Affiliation:
Fundación Neumológica Colombiana, Bogotá 110131, Colombia
Email: ctorres@neumologica.org
ORCID: https://orcid.org/0000-0003-0004-8955
Affiliation:
Fundación Neumológica Colombiana, Bogotá 110131, Colombia
ORCID: https://orcid.org/0009-0005-9590-1200
Affiliation:
Fundación Neumológica Colombiana, Bogotá 110131, Colombia
ORCID: https://orcid.org/0000-0003-2153-0492
Explor Asthma Allergy. 2024;2:219–232 DOI: https://doi.org/10.37349/eaa.2024.00042
Received: June 15, 2023 Accepted: April 02, 2024 Published: June 12, 2024
Academic Editor: Mario Di Gioacchino, Italian Society of Allergy and Clinical Immunology, G. d’Annunzio University, Italy
There are no plausible arguments to consider that the best evidence-based asthma treatment should be different in low- and middle-income countries (LMICs). A few decades ago, the recognition of asthma as an inflammatory disease of the airways positioned the inhaled corticosteroids (ICS) as the cornerstone of the treatment of this disease, maintaining bronchodilators, especially the short-acting beta-agonists (SABA), as symptom-reliever medications for use as needed. However, adherence to regular use of ICS is very low, especially in LMICs, favoring the overuse of SABA, which has been related to an excess of exacerbations and mortality. Recently, the Global Initiative for Asthma (GINA) strategy has recommended the mandatory use of ICS every time a bronchodilator is used as needed (for symptoms relief), whether only as needed or with a background of regular dose of ICS, and has named it: anti-inflammatory reliever (AIR) therapy. This form of therapy, which has been related to a significant reduction of asthma exacerbations, is very attractive for LMICs where patients do not have guaranteed a proper medical follow-up and the access to on-the-counter medications is high. However, the implementation of AIR therapy in LMICs will face many of the already recognized barriers for the diagnosis and treatment of asthma in these countries, especially related to limited access to care in very different health systems, low education level of patients and communities, insufficient health personnel training in asthma in primary care, the unfordable cost of medications, and the lack of political commitment. This review analyzes some of these challenges and strategies for facing them in LMICs.
Asthma is one of the major problems for worldwide public health. The Global Burden of Disease Study (GBD) estimated that in 2019, there were 262 million people affected by asthma, corresponding to an age-standardized rate of 3,416 cases per 100,000 inhabitants [1]. There is a wide geographic variation of the asthma prevalence [1–3]. Although the reasons explaining this variation remain unclear, the greatest prevalences correspond to high-income developed countries (HIC), but with a higher increase in the recent years in low- and middle-income countries (LMICs) [1, 2, 4]. Regardless of the prevalence of asthma in LMICs is, overall, lower than in HIC, the asthma burden in those countries is significantly higher [1, 5–7]. The disadvantaged conditions in LMICs constitute a challenge for facing asthma [5, 8, 9], both in adults and children [10].
For many years, asthma treatment predominantly relied on bronchodilators [11]. Once asthma was recognized as an inflammatory disease of the airways, the most recommended maintaining therapy became inhaled corticosteroids (ICS) on regular daily dose (controller) with bronchodilators, mainly short-acting beta-agonists (SABA), for symptoms relief (rescue/reliever) [11, 12]. However, until recently, only SABA, without ICS, remained as an option for so called intermittent asthma (occasional and short duration symptoms) [13]. The low adherence to the regular use of ICS [14] and the easy accessibility to SABA have contributed to its worldwide excessive use in asthma [12], particularly in LMICs [5].
