that promotes eosinophil infiltration plays a predominant role in the EoE pathophysiologic pathway; it is the highest up regulated gene in EoE genome and is completely associated with this disease [5]. Macroscopic, this disrupted epithelium is observed with typical endoscopic findings like edema, rings (“trachealization”), linear lines (furrowing), and strictures [19, 20].
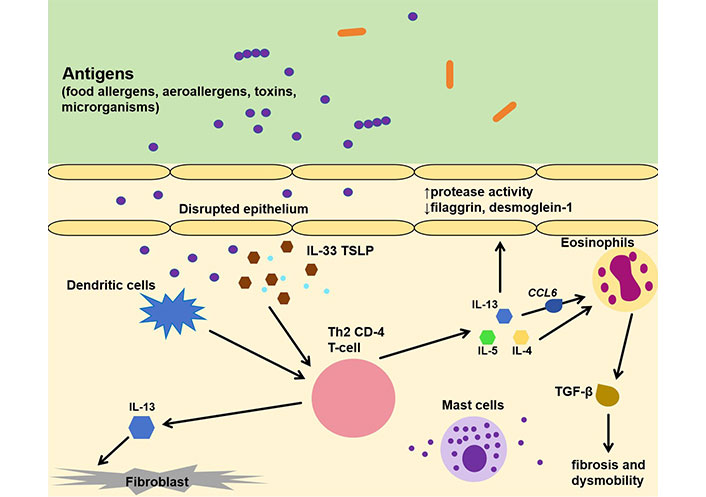
Immunopathology of EoE. IL-33: interleukin 33; TSLP: thymic stromal lymphopoietin; Th2: type-2 helper T; CCL6: C-C motif chemokine ligand 6; TGF-β: transforming growth factor-beta. ↑: elevated; ↓: decreased
IL-4 promotes the differentiation of Th2 cells, while IL-5 is a determining factor for eosinophil maturation and survival [21], as well as their release from the bone marrow. IL-13 seems to be the dominant type-2 cytokine, directing the histologic changes and the eosinophil mediated inflammatory responses. IL-13 is highly up regulated in individuals with EoE [22] and is also produced in the esophagus by infiltrating immune cells. It promotes barrier dysfunction through down-regulation of desmoglein-1 and fillagrin, inflammatory cell proliferation, and decreased epithelial cell differentiation [5, 17]. These molecules shape the interest of contemporary studies that aim to develop biologic agents against them, with anti-IL-13 and anti-IL-4 (dupilumab) being the ones with the most promising therapeutic results [23].
Gastroesophageal reflux disease (GERD) increases the permeability of esophageal mucosa, allowing environmental antigens, food allergens, and aeroallergens to pass through the esophagus and activate the inflammatory response that characterizes EoE. Proton pump inhibitors (PPIs) are an established treatment option for EoE and one of the reasons that patients respond well to this treatment is because they eliminate the GERD symptoms of the patients that have both disorders. Additionally, it was discovered that PPIs block the production of eotaxin-3, which contributes to the pathogenesis of EoE, and that mechanism may explain why some patients with typical EoE symptoms and little evidence of GERD respond well to PPIs [24].
In healthy individuals, eosinophils are found in all lengths of the GI tract apart from the esophagus [21]. Eosinophil activity in EoE has been explained in various studies and it is suggested that eosinophilia in the esophagus is a protective response to environmental antigens, promoting immunoregulation, defense, and repair, that becomes problematic over time promoting tissue remodeling and fibrosis. In the early phases of EoE, the eosinophil recruitment has a protective role in epithelial barrier maintenance, defense, and tolerance, while in chronic stages eosinophilia has pathologic results promoting inflammation and fibrosis [25]. In EoE, the eosinophil levels in the epithelial mucosal according to some researchers correlate with the disease severity, and the lower the eosinophil level the better the response to treatment [26]. Other studies, however, explain that the eosinophil counts in esophageal mucosal do not influence the disease progression [27].
