Affiliation:
1Department of Food, Nutrition & Dietetics, School of Allied Health, La Trobe University, Melbourne 3086, Australia
Email: sassipap@hotmail.com
ORCID: https://orcid.org/0000-0003-1169-7141
Affiliation:
2Department of Experimental Physiology, Medical School, National & Kapodistrian University of Athens, 11527 Attiki, Greece
ORCID: https://orcid.org/0000-0002-9440-7797
Explor Asthma Allergy. 2024;2:245–286 DOI: https://doi.org/10.37349/eaa.2024.00044
Received: September 27, 2023 Accepted: March 04, 2024 Published: June 27, 2024
Academic Editor: Désirée Larenas-Linnemann, Hospital Médica Sur, Mexico
The article belongs to the special issue The Global Picture of Asthma after Guideline Changes and the COVID Pandemics
Over the last two decades, the emergence of lethal virulent strains of coronavirus (CoV), including the severe acute respiratory syndrome CoV 2 (SARS-CoV-2), which is responsible for the coronavirus disease 2019 (COVID-19) pandemic, has become a matter of great attention to the scientific community. Despite the implementation of preventive measures throughout the world, the spread of this disease and associated co-morbidities and mortality continue in all countries, continents, and populations of all ages. COVID-19 is highly contagious. Clinical manifestations are diverse and range from asymptomatic, mild to severe, life-threatening complications in the elderly and patients with underlying conditions such as cardiovascular disease, diabetes, obesity, and asthma. In addition, viral infections can trigger asthma attacks. To date, there is no specific treatment schema to combat COVID-19 disease. Current patient care revolves around disease severity and supportive treatment of symptoms from home-rest in mild disease to anti-viral therapy, oxygen support, anti-inflammatories, and anti-coagulants in severe COVID-19. Regarding prevention, the World Health Organization recommends vaccination, social distancing, quarantine, the wearing of surgical masks, and handwashing. In many countries, vaccination is optional, and given that parents are often reluctant to vaccinate themselves and their children for fear of side effects, identifying ways to enhance or support the immune system to prevent infection or improve recovery in vulnerable populations is worth investigating. Furthermore, research has focused on the pharmacological management of COVID-19 symptoms and much less has been published on nutrition therapy. Therefore, the scope of this review is to summarize the latest evidence on the use of vitamin D to support the metabolism and the immune system of asthma patients during the COVID-19 pandemic. A brief overview of asthma and COVID-19 pathophysiology, COVID-19 treatment guidelines for asthma patients, and the role of vitamin D in lung health, including the optimal blood level required to enhance immunity, will be suggested.
Upper respiratory tract infections are common during the winter months, and are usually mild and treated with over-the-counter medications. Since the end of 2019 with the Wuhan outbreak, the world has been battling a major public health crisis against the novel coronavirus (CoV) disease defined as coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome CoV 2 (SARS-CoV-2) [1]. This respiratory illness is considered one of the greatest challenges in the history of mankind.
In the 1960s, the first CoV infection (including α-strains: 229E and NL63 and β: OC43 and HKU1) was isolated from the nose and lungs and found to be responsible for non-complicated infections of the upper and lower tract infections, including the common cold [2]. Human CoVs are rapidly transmitted from one human to another via airborne droplets and aerosols when talking, coughing, and sneezing [2]. Over the last two decades, the emergence of lethal, virulent, and aggressive strains of CoV, namely severe SARS-CoV-2, has become a matter of great attention to the scientific community [1, 2]. Given its rapid acceleration worldwide, in March 2020, the novel COVID-19 infection was announced by the World Health Organization (WHO) as “a global pandemic” [3]. According to WHO statistics, to date, in the European region alone, mortality from this disease has exceeded 2 million, and about 277 million confirmed cases have been recorded [4]. Dissemination via international travel and the immune naivety of populations have been the main routes of transmission throughout all countries and nations across the globe, inflicting populations of all ages, ethnicities, and socio-economic backgrounds [2]. COVID-19 is associated with considerable morbidity and mortality, causing major havoc in daily family life and depression.
This respiratory disorder imposes a colossal economic burden primarily on elderly severe COVID-19 patients with co-morbidities and the national health system due to the need for hospitalization, intensive care unit (ICU) admission, mechanical ventilation, loss of productivity days, and mortality [5]. Consistent across studies performed in Europe, the United States (US), and Asia, the cost for COVID-19 patients admitted to the ICU was higher than for non-ventilated inpatients [5]. Overall, the cost of mechanical ventilation ranged from $2,000 to $3,500 per day [5]. In the US alone, a 20% COVID-19 infection rate yielded a total direct medical cost of $163.4 billion amidst the pandemic [5]. Interestingly, UK statistics quote that the lock-down period (14-day quarantine, social distancing, and indefinite closing of schools and universities) cost the government £668.4 billion [5, 6]. In Italy, the total cost of lost productivity due to absenteeism from work was approximately €100 million [5, 7]. On a global level, it has been estimated that the total cost for COVID-19 mortality was $3.5 trillion, with the highest cost incurred in the US at $1.4 trillion (that is about 40% of the total worldwide expenditure) [5, 8].
The first line of preventive strategies to mitigate the spread of the disease are isolation, quarantine, social distancing, the wearing of surgical masks, and handwashing [9]. Clinical manifestations among patients are diverse, ranging from asymptomatic, mild lower respiratory tract infections (including fever, cough, dyspnea, myalgia, confusion, headache, sore throat, rhinorrhea, chest pain, diarrhea, nausea, emesis, anosmia, and dysgeusia) to severe, life-threatening complications, namely sepsis, acute respiratory distress syndrome (ARDS), cardiac failure, septic shock [10], and multi-organ failure [2]. Notably, severe viral pneumonia with respiratory failure can cause death [11]. Variability and severity of COVID-19 symptoms are attributed to sex and age differences, along with demographic factors [2]. Amounting evidence substantiates subgroups at high risk for severe COVID-19 outcomes include males, the elderly, black or South Asian ethnicity, and those having co-morbidities such as cardiovascular disease, diabetes, obesity, chronic respiratory disease, severe asthma, and immuno-compromising conditions [12]. The co-existence of chronic disease exacerbates and intensifies the inflammatory response to viral infection and increases the risk for adverse outcomes and mortality. More specifically, mortality risk ranged from 2.40 in the 60–70-year-old age group to 20.60 in the over 80-year-olds [12], is about 1.7 times higher in males than in females, 1.48 times higher in individuals of black and South Asian ethnicity, 1.40 times higher in those presenting with obesity (BMI 30–35 kg/m2), and 1.92 times higher in the morbidly obese (BMI > 40 kg/m2) as compared to the normal-weight [12]. Regarding co-morbidities, the mortality rate is 1.13 times higher in patients with asthma, 1.17 times for chronic heart disease, 2.16 times for stroke or dementia, 1.95 times for diabetes, 1.72 times for recently diagnosed cancer, and 2.52 times for reduced renal function [12]. Sex disparities in COVID-19 clinical outcomes might be potentially due to protective mechanisms inherent to the female sex, which include immunological states [13], hormonal and oestrogen-specific effects [13], lower expression of the angiotensin-converting enzyme 2 (ACE2) receptors in the airways [13], lower prevalence of comorbidities [14, 15], and smoking habits [15, 16]. Furthermore, in reference to CoV strains, temporal trends show that men are more susceptible to SARS-CoV-2 infection [1, 17] and females to SARS-CoV-1 [18], while the MERS-CoV strain prevails in the Middle East [19]. As for population-specific outbreaks, a large body of evidence assessing COVID-19 infection in pediatric asthma patients demonstrated that, compared to adults [1], children suffered with milder symptoms and had a better prognosis [20]. However, children are 2.5 times more likely to be infected with the Delta variant than adults over 50 years [21].