The overuse of SABA has been related to high risk of exacerbations and mortality [15]. Since 2019, the Global Initiative for Asthma (GINA) has recommended the use of ICS whenever SABA are taken, whether in combination in a single device or in separate inhalers, and no longer recommends the SABA-only therapy [13, 16]. To note, from 2021, GINA proposes two separate tracks for the personalized asthma management of adults and adolescents older than 12 years: a preferred, using ICS/formoterol [a rapid-onset long-acting beta-agonist (LABA)] as reliever, and an alternative one with as needed ICS-SABA or SABA (always accompanied by ICS) as reliever, introducing the concept of anti-inflammatory reliever (AIR), whether as needed only or with regular doses of ICS [17]. These recommendations represent the most relevant change in asthma management for decades [13, 18]. Regardless of the controversy over whether the preferred track is better or not [12], the AIR proposal is an option in LMICs whose implementation represents a great challenge because of many already recognized and unsolved barriers [4–10, 19–26]. In 2023, the World Health Organization (WHO) included in the list of essential medicines for asthma the ICS/formoterol combination in addition to the ICS and SABA [27].
This review is focused on some particularities of asthma in LMICs and the challenges for implementing the AIR management in these countries.
Beyond of the multiple barriers and gaps for the proper asthma management in LMICs, mainly related to its socioeconomic conditions [5, 8, 9, 22, 25], the risks, characteristics and behavior of asthma could differ in LMICs from those in HIC due to a higher prevalence of low birth weight, early and undertreated infections, early exposure to helminths, higher exposure and lower protection in workplaces, and higher household air pollution, frequently due to solid/biomass fuels used for cooking or heating the households [6, 26, 28–33]. A significant proportion of LMICs are located in tropical regions, where early exposure to helminth infections and biomass smoke is frequent, and changes in behavior and sensitization to house dust mites could influence the development, phenotypic distribution, treatment, and clinical expression of asthma [28, 34, 35].
Some decades ago, it was suggested that the prevalence of asthma in LMICs/developing countries could be lower than in HIC because of the different early exposure to infections, lower in HIC (the hygiene hypothesis) [36]. This hypothesis has been extensively revised and many other factors could be involved in explaining differences between atopic/allergic and asthmatic populations beyond the early exposure to infections [37].
On the other hand, LMICs have an important burden of air pollution, especially in rural areas, where access to clean domestic energy is limited. Orellano et al. [38] found a higher asthma prevalence in polluted environments and described that air pollution is a risk factor for asthma onset (OR: 1.34 [1.17–1.54]) in a developing region.
Similarly, LMICs have a greater burden of occupational lung diseases. Due to of the poor socioeconomic conditions and the weak professional education, people accept informal jobs with bad working conditions and high exposure to harmful particles and fumes. Often, workers do not have access to labor security and basic protection implements perpetuating asthma triggers [39]. Each country should ensure that companies are fulfilling workers’ laws and providing them with mandatory safety implements, inclusive, there must be health monitoring programs for workers at risk of developing occupational illness.
However, despite the aforementioned differences in risk factors for the development and exacerbation of asthma in LMICs, there is no evidence that the disease itself is significantly different and should be treated differently in these countries [5], and consequently the 2023 GINA AIR recommendations [17] and its implementation should be considered as an option for LMICs.
The burden of asthma morbidity and mortality is significantly higher in LMICs, where many children, adolescents, and adults lack of access to early diagnosis and effective and affordable asthma care [1, 5, 6, 10, 24, 40–42]. LMICs contribute with 96% of global asthma-related deaths and 84% of global disability-adjusted life-years [43] with around 90% of asthmatic patients not being well-controlled [40].
Although ICS are the cornerstone of the asthma treatment, the adherence to its regular use is very low, varying from 22% to 63%, with improvement up to and after an exacerbation [14]. Overall, 24% of exacerbations and 60% of asthma-related hospitalizations could be attributed to poor adherence [14]. Poor adherence is higher in LMICs [5, 9, 24] and in low-resource settings, reaching 80% [44]. In these countries and settings, the proper follow-up by physicians to adjust medication is difficult, with only 40% of children having regular assessment by primary care physicians and less than half receiving their prescribed inhaled controller agent [45]. The global low adherence to the regular use of ICS and the overuse of SABA, particularly in LMICs are some of the reasons that motivate the recommendation of the mandatory use of ICS every time a bronchodilator is used and the emergence of the concept of the AIR therapy.