Mast cells are normally present within the esophageal mucosa. In active EoE, they infiltrate the esophageal epithelium, resulting in their degranulation and activation. Clinically, in some studies, is referred that the patients that present pain symptoms are those with higher mast cell levels [5]. However, their role is not completely understood.
It is identified that food allergens are the most important triggers of EoE [5], and the elimination diet is the most common therapeutic approach to treat these patients [28]. Milk, eggs, and wheat are the most common food allergens that cause EoE and soy, nuts, fish, and shellfish are described less frequently [29]. There is a debate about the role of IgE-mediated food allergy (FA) in the pathogenesis of EoE. The prevalence of EoE in patients with IgE-mediated FA was found to be higher than that reported in the general population (4.7% vs. 0.04%) [30]. However, omalizumab, an anti-IgE biologic agent, currently used for long-term treatment of eosinophilic asthma [31], has been proved to be an ineffective treatment for patients with EoE [17, 32]. Some studies have been performed about the role of another globulin, IgG4. IgG4 is associated with chronic autoimmune diseases with an inflammatory profile and some studies report higher levels of it in esophageal tissue in EoE individuals and a higher total IgG4 serum level in reaction to specific food. However, there is poor literature on this topic [33].
Patients undergoing oral immunotherapy (OIT) for IgE-FA can develop EoE at a biopsy proven rate of 5.2% for peanut allergy [34]. This rate is probably underestimated because not all the individuals with GI symptoms during OIT undergo an endoscopy [5]. However, in a 2-year clinical OIT trial in individuals with peanut allergy, asymptomatic eosinophilia was observed due to the antigen exposure in OIT and fewer cases of EoE [25]. Studies with small sample sizes also indicate that the histological abnormalities and eosinophilia during OIT-EoE are resolved after the first year of OIT [25], but larger studies are required in this field.
The unresponsiveness of many individuals to the elimination diet led to the speculation that other factors may contribute to EoE pathogenesis [34]. Several data from animal studies and clinical pediatric and adult studies suggest that aeroallergens can also trigger EoE, while a relationship between EoE disease activity and seasonal aeroallergen exposure has been identified [35, 36]. The esophagus of individuals with EoE may have a compromised epithelial barrier, allowing aeroallergens to encounter the immune cells present in the lining of the esophagus [37]. This exposure triggers an immune response, characterized by the infiltration of eosinophils into the esophageal tissue. There is little literature exploring the safety and efficacy of subcutaneous IT (SCIT) and sublingual IT (SLIT) for EoE treatment [38]. In summary, aeroallergens, based on current data, have a prominent role in EoE pathogenesis only in a minority of individuals and SLIT and SCIT is suggested to be used only in patients with EoE and allergic rhinitis or asthma. There is little literature exploring the safety and efficacy of SLIT and SCIT for EoE treatment.
The EGIDs tract present with a variety of signs and symptoms. Lately, their incidence and prevalence have increased, but they are still considered as rare diseases in the general population [39, 40]. What distinguishes them from EoE is the role of the eosinophils [3]. Apart from the esophagus, there is a normal eosinophilic infiltration in the GI tract mucosal, that is associated with the defense of the GI system. Therefore, to diagnose these conditions, higher levels of eosinophil infiltration than those observed in EoE are required, in combination with upper GI symptoms [2, 3, 40].
EoG is the best characterized non-EoE EGID but still remains a rare disorder. EoG has a prevalence of 6.3 persons/100,000 persons and a female predominance, unlike EoE [39]. The diagnosis of EoG requires upper GI symptoms—abdominal pain or cramping, bloating, hematemesis early satiety, nausea, vomiting [40], and the presence of 30 eos/hpf in at least five high-powered fields, after the exclusion of secondary causes (Table 1) [3]. Furthermore, it is presented with hypoalbuminemia, anemia, and peripheral eosinophilia [2].