Despite the implementation of preventive measures throughout the world, the spread of this disease continues in all countries, continents, and populations of all ages. To date, there is no specific treatment schema to combat COVID-19 disease. In April 2020, the Food and Drug Administration (FDA) authorized the use of anti-malarial drugs chloroquine and hydroxychloroquine to treat COVID-19 [22]. Adverse health risks, including arrhythmias and premature death in patients, were reported after the use of these drugs [22]. Furthermore, the efficacy and safety of these treatments have not been confirmed in pediatric populations. Regarding prevention, vaccination, social distancing, quarantine, the wearing of surgical masks, and handwashing have been recommended by WHO [9]. Vaccination and anti-viral therapy can reduce disease severity, mortality risk, and the need for hospitalization [9, 23] in the elderly and in patients with underlying conditions such as chronic lung disease, asthma, cardiovascular disease, obesity, diabetes, and immune-suppressed [14, 23]. In some European countries, vaccination is optional or mandatory for high-risk groups above 50 years (for example, in Greece and Italy) [24]. Many adults and parents are often reluctant to vaccinate themselves and their children for fear of potential side effects and uncertainty about the efficacy of the vaccine in preventing infection [25, 26]. Earlier, COVID-19 vaccines were designed to provide protection specifically for one strain [27]. Given that the SARS-CoV-2 virus is subject to mutation and new variants continue to emerge [28, 29], identifying ways to enhance the immune system in order to prevent infection or improve recovery in vulnerable populations is worth investigating. In addition, research has focused on the pharmacological management of COVID-19 symptoms, but much less has been published on nutrition therapy. The scope of this narrative review was to summarize the latest evidence on the use of vitamin D to support the metabolism and the immune system of asthma patients during the COVID-19 pandemic. Firstly, the topic will be introduced by providing a brief overview of asthma and COVID-19 pathophysiology, including COVID-19 treatment guidelines for asthma patients, followed by the therapeutic potential of vitamin D in relation to the modulation of the immune response associated with COVID-19 infection and asthma. Finally, the dosage and mode of vitamin D supplementation in the treatment of severe COVID-19 will be discussed, and the optimal blood level of vitamin D required to enhance immunity will be suggested.
Asthma is defined as a complex, heterogeneous inflammatory disorder of the airways [30]. During an asthma exacerbation, an allergen triggers a series of events, including narrowing of the airways, increased mucus production, persistent inflammation, reversible airway obstruction, and hyperresponsiveness of varying intensity that lead to characteristic symptoms of wheeze, shortness of breath, cough, and chest tightness [31]. Secular trends show that approximately 300 million people worldwide suffer from this condition [32]. It is more common in children and the elderly due to deficits in lung function [31]. Variations in prevalence exist according to environmental factors, access to medical treatment, and the country’s socio-economic development [33, 34]. It is higher in industrialized developed countries of high income (such as the UK, Australia, New Zealand, and the US), but is currently rising in developing low-middle income countries (Africa, Eastern Mediterranean), most likely attributed to poor housing conditions, overcrowding, damp environments, and second-hand smoking [33, 34]. On a global scale, asthma ranks among the top 20 chronic diseases for disability-adjusted life years in children and among the top 10 in mid-childhood, ages 5–14 [34]. Approximately two out of three cases are diagnosed during childhood and adolescence [31]. Prevalence is higher in boys pre-adolescence, after which the trend is reversed [35]. The etiology of asthma is unknown. A combination of genetic, environmental, and lifestyle factors contribute to asthma induction, including atmospheric pollution, cigarette smoking, allergies, physical exertion, viral infections, obesity, dampness, mold, and a high-fat diet [36], to name a few. Strikingly, 80% of children hospitalized for an asthma attack had a viral infection at the time of admission [37]. Asthma exacerbations have been reported in juvenile cases following infection with rhinoviruses [37], influenza [38], adenoviruses [39], and CoVs [39]. Severe asthma or poorly controlled asthma in children and adolescents is associated with hospital admissions, emergency department visits, missed days from school and work, reduced academic performance [40], impairment of every-day living, decreased physical condition [29], chronic fatigue, and sleep problems [41]. Given that subjects with chronic pulmonary disease, including asthma, are more prone to respiratory infections, presumably, these patients would be ideal candidates for COVID-19 infection [14].
From a molecular perspective, similarities exist between COVID-19 and asthma. Both conditions target the lungs and have common molecular pathways. The immunopathophysiology of asthma involves the activation of innate and adaptive immune systems, which stimulate chronic inflammation in the airways and consequently airway edema, mucus hypersecretion, and eventually remodeling of the small airways [42]. It is believed that the imbalance between T helper cell type-1 (Th1)/Th2 pro- and anti-inflammatory cytokines, an increased cellular influx into airways, combined with chronic oxidative stress and hyperreactivity prime the background for an asthma attack in individuals with a genetic predisposition [for example, A disintegrin and metalloproteinase domain 33 gene (ADAM33) is a mediator of cell-matrix interactions via proteolytic release of surface proteins and the cleavage of extracellular matrix components related to airway remodeling [42]]. More specifically, Th2-driven pro-inflammatory cytokines [interleukin (IL)-4, IL-5, IL-9, and IL-13] stimulate B cells to release IgE which activates mast cell degranulation and secretion of histamine and leukotrienes causing bronchoconstriction, while IL-9 and IL-13 contribute to mucus production, and Th17 induce airway remodeling via IL-17 and IL-22 [42]. In response to viral infections, Th1 is activated with a corresponding up regulation of interferon (IFN)-γ and IL-27, which not only aid in the elimination of pathogens but also in airway inflammation [42].