According to 2023 GINA Report, reliever is defined as a medicine used for symptom relief or used before exercise or allergen exposure [17]. As GINA now recommends the use of ICS whenever a beta-agonist (formoterol or SABA) is taken, the anti-inflammatory effect is guaranteed with the use as needed of these combinations, introducing the concept of AIR treatment: ICS plus a rapid onset LABA (formoterol) (track 1) or ICS-SABA (track 2) [17]. For the combination of ICS-formoterol, GINA poses two options: 1) as-needed low-dose only (steps 1 and 2), called AIR-only, and 2) maintenance and reliever therapy (MART) for steps 3, 4, and 5. In each case, it is necessary to inform the patient about the maximum allowed reliever dose of ICS/formoterol for adults: 12 inhalations in a single day [formoterol: 72 µg (54 µg of delivered dose)] [17]. The use of as needed SABA only without ICS is no longer recommended [16].
These recommendations are based on the evidence that SABA monotherapy has been associated with increased bronchial hyperresponsiveness, rebound bronchoconstriction and worse disease outcomes [11, 15, 46–48], despite which their overuse is widespread worldwide [49], particularly in LMICs [5, 21, 46]. Recently, a systematic review by Crossingham et al. [50] found that ICS-formoterol on demand was better than SABA on demand in terms of severe exacerbations, emergency room visits, and hospitalizations reduction.
The as-needed/reliever use of ICS (AIR treatment) for asthma must be distinguished from the episodic use during and after exacerbations [51].
According to the severity of the disease, there are several studies to evaluate the response to the ICS/albuterol option: in a single inhaler, the BEST [52] and the MANDALA [53] studies for mild and for moderate/severe asthma, respectively, and in separate inhalers, the BASALT [54] and the PREPARE [55] studies for mild and for moderate/severe asthma, respectively.
The BEST study, a 6-month, randomized, placebo-controlled, double-blind protocol conducted in 455 adults with mild asthma, demonstrated that ICS/SABA (beclomethasone/albuterol) in a single inhaler on-demand (prn) was as effective as regular dose of ICS plus SABA prn in terms of peak flow and asthma exacerbations, with a lower cumulative ICS dose (18.5 ± 25.3 mg) than in the group receiving regular beclomethasone therapy (77.0 ± 17.4 mg, P < 0.001) or the group receiving regular combination therapy (77.1 ± 17.6 mg, P < 0.001) [52]. Likewise, the BASALT study found that the same combination (beclomethasone and albuterol) in separate inhalers used on-demand (prn) had a lower cumulative dose of ICS than the regular dose of ICS plus SABA group [54]. This kind of combination, mainly in separate inhalers, is highly available and included in the basic medicines lists in many of the LMICs [24] and the list of essential medicines of WHO [27].
The MANDALA study, conducted in 3,132 subjects older than 12 years with moderate to severe uncontrolled asthma, compared albuterol/budesonide prn (180 μg/160 μg, high-dose group), albuterol/budesonide prn (180 μg/80 μg, low-dose group) and albuterol-alone prn (180 μg). The risk of asthma exacerbations was lower (26%) in the albuterol/budesonide prn (180 μg/160 μg) group compared to the albuterol-alone group [53]. The availability of this combination is limited in LMICs and its use on demand is not generally approved.
The PREPARE was a pragmatic, randomized, open-label study aimed to evaluate whether a patient-activated relief-activated inhaled corticosteroid (PARTICS) strategy, consisting in the use of ICS for every use of SABA reliever medication, can improve asthma outcomes. The implementation of the PARTICS strategy led to a reduction of severe exacerbations of asthma with an associated increase of inhalers containing ICS compared to standard care [55]. This study is relevant to our discussion because it was focused on a cohort of 1,200 African-American/Black and Hispanic/Latino adults from low-resource setting which can be representative of LMIC conditions.