Diseases associated with gastrointestinal (GI) eosinophilia for each eosinophilic GI disease (EGID)
| EGID | Related diseases |
|---|---|
| Eosinophilic esophagitis (EoE) [12] | Achalasia |
| Crohn disease | |
| Celiac disease | |
| Parasitic infection | |
| Idiopathic hypereosinophilic syndrome (HES) | |
| Vasculitis | |
| Drug hypersensitivity | |
| Connective tissue disease | |
| Gastroesophageal reflux disease (GERD) | |
| Eosinophilic gastritis (EoG) and eosinophilic enteritis (EoN) [64, 65] | Malignancy |
| Crohn disease | |
| Celiac disease | |
| Helicobacter pylori | |
| Autoimmune gastritis | |
| Radiation enteritis | |
| Drug reaction | |
| Eosinophilic colitis (EoC) [40, 65, 66] | Inflammatory bowel disease (IBD) |
| Crohn disease | |
| Celiac disease | |
| Helminthic infections (e.g., pin worms, hookworms) | |
| Idiopathic HES | |
| Autoimmune diseases (scleroderma, Churg-Strauss) | |
| Drug reaction |
The term eosinophilic gastroenteritis (EoGE) refers to simultaneous occurrence of eosinophilic inflammation in the stomach and small intestine. However, recently, they are considered as separate entities [1, 3] even though the co-occurrence of these disorders is a very common phenomenon.
EoN can be sub-categorized into EoD, eosinophilic jejunitis, and eosinophilic ileitis. To diagnose this disorder, more than twice the normal number of eosinophils in the intestinal lamina propria is required (duodenum ≥ 52 eos/hpf, ileum ≥ 56 eos/hpf). This rare entity manifests itself with diarrhea and/or pain that may or may not present with certain foods leading to malabsorption [40]. However, it may present as asymptomatic eosinophilia combined with hypoalbuminemia or anemia [40]. It is believed that it rarely exists as a unique form of EGID, observed in patients with at least another affected area in the GI tract [2, 3]. The latest guidelines suggest that when multiple cites of the GI are involved, they all must be referred by the segment involved, for example, EoD and EoG [40].
The pathogenesis of EoG, EoGE, and EoN is not well understood. There are a lot of clinical similarities, but also differences, with EoE [41]. The genome-wide transcript profiles of these patients present significant elevated ILs (IL-4, IL-5, IL-13, IL-17) which reveal a strong association with type 2 allergic response.
An atopic predisposition is likely since 50 percent to 70 percent of non-EoE EGID patients also have asthma and/or allergic rhinitis [42] and elevated serum IgE levels [41].
Patients with non-EoE EGIDs have significant peripheral eosinophilia in comparison with individuals with EoE, where peripheral eosinophilia is not always present [41]. It has been proposed that the increased gut permeability permits eosinophils to easily penetrate the gut surface, entering the systemic circulation. Of note, the occurrence of other atopic conditions that may simultaneously exist with EoG may be responsible for the observed peripheral eosinophilia that characterizes the disease. The symptoms vary, depending on the layer of the epithelium wall that is infiltrated by the eosinophils. Therefore, mucosal infiltration is defined by nausea and vomiting, muscularis form leads to wall thickening and obstruction and serosal form leads to ascites [3]. Common features in biopsies in patients with these EGIDs are among others abundant eosinophils in mucosal and sub mucosal, an increased number of eosinophils in lamina propria and intraepithelial eosinophil abscesses. In EoG biopsies, a significant amount of mast cells and forkhead box P3 (FOXP3)-positive lymphocytes in comparison to controls is observed [2].
Regarding the molecular analysis of EoG transcriptome, a meta-analysis from Shoda et al. [43] suggests 18 differentially expressed genes that separate the individuals with active EoG from the control subjects. A sub-categorization of these genes indicates 8 up regulated genes [CCL26, CCL18, IL-13 receptor subunit alpha 2 (IL13RA2), IL-5, Charcot-Leyden crystal (CLC), etc.] associated with cytokines, cell adhesion, and eosinophilia and 10 down-regulated genes: [bone morphogenetic protein 3 (BMP3), solute carrier family 26 member 7 (SLC26A7), collagen type II alpha 1 chain (COL2A1), ATPase H+/K+ transporting alpha subunit (ATP4A), somatostatin (SST), etc.] [43].