In the same manner, the progression of COVID-19 pathogenicity is the result of a complex interplay between innate and adaptive immune mechanisms, which orchestrate SARS-CoV-2 infection and contribute to organ-specific tissue damage [43]. Emerging evidence from extensive and rigorous research suggests that SARS-CoV-2 infection consists of three phases: viral replication, immune hyperactivity, and pulmonary destruction [43]. Noteworthy, the expression of SARS-CoV-2 host cell entry receptor and entry-associated proteases in human tissue prevails throughout the cardiovascular, endocrine, respiratory, digestive (liver, pancreas), nervous, and muscle systems, which explains the wide range of symptoms and sites of multi-organ failure [43, 44]. In a nutshell, the SARS-CoV-2 is a single-stranded RNA virus bounded by a nucleocapsid (N) protein and three surface proteins: membrane (M), envelope (E), and spike (S). This pathogen gains entry into the human lungs via the nose after which it attaches to ACE2 receptors on the cell surface of alveolar cells located in the lungs [43]. ACE2 binds to the receptor-binding domain (RBD) of the SARS-CoV-2 S protein. Cleavage of the “S” protein by serine proteases, transmembrane serine protease 2 (TMPRSS2), or cathepsin B and furin present in cell membranes primes the viral S protein for fusion to the host cell membrane and enters by endocytosis [43]. It is believed that the high infectivity rate of SARS-CoV-2 is ascribed to the presence of the furin molecule in the S protein [43]. Inside the cell, the S protein divides into S1 and S2 subunits, where S1 regulates virus-host and cellular tropism mediated by the RBD, and S2 facilitates virus-cell membrane fusion via two tandem domains’ heptad repeat 1 (HR1) and HR2 [43]. Following fusion, the viral RNA genome is released into the cell cytoplasm, where replication is initiated and transcription of the virus and non-structural proteins occurs. Virion components are assembled on the endoplasmic reticulum of the Golgi body and new virions are released into the circulatory system via exocytosis, infecting cells of the upper respiratory tract, lower airways, and enterocytes of the gastrointestinal system, potentially leading to respiratory infection, severe inflammation, acute lung injury, and manifestations of symptoms [43]. A schematic representation of SARS-CoV-2 pathogenicity is illustrated in Figure 1.
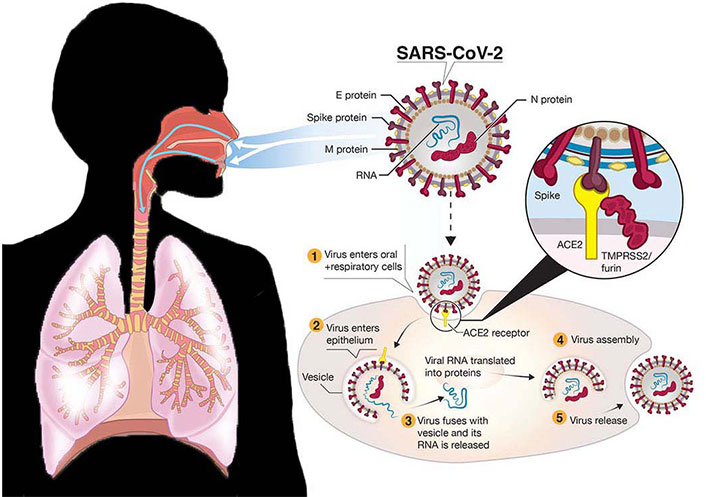
Schematic representation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. E: envelope; M: membrane; N: nucleocapsid; ACE2: angiotensin-converting enzyme 2; TMPRSS2: transmembrane serine protease 2
Note. Adapted from “A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic” by Funk CD, Laferrière C, Ardakani A. Front Pharmacol. 2020;11:937 (https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2020.00937/full). CC BY.
Evidence delineates that these proteins might interfere with IFN pathway activation through a variety of mechanisms, including prevention of pattern recognition receptor (PRR) recognition, inhibition of signaling pathways, and host mRNA and protein degradation, thereby promoting viral replication and amplifying the inflammatory response [43]. Furthermore, SARS-CoV-2 binding induces down regulation of ACE2, imbalance of the ACE/ACE2 ratio, and dysregulation of the renin-angiotensin-aldosterone system (RAAS), which combined with intracellular reactive oxygen species (ROS) in neutrophils and hypoxia-induced hyper viscosity evoke vascular endothelial dysfunction and activation of coagulation pathways, platelet aggregation, and ultimately vascular thrombosis [43]. The reduction in expression of ACE2 receptors by SARS-CoV-2 is associated with acute lung injury and disease pathology [43]. A prominent feature of severe SARS-CoV-2 infection is the systemic hyper-inflammatory state created by the production of high levels of pro-inflammatory cytokines [IL-2, IL-4, IL-10, IL-6, IL-7, tumor necrosis factor-alpha (TNF-α), IFN-γ-induced protein 10 (IP-10)] and chemokines [chemokine C-C motif ligand-2 (CCL2), granulocyte-colony stimulating factor (G-CSF), chemokine C-X-C motif ligand-10 (CXCL-10)] or “cytokine storm” and subsequently a delayed B-cell response and lymphocytopenia of T cells (predominantly in adult patients) [1], which correlates with disease stage and severity in COVID-19 patients [43]. Therefore, severe hypoxia, increased oxidative stress, and intensified systemic inflammation in the airways directly damage the pulmonary capillary mucosa, promote alveolar edema, impair the alveolar structure, and consequently result in dysfunction of pulmonary ventilation [43]. Altogether, these conditions would trigger exacerbations in asthma patients and worsen symptoms in those with severe asthma.
At the onset of the COVID-19 epidemic, there were concerns within the medical community about the impact of COVID-19 on asthma patients due to their increased susceptibility to viral respiratory infections [36] including the deficient and delayed immune response that can give rise to severe asthma exacerbations [45]. COVID-19 causes breathing difficulties, ranging from mild to critical, with older adults and people who have chronic comorbidities, such as hypertension, pulmonary disease, obesity, heart disease, cancer, and diabetes, carrying a higher risk of severe symptoms [14]. Moreover, diagnosing COVID-19 in asthma patients presents a challenge due to the overlap of symptoms between these two conditions, which can lead to misdiagnosis of COVID-19 for asthma and delays in medical care access. Paradoxically, meta-analyses suggest that there is no strong evidence that asthma patients are more vulnerable to COVID-19 infection than the general population [46–48]. During the first wave of the COVID-19 lockdown period, studies conducted in adults and children reported a reduction in asthma exacerbations, hospital and ICU admissions, and ventilation use with no increase in mortality [47–51]. No significant differences were found between the healthy population and subjects with asthma [47, 48]. In fact, the risk of acquiring SARS-CoV-2 was lower in those with asthma as compared to non-asthmatics [47, 48]. Even in severe asthma patients, a low incidence of COVID-19 has been observed, and there was no association with a higher risk of SARS-CoV-2 infection or poor outcome [50, 52]. Interestingly, children experienced fewer upper respiratory tract infections and fevers than in the preceding year [53]. This could be attributed to a number of factors: social isolation and distancing, better hygiene measures, school closures, the wearing of masks to reduce contamination, virtual consultations, and adherence to asthma therapy [54, 55] including inhaled corticosteroid use due to anti-viral effects [56]. Secondly, children are exposed to a diversity of pathogens during the early years of life and therefore have acquired immunity to common CoVs responsible for mild upper respiratory tract infections, namely the common cold and bronchiolitis [57, 58]. In addition, higher exposure to common pathogens favors increased mucosal colonization by viruses [58].