The evidence supporting the MART strategy is based on several studies with more than 30,000 patients, showing that it significantly reduces severe exacerbations when compared to ICS, ICS-LABA plus SABA on demand, with a similar level of symptom control [50, 56–60]. Similar results have been found with budesonide and beclomethasone, achieving lower exposure to oral corticosteroids (OCS) and less SABA use, both in low and high T2 asthma. A possible limitation in LMICS is that the studies have been done using dry powder inhalers, which have a higher cost than pressurized metered-dose inhaler (pMDI). At present, the MART strategy is approved in 120 countries and AIR-only in 48 countries. The evidence is low in patients with persistent airflow limitation. There is only one study in the emergency department with ICS-formoterol [61] and there are no studies with mometasone-formoterol or with fluticasone propionate-formoterol which could have different cost in some countries.
Several factors, such as the poor adherence to ICS, the high outdoor, indoor, and occupational exposures, and the low rates of vaccination, contribute to an increased risk of exacerbations in LMICs. Some air pollutants (O3, NO2, PM2.5) have been related to airway hyperresponsiveness, inflammation, and oxidative stress, important characteristics for asthma development and exacerbations [62]. Other factors, as the lack of educational programs for patients, the limited access to the health services, and insufficient skills of the health workers are very common in LMICs and contribute to the poor outcomes of exacerbations (Figure 1) [33].
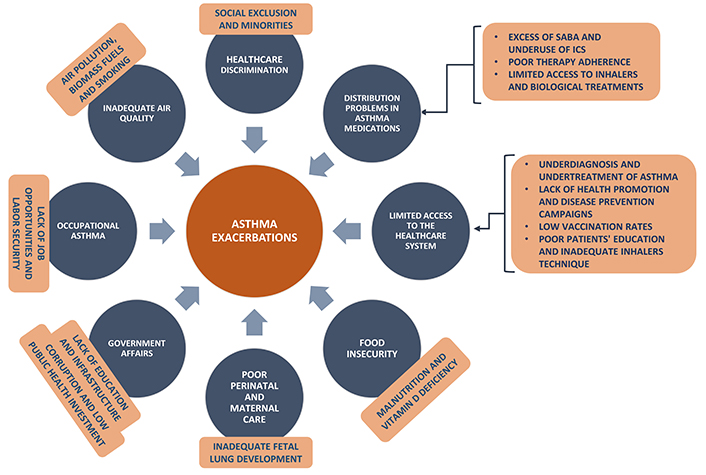
Factors that lead to poor asthma control in low- and middle-income countries (LMICs)
In addition to relief symptoms, the other main goal of the treatment of asthma is the reduction of future risks, which includes: prevent exacerbations and deaths, minimize the adverse events, mainly due to OCS/systemic corticosteroids, and maintain the best lung function [17]. Preventing exacerbations reduces asthma deaths, OCS use and the negative impact on quality of life, contributes to maintaining the lung function, and decreases the health resources use, particularly derived from emergency room visits and hospitalizations. Moreover, reducing exacerbations avoids the disruption of daily activities and the school and work absenteeism [63]. Deaths from asthma represent the “tip of the iceberg” of the global burden of asthma [1], 96% of them occurring in LMICs [43].
On the other hand, asthma exacerbations require the increase of reliever medication and the use of bursts of OCS. The cumulative dose of OCS is related to short-term adverse events, such as pneumonia, pulmonary thromboembolism and sleep disturbances [64], and to long-term dose-dependent effects, like osteoporosis, diabetes, cataracts, and depression [65, 66]. One of the most important actions for facing the burden of asthma in LMICs is to prevent exacerbations of asthma and consequently, excessive use of OCS and SABA.
The AIR treatment reduces the exacerbations of asthma and the derived morbidity, including the adverse effects of OCS [51]. As mentioned, because of the low adherence to the regular use of ICS and the overuse of SABA, the AIR treatment, in which patients receive ICS (or additional ICS, if already they are prescribed with regular doses of OCS) whenever they take their reliever inhaler, guarantees the anti-inflammatory action and the adjustment of their ICS intake according to symptom fluctuation [51, 67].