EoC is the rarest (3.3 persons/100,000 persons) [39, 44, 45] and least described EGID and there is poor literature regarding the pathogenesis and treatment of this disease. The diagnosis of EoC remains a challenge because there are a lot of pathologies in gut that may be accompanied by elevated eosinophil count, like inflammatory bowel disease (IBD) (Table 1). However, the required eosinophil count ranges from 100 eos/hpf in the proximal colon, 84 eos/hpf in the transverse and descending colon, and 64 eos/hpf in the recto-sigmoid colon [3]. The symptoms may vary, with bloody diarrhea, abdominal pain, and anemia being among the most prevalent [44]. The pathogenesis of EoC is not a well understood issue. Individuals with a family history of allergic conditions may indicate greater risk. Evaluation of the transcript profile of patients with EoC revealed 987 differentially dysregulated genes in active EoC to be involved. It is noteworthy that some of them are also predominant in IBDs. CLC was found to be the most highly induced gene in EoC [46]. There are also some overlaps with the EoE and EoG genes, but the down-regulated genes in EoC did not overlap with other EGIDs. Furthermore, 48% of EoC patients have at least one more atopic condition, underlying the atopic profile of these patients [3]. There is minimal evidence for strong type 2 allergic inflammation appearance in comparison to EoE and EoG. In conclusion, EoC is probably defined as a distinguished condition from other EGIDs. The analysis of EoC transcriptome provides new data on the pathogenesis of the disease, but more studies are required to reveal it in this developing disorder [46].
The main goals of EoE’s treatment are to improve clinical symptoms and to prevent disease progression and complications [17, 47].
PPIs can be selected as an effective first-line treatment for certain patients with EoE. The efficacy of PPIs both in adults and children is variable and there are no randomized, placebo controlled clinical trials assessing their effectiveness in EoE. However, their long-term safety profile makes them an appealing first-line treatment [48]. A meta-analysis on PPIs in EoE that included 33 studies and 619 patients, documents a histologic remission in 50.5% of cases, with a clinical response rate of nearly 60%. However, the authors suggested that these findings should be used with caution because of several biases [47]. A recently published analysis from a European EoE registry in real-world practice concluded a clinical and histological remission rate with first-line PPI therapy (50.2%) compared to first-line therapy with topical corticosteroids (67.7%) [49].
Vonoprazan, a potassium-competitive acid blocker (P-CAB) with a different molecular structure from PPIs, is proposed as an alternative treatment not only for naive EoE but also for PPI-resistant EoE patients. More studies need to be conducted regarding this treatment for severe cases of EoE [50].
Oral topical corticosteroids, mainly fluticasone and budesonide, are considered an effective treatment option for EoE, even though they failed to induce histologic remission in approximately one-third of treated patients [48]. Topical use of budesonide or fluticasone has no inferior therapeutic results than PPIs such as esomeprazole. There is a variability in the way that swallowed topical corticosteroids are administered, resulting in differences reported among studies considering their effectiveness [51, 52]. Orodispersible tablets containing budesonide (OBT) have been approved by the European Medicine Agency [51] and their efficacy has been confirmed in double-blind clinical trials. Fluticasone propionate orally disintegrating tablets were superior for histologic and endoscopic responses and for the reduction in dysphagia frequency vs. placebo in a recent randomized double-blind placebo-controlled trial [51]. Oral corticosteroid preparations are generally well tolerated, however, questions remain regarding their long-term safety and the risk of adverse events, such as local viral infections or fungal diseases and/or adrenal insufficiency [46, 53].