From a molecular perspective, there are several potential mechanisms that have been proposed to explain the phenomenon of reduced SARS-associated CoV morbidity in patients with asthma. Given that ACE2 receptors serve as entry points for SARS-CoV-2 binding to the host cell, there is evidence that asthma patients tend to have lower ACE2 expression in airway cells [59] and therefore are less susceptible to COVID-19 infection. Notably, the expression of ACE2 receptors in the nasal epithelium and lower airways is age-dependent, and the existence of these receptors is lower in children than in adults [59, 60]. Apart from the anti-inflammatory effects of inhaled corticosteroids, the mainstay of asthma symptom treatment is the reduced sputum ACE2 expression caused by these agents, thus inhibiting SARS-CoV-2 replication [56]. With respect to asthma pathogenesis, the state of chronic inflammation in the lungs arising from insults by allergens might lead to immune tolerance and protection against the development of hyperinflammation that drives COVID-19 progression [61]. Mucus hypersecretion, another hallmark of asthma induction, is rich in glycoproteins, namely polymeric mucins MUC5AC and MUC5B, matrix gel-forming molecules in airway mucus that function as a first-line mechanism against viral infection [62, 63]. Therefore, one might speculate that the profuse production of mucus could inhibit the SARS-CoV-2 virus from penetrating to the alveolar type II (ATII) cells, which predominately express ACE2 in the lungs. In the event of an asthma exacerbation, eosinophil recruitment and accumulation signify the onset of airway inflammation and bronchoconstriction, leading to characteristic symptoms of asthma [31]. Experimental studies indicate a potential role of eosinophils in promoting viral clearance and antiviral host defense [64]. In this context, patients with eosinophilic asthma (Th2-asthma phenotype) possessing high levels of eosinophilia in the airways could confer protection against the hyper-inflammatory response associated with severe COVID-19 morbidity and mortality [65]. Collectively, these mechanisms could reduce the viral load, and as a consequence, limit SARS-CoV-2 replication, attenuate airway inflammation, and confer prophylaxis for COVID-19 in asthma.
Severe asthma, as defined as having uncontrolled or partially controlled asthma despite therapy, prevails in 5–10% of patients [66]. Unlike mild stable asthma patients, the scenario is different. Data from the electronic health records of 17 million British subjects revealed that hospitalized patients with severe asthma and taking high-dose inhaled corticosteroids were associated with a higher risk of mortality from COVID-19 [12, 67]. In the same line, an Italian study noted that severe asthma predicted cases requiring ventilation or having a worse COVID-19 outcome (death) [68]. Previous research has documented that in severe SARS-CoV-2-infected patients, eosinopenia is a common observation [69]. Therefore, severe asthma patients with a Th2-low eosinophil phenotype could have a higher susceptibility to severe COVID-19 disease. Future studies are warranted to investigate whether the assessment of blood eosinophil concentrations as part of routine patient care is predictive of severe COVID-19. Identifying asthma patients with eosinopenia would enable clinicians to take drastic action at the early stage of the disease, thus resulting in improved outcomes.
According to the Global Initiative for Asthma (GINA), continuing the prescribed asthma therapy (including inhaled and oral corticosteroids) in order to maintain optimal asthma control is crucial in reducing the risk of future exacerbations and the need for urgent healthcare during the course of the pandemic [70, 71]. Written action plans instructing patients on the steps to follow in the event of an asthma attack have been effective in improving health outcomes and reducing emergency visits including hospitalization [70].
It is believed that the sequelae of COVID-19 pathogenesis are initially fueled by SARS-CoV-2 replication during the early stages of host infection, followed by an exaggerated immune and inflammatory response that leads to epithelial cell damage, vascular endothelial dysfunction, and the development of thrombosis [43]. Pharmacotherapy has been designed to counteract these characteristic features. Therefore, anti-viral therapies targeting SARS-CoV-2 replication early in the course of the disease will have a favorable clinical outcome. On the other side of the coin, anti-inflammatory, immunosuppressive anti-thrombotic medications will be most effective as the disease progresses to a severe condition as manifested by respiratory distress and thrombosis due to a state of hypoxemia and endothelial dysfunction [43].
Bearing in mind that the existing data supports the concept that asthma is not a potent risk factor for SARS-CoV-2 infectivity, then the management of COVID-19 in asthma patients follows the same procedure as for the general population. Treatment options are based on strong evidence from clinical trials undertaken in unvaccinated, high-risk subjects (> 50 years), with immune-compromised or underlying chronic conditions (cardiovascular or kidney disease, obesity, diabetes, and chronic pulmonary disease) [72]. Current patient care revolves around the classification of patients according to disease severity (mild, moderate, severe, or critically ill), oxygen requirements, and supportive treatment of symptoms [72]. Severe outcomes of COVID-19 disease are defined by hospital and ICU admissions, ventilatory support, or death. The objective of pharmacotherapy is to aid recovery and prevent progression to serious disease, hospitalization, and ultimately, mortality.
In ambulatory patients with mild to moderate disease, the National Institutes of Health (NIH) recommends the use of common antipyretics and analgesics to relieve symptoms of fever, headaches, myalgia, and cough, combined with rest and maintenance of fluid intake to prevent dehydration [72]. In outpatients with mild-moderate symptoms not requiring supplemental oxygen but who are at high risk of developing severe COVID-19, anti-viral drugs are indicated [73–75]. Spinner et al. [73] demonstrated that middle-aged outpatients with moderate COVID-19 prescribed 5 days of remdesivir (an RNA polymerase inhibitor with potent anti-viral activity) had a statistically significant difference in clinical status. Comparably, in the PINETREE study, Gottlieb et al. [74] reported that 3 days of administration of intravenous remdesivir in non-hospitalized, un-vaccinated patients of high risk (≥ 60 years, having obesity or one other underlying condition, e.g., diabetes or hypertension) with mild to moderate COVID-19 were associated with an 87% lower risk of hospitalization or death. Hammond et al. [75] using data from the EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients) study, showed that administration of oral ritonavir-boosted nirmatrelvir (a 2 main protease inhibitor) every 12 h for 5 days to high-risk, non-hospitalized, unvaccinated adults with COVID-19 (mean age 45 years) resulted in an 89% lower risk of progression to severe disease in terms of hospitalization or 28-day mortality. In a real-life setting, a recent population-based cohort study showed that subjects (vaccinated/non-vaccinated) treated with ritonavir-boosted nirmatrelvir had 44% decreased odds of hospitalization for COVID-19 or all-cause mortality, and 51% lower odds for mortality alone [76]. The therapeutic efficacy of nirmatrelvir-ritonavir has also been documented in populations at low risk for COVID-19 [77]. A subgroup analysis of data from the EPIC-SR (EPIC in Standard-Risk Patients) study revealed that treatment of vaccinated patients at low-risk for COVID-19 (at least one risk factor for severe disease) with nirmatrelvir-ritonavir resulted in a 57% reduction in hospital admissions and death, although non-significant, and a 62% decrease in COVID-19-related medical visits daily. In an observational, retrospective cohort study based on data from electronic medical records, Arbel et al. [78] confirmed the effectiveness of nirmatrelvir against the Omicron variant in the over-65 year age group, particularly in those with no immunity.