There are serious limitations to accessing the inhaled medicines, especially to ICS (alone or combined with LABA or SABA) for a proper management of asthma in LMICs [5, 8, 19, 23, 24, 68, 69]. Although there is a wide variation between the different LMICs, the determinants of these limitations include factors depending on the patients and their families, the community, the primary care personnel, the health systems and their regulatory normative, and the commitment of governments and the private pharmaceutical industry. Some countries have not included ICS in their health system medicine lists, and many do not include ICS/LABA (ICS/formoterol or other LABA) or ICS/SABA [5, 24].
This complex situation has been recognized for several years. Almost two decades ago, the International Union Against Tuberculosis and Lung Disease (The Union), based on the experience of the Global Drug Facility (GDF) for tuberculosis, proposed and launched the Asthma Drug Facility (ADF) strategy for facing the non-availability of essential asthma medicines and prohibitive pricing in many LMICs [19]. Unfortunately, after significant efforts, the ADF had to be discontinued mainly because of the lack of political commitment of the countries [19].
The recent systematic review by Stolbrink et al. [24], about the availability, cost, and affordability of essential medicines for asthma and COPD in LMICS included data from 60 countries. Six of 58 countries met the 80% availability target for SABA, only three of 48 countries for ICS, and zero of four for ICS/LABA [24]. They found that the cost of SABA is approximately half that the ICS or ICS/LABA [24]. Consequently, people with asthma in LMICs rely heavily on inhaled SABA alone, or oral preparations of salbutamol, theophylline, or prednisolone instead [6].
A study in three LMICs surveyed a significant number of pharmacies in Nepal, Perú and Uganda [70]. Most of them (> 90%) were private. The availability of any medication for respiratory disease was higher in private (93.3%) compared to public (73.3%) pharmacies. Salbutamol monotherapy was the most commonly available respiratory medication among the three sites while beclomethasone was only available in Perú (33.7%) and Nepal (22%). The availability of ICS/LABA in private pharmacies varied from 0% in Uganda to 6.7% in Perú, to 87.3% in Nepal. ICS/LABA were not available in public pharmacies in Uganda and Perú [70].
WHO defines a list of essential inhaled medicines for respiratory diseases that provide wide spectrum for effective treatment, which include those necessary for AIR therapy [27]: budesonide/formoterol and ICS/formoterol. Also, it includes budesonide, beclomethasone, ciclesonide, flunisolide, fluticasone and mometasone, and combinations as fluticasone/salmeterol, fluticasone/formoterol and fluticasone/vilanterol [27].
In LMICs, the most frequently used products for asthmatic patients, such as fluticasone/salmeterol, budesonide/formoterol, budesonide, beclomethasone and albuterol, have very variable prices, but the cost of the single drugs is consistently lower than the combinations. Some inhaled drug prices are not affordable for countries (patients and health systems) with low purchasing power. Coverage by the public health systems and public subsidies also have wide variability between countries, and among the countries between public and private subsystems, generating great inequity [69].
The Global Asthma Network [4] reveals that SABA are used in 29% to 85% of all asthmatic patients, ICS in only 12% to 51%, oral SABA in 37%, and theophylline in 20%. Forty-four to 60% of individuals with severe asthma symptoms do not use ICS. The systematic review by Stolbrink et al. [24], including data from 60 LMICSs, showed that SABA met the target of 80% availability in only 6 countries.
The SABINA III study, which evaluated SABA prescriptions and their relationship with asthma clinical outcomes, enrolled 8,351 patients in 24 countries, 45% of whom had one or more exacerbations in the past 12 months. Patients who were overprescribed with more than three canisters of SABA per year counted by 38% and 20% of them were buying SABA without prescription [48].