Dietary interventions on EoE include empiric elimination diets and the use of elemental formulas. The latter has shown the highest potential for induction of remission, but they have an unpleasant taste, a breadth of avoidance, and cost and social limitations that raise concern. Alternatively, empiric elimination diet in the form of six-food elimination diet (6-FED) that is based on the elimination of milk, egg, wheat, soy, peanut, and seafood has been proven effective in inducing remission [13, 54, 55]. If 6-FED is applied correctly, 50–71% of patients will accomplish remission of EoE. Less restrictive diets with either 4-FED or 1-FED elimination have been studied with a rate of histologic remission of 49.4% for the 4-FED and 51.4% for the 1-FED. These results support a trend toward less restrictive diets [56]. The success of elimination diet as a treatment for EoE depends on patient’s will to adhere to the diet and to comply with the doctor’s instructions. Chronic dietary therapy can cause some adverse events such as nutritional deficiency, eating disorders, or psychological issues [54].
There is no firm evidence regarding the effectiveness of agents like montelukast, a leukotriene receptor antagonist, cromolyn, a mast cell stabilizer, and azathioprine, an immunomodulator, so it is suggested to be used only in the context of a clinical trial [48].
For non-EoE EGIDs (EoG, EoN, EoC) there is a lack of data about treatment, mainly because of the small number of patients diagnosed with these diseases. Evidence to support the use of glucocorticoids is limited to a small series of patients. Patients treated with an initial dose of 20–40 mg/day of prednisolone with gradual tapering during the next weeks, responded within two weeks of treatment and achieved remission, although more than 50% of patients who attained remission relapsed with lower doses [41, 57]. The long-term sequelae of the chronic use of glucocorticoids in these patients prevent them from being a sustainable treatment option.
Topical glucocorticoids are efficient on EoE and there are few case reports that state the same about other EGIDs. PPIs can also be a less effective therapeutic option, as well as elimination diet. In several cases, histamine 1 antagonists or leukotriene receptor antagonists can be effective, but there are no studies to confirm this evidence [41, 58]. Finally, in EoC, little evidence suggests mesalazine as an effective treatment option, with surgery to be limited only in cases with severe complications of the disease [47].
Targeting key components of the molecular pathways involved in the pathogenesis of EoE, such as Th2 cells, eosinophils, mast cells, and the cytokines IL-4, IL-5, IL-13, and TSLP, provide the way for a more personalized approach to the treatment of EoE [59].
Dupilumab is a fully human monoclonal antibody that binds with the shared receptor of IL-4 and IL-13 cytokines, blocking the type-2 inflammation that predominates in the pathogenesis of EoE. In May 2022, the U.S. Food and Drug Administration approved dupilumab to treat EoE in adults and pediatric patients of 12 years and older. The efficacy and safety of dupilumab was studied in a randomized, double-blind, multicenter, placebo-controlled trial that lasted for 24 months. The two primary endpoints were histologic remission (less than 6 eos/hpf) and the change from baseline in the Dysphagia Symptom Questionnaire (DSQ) [59, 60]. Histologic remission was achieved in 60% of patients who received dupilumab weekly and in 5% of patients who received placebo (P < 0.001). The DSQ scores improved with weekly dupilumab as compared with placebo, with differences of –12.32 (95% CI, –19.11 to –5.54; P < 0.001). The most frequent adverse event was injection-site reaction, while the incidence of conjunctivitis was low [59]. Although dupilumab improves short-term disease outcomes, long-term outcomes regarding the natural course of the disease remain unknown. More data are needed regarding the ideal phenotypes and endotypes in which dupilumab could be more beneficial, taking into consideration the high cost of the drug [61].
Considering the limited therapeutic arsenal for EoE, there is growing interest for the discovery of more biologic agents that could be beneficial for patients with EoE. Even if dupilumab is the only biologic that has been approved as a therapeutic option in EoE, there is a variety of ongoing studies about other biologic agents that could be approved in the near future [32].