WHO approves the use of remdesivir for the treatment of mild-moderate COVID-19 in both hospitalized and non-hospitalized adults and pediatric patients (≥ 28 days with weight ≥ 3 kg) at high risk of progression to serious disease [79, 80]. Based on the robust scientific evidence mentioned above, for high-risk outpatients, it is recommended that remdesivir be administered for 3 days and that treatment be initiated within 7 days of symptom onset. In the case of hospitalized patients (under no mechanical ventilation), the recommended treatment duration is 5–10 days. For hospitalized patients requiring invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO), remdesivir should be initiated immediately after diagnosis of COVID-19 infection for a time period of 10 days [80]. Similar guidelines are suggested for ritonavir-boosted nirmatrelvir, twice per day for 5 days, within a 5-day onset of symptoms. Ritonavir-boosted nirmatrelvir has been approved by the FDA for use in non-hospitalized adults and adolescents 12 years to 17 years (with a weight ≥ 40 kg) with mild-moderate COVID-19 who are at high risk of severe disease [81]. Given the high efficacy of ritonavir-boosted nirmatrelvir and that it is available as an oral medication (unlike remdesivir, which is administered intravenously), the NIH advocates the use of this drug as the first line of treatment. In the case of unavailability or clinical inappropriateness due to drug-drug interactions, then remdesivir is the best option [82].
Convincing evidence from several studies substantiates the clinical benefit of anti-viral drugs in hospitalized patients diagnosed with severe COVID-19 in the presence and absence of respiratory support [83–86] as well as in the immunosuppressed [87].
According to a contemporary meta-analysis that assessed the potential benefits of remdesivir vs. standard care in 10,480 hospitalized patients with COVID-19, pooled analysis showed that remdesivir reduced mortality in hospitalized patients who required no oxygen or low-flow oxygen support [86]. Overall, patients in the remdesivir group had lower odds of all-cause mortality at day 28 or need of mechanical ventilation and higher odds of mechanical ventilation “free days” than patients in the no-remdesivir group. In general, remdesivir recipients presented a better clinical status. These findings are consistent with the WHO COVID-19 treatment guidelines, which recommend remdesivir for patients with severe COVID-19 but not for the critically ill [80]. This recommendation is driven by the results of the WHO-Solidarity study, which was an up-dated meta-analysis of 14,304 eligible adult patients (≥ 18 years) recruited from 454 hospitals in 35 European countries assessing mortality risk in COVID-19 patients taking remdesivir as compared to no drugs (the control) [83]. According to the WHO Solidarity Trial Consortium [83], compared to the standard care group, 17% lower mortality risk or progression to ventilation in patients requiring supplemental oxygen support at baseline was observed in the remdesivir group, and 13% lower mortality risk for patients on supplemental oxygen not requiring mechanical ventilation. There was no significant effect on patients who were ventilated at baseline.
In the ACTT-1 trial (phase 3), a randomized double-blind multinational study of hospital adults (mean age 59 years) with COVID-19, it was reported that compared to the placebo, patients receiving remdesivir had a reduced mortality rate, shorter time to recovery, improvement, discharge as well as stay in hospital [84]. Furthermore, a lower proportion of patients in the intervention group required oxygen supplementation or additional ventilatory support throughout the study duration and of those receiving oxygen at baseline (mechanical or ECMO), the duration of support was reduced. Thus, suggesting that treatment with remdesivir in the hospital setting may prevent the progression of serious disease, improve the clinical status, and shorten the time to recovery and length of hospital stay.
Garibaldi et al. [85] conducted a retrospective, multicenter comparative effectiveness study of 96,859 hospitalized individuals (median age 65 years) positive for SARS-CoV-2 and symptomatic for COVID-19 to investigate the time to improvement in patients receiving at least one dose of remdesivir. Results showed that remdesivir recipients were more likely to achieve clinical improvement by 28 days and patients receiving no oxygen or low-flow oxygen were less likely to die than controls.
Mozaffari et al. [88], in a novel comparative effectiveness study, investigated the effect of remdesivir on mortality among hospitalized patients for COVID-19 requiring supplemental oxygen support that included low- or high-flow oxygen, non-invasive ventilation, mechanical ventilation, and ECMO. The authors reported that treatment with remdesivir was associated with significant reductions in mortality across patients with severe or critical disease requiring high-flow oxygenation, non-invasive ventilation, mechanical ventilation, and ECMO.
Regarding immunocompromised patients, a large retrospective cohort study investigated the effectiveness of remdesivir in hospitalized immunocompromised patients across different variants of COVID-19 (Delta and Omicron) [87]. Administration of remdesivir to this high-risk subgroup of patients was associated with a 25–30% lower mortality risk than in the no-remdesivir group. Noteworthy, the drug was effective in reducing the viral load of both the Delta and Omicron strains.
To summarize, randomized controlled trial (RCT) data have confirmed the effectiveness of early remdesivir administration in reducing time to recovery and mortality among COVID-19 patients in outpatient settings and in hospitalized patients requiring and not requiring ventilatory support. It appears that remdesivir is an effective antiviral drug that improves the survival rate and prevents the progression to a critical illness. One limitation is that remdesivir is available as an injection and is deliverable within the clinical setting. Production of remdesivir in oral form will facilitate physicians in its rapid distribution throughout the public sector. From a futuristic point of view, early intervention in the course of COVID-19 disease could prevent patient and societal burden, increase the survival rate, and reduce overall economic costs.
It is worth mentioning that in outpatients with mild COVID-19 patients or in hospitalized patients not requiring supplemental oxygen, the use of dexamethasone is contraindicated and does not confer survival benefits [89, 90]. Jamaati et al. [91] reported no clinical benefit of dexamethasone administration in COVID-19-induced ARDS with respect to non-invasive/invasive mechanical ventilation, death rate, length of hospital stay, and illness duration. In fact, Crothers et al. [89] demonstrated that dexamethasone was associated with a 76% higher risk for mortality in hospitalized patients (~ 70 years old) in the absence of oxygen support. Contradictory to these findings, in the recovery trial, administration of dexamethasone among COVID-19 inpatients receiving invasive mechanical ventilation or supplemental oxygen alone had a 36% and 18% lower incidence of death, respectively, as compared to those on standard care [92]. However, no benefit was observed among those receiving no respiratory support. In the same direction, Zeng et al. [93], in a meta-analysis of nine (9) RCTs (n = 7,907) that included severe and non-severe COVID-19 patients, found that corticosteroid treatment significantly reduced all-cause mortality in patients with severe COVID-19 infection by 23% but not in the non-severe. In addition, low-dose dexamethasone with an extended treatment course appeared to be beneficial for all-cause mortality in COVID-19 patients. Unexpectedly, in non-severe COVID-19 patients, corticosteroids were found to be associated with a prolonged length of hospital stay.