The overall picture is summarized in low accessibility to inhaled medication in LMICs, especially ICS, alone or in combination, notoriously in the public healthcare system, leading to catastrophic out-of-pocket expenses for patients who prefer to use SABA only due to its greater accessibility and lower cost.
At the beginning of the COVID-19 pandemic, some studies suggested that the early administration of inhaled budesonide reduced the severity of the disease, the likelihood of needing urgent medical care and reduced time to recovery after early COVID-19 [71, 72]. On the other hand, evidence from clinical studies indicated that the ICS routinely taken for asthma could have had a protective role in preventing severe COVID-19 and, therefore, may be a promising treatment for COVID-19 [73]. In addition, some observations suggested symptomatic improvement with the use of budesonide/formoterol in people affected by COVID-19 [74].
These findings could have had an impact on the availability and use of ICS and ICS/formoterol. Asthmatic patients were considered a high-risk population and asked for improving their adherence to the regular use of ICS leading to an increase of use [75]. However, the long-term adherence to ICS does not seem to have changed because of the COVID-19 pandemic [76]. To our knowledge, there is no data on the impact of the COVID-19 pandemic on the low availability and use of ICS and ICS/LABA in LMICs. However, change to video/phone consultations and home administration of biologics was common in severe asthma care during the COVID-19 pandemic and was associated with high satisfaction levels in most cases [77], as well as other digital tools [78], and it is expected to continue after pandemic time.
In summary, it is not clear if the COVID-19 pandemic positively influenced the availability and affordability of ICS and ICS/formoterol medicines for asthma in LMICs, despite asthmatic patients being considered a high-risk population. However, many tools and practices learned during the pandemic continue to be useful and need to strengthen them.
Looking for determinant factors and solutions to the limited access to effective treatment for asthma in LMICs, the stakeholders meeting convened by The Union [23], the World Asthma Report [1] and some expert reviews [8, 79] have identified as the main barriers: 1) the low awareness, knowledge and education on chronic respiratory diseases (CRDs) in general and asthma in particular (from patients and health workers); 2) the limited healthcare infrastructure, including a shortage of healthcare facilities, medical professionals, and essential medical supplies; 3) the high cost and poor availability of asthma medications; 4) the limited data on CRDs burden and treatments; 5) the ineffective procurement and distribution networks; 6) the poor communication of the needs of people with CRDs; 7) the limited financial resources; and, 8) the lack of political commitment.
Some practical examples of this complex situation include the common under-prescription of ICS, which reflects the lack of training and knowledge in asthma management among health personnel, especially in primary care, and the inability of the health systems for a proper long-term follow-up of asthmatic patients. The loss to follow-up seen within 1-year in pilot projects in China, Benin, and Sudan [6, 80–82] varied from 52% to 76%. In general, in LMICs, the understanding of patients and physicians about the need for chronic treatment of asthma is incomplete and the periodic adjustment of medication for asthma control is insufficient. In general, investment and public health priority of asthma is significantly lower than HIV and tuberculosis.
The determinant factors of suboptimal treatment of asthma in LMICs differ significantly between countries, and generalizations based on national income should be avoided [5]. Each country and each region should identify its own barriers, define its prioritization, and address them systematically. Education of general community and asthmatic patients, and training and capacity building of health workers, especially in primary care, are crucial [5, 8]. A proper approach to cultural factors (e.g., beliefs that result in unwillingness to accept the diagnosis of asthma, religious practices and beliefs, roles of traditional health workers, reliance on home remedies or unsupported thoughts about asthma medication risks) is crucial and represents one of the major challenges for optimal asthma care.
According to The Union stakeholders meeting [23], the World Asthma Report [1] and expert opinions [5, 6, 8, 19, 24, 79], to overcome the barriers and effectively face asthma in LMICs, it is crucial to achieving a high level of political commitment and greater investment in asthma services and programs, achieve universal health coverage through health systems strengthening, advance research and development, generate high-quality data to inform policy and practice, and to address underlying determinants of morbidity and mortality. Education of health students and workers for early recognition, a better understanding of asthma, and a systematic application of evidence-based recommendations is a priority task.