Mepolizumab is an anti-IL-5 biologic that has been studied for the treatment of EGIDs including EoE. Although in most studies, mepolizumab led to a reduction in tissue eosinophilia, very few patients accomplished a total histologic remission with < 15 eos/hpf. Symptoms of EoE were limited by mepolizumab, but not totally relieved. No major side effects were observed. Reslizumab, is another anti-IL-5 monoclonal antibody with similar results in patients with EoE. Therefore, none of these two agents is currently approved for the treatment of EoE [62].
Benralizumab, another anti-IL-5 antibody that leads to antibody-mediated cellular cytotoxicity and is approved as treatment for eosinophlic asthma [63] is also studied for the treatment of EoE and other EGIDs. Benralizumab did not show encouraging results in EoE, as in most cases, it didn’t reduce patient’s symptoms, except for some case reports [32, 62].
IL-13 seems to play a crucial role in Th2 inflammatory conditions directing the production, trafficking into tissues, and survival of eosinophils. Blocking this pathway could also sustain a potential therapeutic option. Cendakimab (RPC4046), is a human anti-IL-13 monoclonal antibody that has shown some promising results in studies as a potential treatment of EoE. A significant reduction in esophageal eosinophil count and improvement in clinical dysphagia and endoscopic image were observed [32]. Even though no major adverse events were recorded during these studies, it’s yet to be discovered if this monoclonal antibody is safe for long-term use not only in EoE, but also in non-EoE EGIDs [32, 62].
Siglec-8 is a cell surface receptor expressed only on mature eosinophils and mast cells which, when engaged, causes natural cell death. Lirentelimab is an antibody that targets this receptor and causes tissue eosinophil death, via apoptosis. This biologic is mostly studied in other EGIDs like EoG, EoD, or EoC [62]. Studies have shown a significant reduction in tissue eosinophils count, which was accompanied by an improvement in the symptoms caused by the disease, especially in groups treated with high dose lirentelimab [32].
It has been reported that benralizumab reduces tissue eosinophilia and improves symptoms when used in EoG, being a potential candidate approved as a treatment in this disease entity in the future [32, 62]. Cendakimab could also be an alternative for these diseases and its safety and efficiency for long-term use in these diseases is a field of research [32, 62].
Moreover, there are some ongoing studies that aim to broaden our knowledge of lirentelimab’s efficacy in EGIDs, and if it could be an alternative and efficient option for these patients [32, 64–66].
EGIDs are a perplexing group of chronic conditions characterized by an abnormal immune response within the GI tract. The eosinophil infiltration leads to inflammation of the mucosal tissue remodeling and organ damage. The genetic predispositions combined with the environmental triggers compose the basic etiology of the activation of the immune mechanisms that lead to these diseases. Molecules like TSLP and ILs play a crucial role in the pathogenesis of EGIDs. Regarding the treatment options, PPIs and corticosteroids combined with elimination diets remain the first-line treatment options for the patients, however, due to the histologic and clinical disease heterogeneity, a long-term management of these patients is needed. The biologic agents that were discussed in this review are a very promising management option in order to accomplish more personalized treatments for this heterogenous group of disorders.
6-FED: six-food elimination diet
CCL6: C-C motif chemokine ligand 6
EGIDs: eosinophilic gastrointestinal diseases
EoC: eosinophilic colitis
EoD: eosinophilic duodenitis
EoE: eosinophilic esophagitis
EoG: eosinophilic gastritis
EoN: eosinophilic enteritis
FA: food allergy
GERD: gastroesophageal reflux disease
GI: gastrointestinal
IL-33: interleukin 33
OIT: oral immunotherapy
PPIs: proton pump inhibitors
SCIT: subcutaneous immunotherapy
SLIT: sublingual immunotherapy
Th2: type-2 helper T
TSLP: thymic stromal lymphopoietin
GP and AP: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. AB: Validation, Writing—review & editing. ACS and NR: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Nikoletta Rovina who is the Guest Editor of Exploration of Asthma & Allergy had no involvement in the decision-making or the review process of this manuscript. The other authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