Concerning the critically ill, data from meta-analyses concludes the survival benefits of glucocorticoid treatment in the severely critically ill [94, 95]. Li et al. [94], in a high-quality meta-analysis of 10 RCTs and 71 observational studies that included over 45,000 patients, found that in severely critically ill patients, glucocorticoid therapy was associated with a 12% reduction in all-cause mortality risk from COVID-19 and a 52% reduction for SARS. In SARS, patients’ lower mortality was observed in the severe and critically severe groups, taking early and medium- to high-dose glucocorticoids. Of note, in the severely critically ill, glucocorticoids were not associated with adverse effects of hyperglycemia, nosocomial infection, or delayed viral clearance. Contrastingly, in mild-to-moderately ill patients, the risk of complications was considerably higher.
A prospective meta-analysis of 1,703 critically ill COVID-19 patients from seven (7) randomized trials showed that critically ill patients receiving systemic corticosteroids had 34% lower odds of 28-day all-cause mortality than those receiving usual care or placebo [95]. Most importantly, in critically ill patients, low-dose dexamethasone resulted in clinical benefits in those requiring ventilatory support (mechanical or supplemental). Treatment guidelines for mild-to-severe COVID-19 are summarized in Table 1. The care of the critically ill is beyond the scope of this review, and recommendations are available from the NIH [72].
Therapeutic management of COVID-19
| Hospital status | Treatment | ||
|---|---|---|---|
| Outpatients | |||
| Mild-moderate symptoms (no supplemental oxygen requirements) |
| ||
| Patients at high risk of severe COVID-19 (e.g., elderly, immunocompromised, > 6 months since vaccinated) |
| ||
| Inpatients | |||
| No supplemental oxygen |
| ||
| High risk of severe COVID-19 |
| ||
| Immunocompromised undergoing |
| ||
| a) Conventional oxygen supplementation | |||
| Require minimal conventional oxygen |
| ||
| Most patients (combined therapy) |
| ||
| Patients receiving dexamethasone with respiratory distress and systemic inflammation |
| ||
| b) HFNC oxygen, NIV, MV, or ECMO | |||
| All patientsd |
| ||
COVID-19: coronavirus disease 2019; HFNC: high-flow nasal cannula; NIV: noninvasive ventilation; MV: mechanical ventilation; ECMO: extracorporeal membrane oxygenation. a Recommended 5-day treatment; b treatment therapy to be initiated immediately within 5–7 days of COVID-19 symptomology; c to be administered within 10 days of symptom onset; d add on remdesivir in the immunosuppressed or evidence of ongoing viral replication. +: add on medication
A drawback of pharmacotherapy is that the development of new drugs and vaccines, clinical trials, regulation requirements, and approval from the FDA require time. Therefore, the consideration of more natural therapies, including food and nutrients, is an attractive, non-invasive, and viable option. A healthy lifestyle and nutritious diet may further boost the immune response to combat COVID-19, as well as help to manage asthma symptoms better.
The notion that diet offers the possibility to prevent or cure nutritional deficiencies, disease onset, and promote health was recognized in antiquity, a mere 2,500 years ago, by Hippocrates, the father of medicine: “Let food be thy medicine and medicine thy food.” [96]. Indeed, the prospect that foods routinely consumed in the household can improve the patient’s overall health, provide therapeutic benefits, or act as a panacea for common diseases is captivating.
From a clinical perspective, the increased awareness of the importance of lifestyle intervention and nutritional strategies for overall good health and blocking the natural course of disease pathogenesis has been the turning point for the renaissance of diet and lifestyle patterns. It is well recognized that chronic disorders such as cardiovascular disease, dyslipidemia, diabetes, and obesity are directly related to an unhealthy lifestyle [97]. There is overwhelming evidence that combination therapies including lifestyle modifications (promoting healthy diets, physical activity, smoking cessation, and healthy weight or modest weight reductions) combined with drug therapy are successful in disease prevention, delaying onset, stabilizing, or reversing the disease process in high-risk populations [98–100]. Nevertheless, to date, drug therapy remains the first line of choice for disease management. However, in the case of multifactorial diseases, clinicians are increasingly realizing the limitations of the one-target drug approach. Research in nutrition and lifestyle patterns offers new insight into the pathophysiological mechanisms underlying a disease and molecular targets of interest which can be applied to propose novel pharmacotherapeutic targets or diet therapies for the tailoring of personalized treatments [101]. Lately, the current trend in nutritional science has been the use of bio-active food components, or functional foods (that is, foods that contain other elements apart from nutrients with health-benefiting effects), and dietary supplements to counteract nutrient deficiencies and risk of disease.
Over the last couple of years, there has been tremendous public interest in the role of nutrition in the prevention and management of COVID-19. A plethora of information is available on the internet or social media claiming that certain foods, fad diets, or supplements can combat respiratory infections or prevent disease. Most of the time, this information is false, misleading, and without scientific evidence [102, 103]. So, can diet prevent the COVID-19 infection? Currently, there is no convincing evidence that food, nutrients, or supplements can protect, treat, or promote immunity. Good hygiene, social distancing, and isolation of infected individuals remain the cornerstones of avoiding contamination. WHO advocates consuming a healthy, well-balanced diet to promote good health, enhance immunity, and reduce the risk of chronic disease [104]. A healthy dietary pattern consists of fresh fruit and vegetables, legumes, minimally processed whole-grain cereals, nuts, low-fat meats and dairy products, poultry, fish, and eggs. In addition to drinking water to prevent dehydration and optimal functioning of the body, avoiding excessive intake of sugar, fat, and salt to prevent weight gain, obesity, cardiovascular disease, hypertension, diabetes, and cancer [97]. WHO nutritional guidelines during the COVID-19 outbreak are summarized in Supplementary material.
Nevertheless, dietary habits have a profound impact on our health, and poor dietary habits are linked to the development of major chronic diseases [97]. Nutrients are pivotal in the regulation and strengthening of the immune response by providing nutrients that play a key role in immunity. More specifically, micronutrients, vitamins, minerals, and antioxidants [Cu, folate, iron (Fe), Se, zinc (Zn), and vitamins A, B6, B12, C, and D] are capable of influencing inflammatory mechanisms that constitute innate immunity [105, 106]. A large body of evidence shows that nutritional deficiencies and an insufficient or unhealthy diet impair the immune response, causing loss of the body’s ability to protect against disease, infection [105, 106], chronic obstructive pulmonary disease (COPD) [107], or allergy development along with the elimination of pathogens, thereby resulting in poor resistance to infection. In a recent study, Al-Fartusie et al. [105] documented that Zn, magnesium (Mg), manganese (Mn), chromium (Cr), and Fe were significantly lower in COVID-19 adult patients than in recuperating individuals and healthy controls. With respect to COVID-19, vitamin D deficiency was common in adult and child patients [108, 109]. The same trend was observed for asthma patients independent of age group [110, 111]. Thus, indicating that aberrations in metabolism could be associated with an increasing risk of COVID-19 infection and asthma onset.
This section is intended to summarize the existing mechanistic data regarding vitamin D with reference to COVID-19 and asthma pathways.