Patients in GINA Step 5 or uncontrolled Step 4 and all patients with difficult-to-treat and severe asthma should be part of interdisciplinary and comprehensive asthma programs, regardless of their socioeconomic position, in which systematic education be an essential component focused on how to avoid asthma exacerbations, mitigate modifiable environmental triggers, and use the inhaler correctly.
A fundamental component for improving asthma management in LMICs is the access to essential asthma medicines [23, 24]. The ICS, ICS/formoterol and ICS/LABA should be included in the list of essential medicines in all LMICs and they should be universally available in public and private pharmacies. Political commitment and public-private strategic alliances, involving the pharmaceutical laboratories, are one of the most important and challenging steps.
The two tracks for asthma treatment (one preferred and one alternate) proposed by GINA in 2021 [16, 17] have advantages and disadvantages [12]. Beyond of what are the best conditions and patients for each track, the AIR treatment is a good option for LMICs because it guarantees the use of ICS and its anti-inflammatory effect each time that patients need to relief their symptoms, reducing the risk of asthma exacerbations.
However, the implementation of AIR treatment in LMICs faces the same described barriers with two additional aspects: the recent appear of the GINA recommendation, with insufficient knowledge by physicians, particularly in primary care, and the higher cost of the combinations including formoterol. The first aspect requires of active programs for training health workers in different scenarios, since university studies, and education of patients and caregivers. The second one involves different stakeholders which should look for reducing the high differences in prices between the AIR treatment options and the huge gaps between public and private subsystems.
As mentioned, each country and region should define its particular barriers and solutions and evaluate the risks of the excessive use of SABA in settings with poor adherence to regular doses of ICS when patients use separated inhalers. Beclomethasone is the cheapest inhaled corticosteroid, but is more expensive than salbutamol (SABA). It is necessary to make great efforts to ensure the adherence to the use of beclomethasone, whether in regular doses or each time that SABA is used as reliever, when beclomethasone and SABA are used in separate inhalers.
It is well-recognized that the poor adherence to ICS is related to multiple factors [14, 44]. An individualized approach must be done for each asthmatic patient to choose the best option of treatment. The AIR therapy merges as a good and practical option in LMICs. Each country might identify the most cost-effective ICS-based [20].
The AIR therapy is a good option for LMICs. The evidence strongly supports the superiority of ICS-based reliever therapy over SABA rescue therapy and argues for its widespread adoption globally. However, several obstacles hinder its implementation in LMICs. These barriers include physicians’ lack of awareness of guideline recommendations, underinvestment in healthcare, and limited patient education. Active educational programs aimed at patients and health personnel should promote the discontinuation of the use of SABA alone without ICS.
It is essential to prioritize the comprehensive assessment of each patient’s context, preferences, and treatment goals, addressing modifiable factors and associated comorbidities. Furthermore, optimal compliance with prescribed medications and mastery of inhaler techniques are essential for therapeutic results.
The ICS, ICS/formoterol and ICS/LABA should be included in the list of essential medicines in all LMICs, and they should be universally available in public and private pharmacies. Beyond the educational programs as the cornerstone for facing asthma as public health problem in LMICs, political commitment and public-private strategic alliances, involving the pharmaceutical laboratories, are some of the most important and challenging steps for improving the availability and affordability of asthma medicines and the implementation of GINA recommendations, including the AIR therapy option.
AIR: anti-inflammatory reliever
CRDs: chronic respiratory diseases
GINA: Global Initiative for Asthma
HIC: high-income developed countries
ICS: inhaled corticosteroids
LABA: long-acting beta-agonist
LMICs: low- and middle-income countries
MART: maintenance and reliever therapy
OCS: oral corticosteroids
SABA: short-acting beta-agonists
WHO: World Health Organization
CATD and IPR: Conceptualization, Investigation, Validation, Formal analysis, Writing—original draft, Writing—review & editing. AAM: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