Hypovitaminosis D (25-hydroxy-D [25(OH)D] < 20 ng/mL) [112] inflicts over one billion adults and children collectively worldwide [113]. Approximately 40% of the European population is vitamin D deficient (< 50 nmol/L or 20 ng/mL) [114], 37% of Canadians [115], and 24% of Americans [115]; while 13% of Europeans are severely deficient (< 30 nmol/L or 12 ng/mL) [114], followed by 7.4% of Canadians and 5.9% of Americans [115]. Even in sun-replete Southern and Eastern Mediterranean regions, independent of age, gender, or socioeconomic factors [116]. This is a matter of concern, given that vitamin D deficiency/insufficiency has reached epidemic proportions around the globe. The detrimental effects extend beyond bone health [113] and include a myriad of non-skeletal diseases such as cardiovascular disease [117, 118], diabetes [119], metabolic disorders [120], obesity [121], cancer [122], COPD [123], asthma [124], rhinitis [125], upper respiratory tract infections [126, 127] as well as COVID-19 [128]. Contrary to our expectations, 75% of Turkish adults are D-deficient, followed by 50% of French, 40% of Spaniards, 54% of Greeks, 47% of Israelis, 35% of Italians, and 31% of Cypriots [116, 129]. Systematic reviews undertaken in the European region document that deficiency rates are highest in females, neonates, adolescents, during pregnancy, and in the elderly [116, 130]. Limited sun exposure, clothing, more time spent indoors, population differences in lifestyle and dietary habits, sunscreen use, vitamin D metabolism and absorption, the winter season, and high latitude are factors contributing to vitamin D deficiency [113, 116, 130]. Regarding Northern European countries, hypovitaminosis D is almost negligible, with an impressive 0.7% [131] in Sweden, attributed to fortification policies, vitamin D supplementation, and increased consumption of fatty fish, a rich source of vitamin D [132].
In the human body, the circulating form of vitamin D, 25(OH)D, is widely accepted as the best biomarker of vitamin D status and stored through dietary intake and sunshine exposure [113]. Originally, the Institute of Medicine (IOM) advocated that serum 25(OH)D levels of at least 20 ng/mL (50 nmol/L) were required to maintain bone health and prevent rickets in children [113]. Nonetheless, there is a general global consensus that blood 25(OH)D levels below 25 nmol/L (~10 ng/mL) reflect “severe deficiency” [133]. However, since then, the threshold has been updated by the Endocrine Society’s Practice Guidelines Committee based on meta-analyses of falls and currently endorses that serum 25(OH)D levels of at least 30 ng/mL or 75 nmol/L (defined as vitamin D sufficiency) is necessary to reduce the risk of falls and to provide health benefits for chronic disease, and the preferred range is 40–60 ng/mL (100–150 nmol/L) which is easily obtained by sun exposure, supplementation, and consumption of products fortified with vitamin D [112, 134]. In terms of supplementation, at least 1,000 International Units (IU) per day of vitamin D may be necessary to raise 25(OH)D > 30 ng/mL [112]. The recommended vitamin D intake for infants 0–12 months is 10 μg (400 IU), 1–70 years, including during pregnancy and lactation, 15 μg (600 IU), and > 70 years, 20 μg (800 IU) [113].
Vitamin D is a steroid hormone possessing both skeletal and non-skeletal actions [135]. The role of vitamin D in preventing osteoporosis and fractures in adults and rickets in children is well established [113]. A multitude of observational studies support a relationship between 25(OH)D deficiency and cardiovascular disease [136], asthma [137, 138], COPD [139], bronchiolitis [140], pneumonia [141], cancer [142], and diabetes [143]. The presence of the vitamin D receptor (VDR) and 1α-hydroxylase in a variety of tissues and cells of the human body, including the skin, skeletal muscle, adipose tissue, pancreas, immune cells, lungs, blood vessels, brain, breast, cancer cells, and the placenta, would account for the non-skeletal actions and health benefits [135, 144]. Activation of the VDR by 1,25-dihydroxyvitamin D [1,25(OH)2D, (or calcitriol)] results in a multitude of biological functions, including immune-modulating effects [144].
Paralleling the emergence of the COVID-19 pandemic, the therapeutic role of vitamin D in severe upper respiratory tract infections has sparked growing attention from the scientific community. Liu et al. [145], in a meta-analysis of pooled data from 361,934 participants showed that vitamin D deficiency (< 20 ng/mL) or insufficiency (< 30 ng/mL) was associated with a 43% increased risk of COVID-19. Specifically, COVID-19-positive subjects presented with lower vitamin D levels than COVID-19-negative counterparts.
Hurst et al. [106] conducted a cross-sectional study in 388 hospitalized subjects with severe respiratory tract infections (COVID-19: n = 295; influenza A: n = 93) and 139 survivors of critical illness pre-COVID-19 pandemic. Results showed that vitamin D insufficiency [25(OH)D 25–50 nmol/L] and deficiency (< 25 nmol/L) predominated in all hospitalized subjects, including critical illness survivors, regardless of the type of respiratory infection. Interestingly, in COVID-19 and influenza patients, total 25(OH)D measured early in illness was significantly lower in subjects that received invasive mechanical ventilation [19.6 nmol/L vs. 31.9 nmol/L (P < 0.0001) and 22.9 nmol/L vs. 31.1 nmol/L (P = 0.0009), respectively].
Alipio [146], in a retrospective study of 212 COVID-19 cases (mild n = 49, ordinary n = 59, severe n = 56, critical n = 48) found that vitamin D deficiency (< 20 ng/mL) was highest in severe COVID-19 subjects [n = 77, 25(OH)D = 17.1 ng/mL]. For each increment in 25(OH)D, the odds of having a mild disease were 7.94 times higher than severe disease and 19.61 times higher than critical disease.
Similarly, Maghbooli et al. [147], in a retrospective study of 235 COVID-19 patients (severity: mild-moderate n = 64, severe-critical n = 172) with a mean age of 59 years old, reported a significant inverse association between 25(OH)D concentrations and COVID-19 severity, mortality, inflammatory markers C-reactive protein (CRP), and lymphocyte counts. Compared to patients 25(OH)D-deficient, those having 25(OH)D > 30 ng/mL (D-sufficient) were associated with reductions in CRP levels, a reduced risk of unconsciousness, and a hypoxic state (as defined by arterial blood oxygen saturation < 90%), including a lower mortality rate and increases in lymphocyte counts. However, there were no significant differences in the length of hospital stay and ICU admission between patients who were vitamin D-sufficient vs. D-deficient (< 30 ng/mL).
In line with these studies, meta-analyses support the prophylactic effect of vitamin D supplementation on COVID-19 mortality and disease severity [148–151]. Argano et al. [148], in a recent meta-analysis of five RCTs, demonstrated that daily doses of vitamin D supplementation ranging from 5,000 IU/day to 21,280 IU/day for a time period of 2 weeks to 1 month reduced mortality risk and ICU admission in adult patients (20–75 years) hospitalized for COVID-19. Pal et al. [151], in a meta-analysis of 13 studies (10 observational, 3 RCTs) that pooled data from 2,933 COVID-19 cases, demonstrated that vitamin D supplementation was associated with 59% lower odds of ICU admission and mortality and 73% lower odds of adverse outcomes, specifically in patients with moderate to severe COVID-19 requiring hospitalization. Subgroup analysis revealed that vitamin D supplementation conferred favorable clinical outcomes when it was administered to patients’ post-COVID-19 diagnosis as compared to pre-COVID-19 diagnosis. This is in agreement with two other meta-analyses of randomized and non-randomized studies performed in adult COVID-19 patients reporting a beneficial effect of vitamin D supplementation on ICU admission, COVID-19-related mortality rates, and PCR positivity [149, 150]. Given the promising data on the protective effect of vitamin D supplementation on COVID-19 outcomes, further high-quality studies are needed to confirm these findings.
In contrast, data on the efficacy of vitamin D supplementation in hospitalized pediatric patients infected with COVID-19 are lacking. Only one RCT to date has been conducted by Zurita-Cruz et al. [152]. In this study, 45 patients with SARS-CoV-2 (aged 1 month to 17 years) requiring hospitalization and oxygen therapy were included and randomized to the vitamin D supplementation group (intervention, n = 20) vs. the control group (no supplementation, n = 25). Of note, in both groups, children had serum concentrations < 20 ng/mL. The intervention group was supplemented with 1,000 IU/day (for infants < 12 months) and 2,000 IU/day (children aged 1–17 years). Data analysis revealed that two children in the intervention group progressed to oxygen ventilation compared to nine in the control group; while for mortality outcomes, one patient vs. six, respectively.
However, Xiao et al. [153], in a systematic review of seven RCTs examining vitamin D supplementation for the prevention of childhood acute respiratory infections, reported no associations between vitamin D supplementation and reduced risk of acute respiratory infections in children under 18 years. However, in children diagnosed with asthma, vitamin D supplementation resulted in a 74% reduction in the risk of asthma exacerbations triggered by respiratory infections. Possible sources for the null effect of vitamin D and respiratory infections could be owing to the small number of studies included, diversity in endpoints analyzed, high heterogeneity among studies, and publication bias. The authors concluded that there is a lack of evidence supporting the use of vitamin D supplementation for the prevention of respiratory disease in healthy children but that it may be beneficial in children with asthma.
Contradictory to the data documented by Xiao et al. [153], Jolliffe et al. [154] using data from 46 RCTs undertaken in all ages (n = 49,419 aged 0–95 years) and a variety of geographical settings, reported that vitamin D supplementation (400–1,000 IU daily for 12 months) reduced the risk of acute respiratory infections in children < 16 years as compared to the placebo. This is consistent with an earlier meta-analysis of individual participant data from 25 RCTs conducted by Martineau et al. [155]. Pooled analysis of data from 10,933 subjects (ages ranged from 0–95 years) showed a reduced risk of acute respiratory infection in those receiving daily or weekly vitamin D supplements as compared to bolus. Additionally, vitamin D supplementation reduced the rate of asthma and COPD exacerbations requiring corticosteroid treatment. Furthermore, the protective effect of vitamin D appeared to be stronger in subjects with 25(OH)D concentrations < 25 nmol/L, independent of age. These findings suggest that frequency, dose, and duration of supplementation may be key factors protecting against acute respiratory infections in both children and adults [155].
From another perspective, Asyary and Veruswati [156] demonstrated that sunshine exposure (the main source of vitamin D in humans) increased COVID-19 recovery rates in adult patients. In this context, given that UV intensity is highest in countries in close proximity to the equator, Whittemore [157] conducted a novel correlation analysis of 88 countries to explore the relationship between latitude and COVID-19 fatality rates. Results showed a significant positive correlation between lower COVID-19 mortality rates and a country’s proximity to the equator (r2 = 0.16, P < 0.001). In fact, 16% of the variation in mortality rates can be accounted for by the country’s latitude. A plausible explanation is the correlation between sufficient endogenous vitamin D synthesis derived from ample sunlight exposure in countries located close to the equator and reduced mortality from COVID-19 as compared to populations residing in the northern hemisphere [157]. The potential of sunlight exposure to accelerate recovery and survival from a vicious COVID-19 disease is inspiring.
Overall, the aforementioned studies laid the foundation for the hypothesis that vitamin D might have a critical role in COVID-19 susceptibility, disease progression, and severity. This concept was further strengthened by emerging evidence from recent studies depicting the detrimental effect of vitamin D deficiency on COVID-19 disease progression and outcome [128, 158]. Chiodini et al. [128] in a recent meta-analysis of 1,403,715 cases (ages ranged from 35 years to 86 years), revealed that severe vitamin D deficiency (< 25 nmol/L), deficiency (< 50 nmol/L), and insufficiency (< 75 nmol/L) were associated with 2.63, 2.16, and 2.83 increased odds of ICU admission for COVID-19, respectively; 2.60, 1.84, and 4.15 increased odds of COVID-19-related mortality, respectively; 1.68, 1.83, and 1.49 increased odds of SARS-CoV-2 infection, respectively; and 2.51, 2.38, and 1.82 increased odds of hospitalization for COVID-19, respectively.
Regarding children, a recent meta-analysis of six studies performed by Shah et al. [158] documented that almost 50% of pediatric patients with COVID-19 were vitamin D deficient. Low levels of vitamin D significantly increased the odds of severe disease by 5.5. Notably, children and adolescents with vitamin D deficiency had a higher risk of COVID-19 infection than peers with normal serum vitamin D concentrations and a worse disease outcome (increased inflammation and fever, need for hospitalization, and ICU admission).
In pediatrics, vitamin D deficiency is a strong predictor of asthma and respiratory infections in children and adolescents [159–161]. Epidemiologic data suggest that sub-normal vitamin D levels in pediatric patients are associated with a higher risk of respiratory infections [161], asthma exacerbations [162–164], hospital admissions [162, 163], diminished lung function [164–166], corticosteroid use [166], uncontrolled asthma [137, 165, 167], and increased asthma severity [137, 168]. In fact, serum 25(OH)D concentrations < 75 nmol/L were associated with a 50% increased risk of respiratory tract infections in children and adolescents [161]. The same conclusion was derived from a contemporary systematic review conducted by Raju et al. [169] investigating the relationship between vitamin D deficiency in children and susceptibility to respiratory infections. Pooled analysis of data from 10 studies (eight case-control, one RCT, and one cohort study) revealed that in 70% of studies, children with hypovitaminosis D had a higher susceptibility to developing respiratory infections [169]. An earlier large population-based study of the National Health and Nutrition Examination Survey (NHANES) which included 18,883 pre-adolescent children ≤ 12 years old, found that low vitamin D status was associated with upper respiratory tract infections, especially among patients with asthma [170]. More specifically, 24% of children with 25(OH)D < 10 ng/mL (severely deficient [171]) reported having a recent upper respiratory tract infection, 20% of those with D levels from 10 ng/mL to < 30 ng/mL, 17% with levels ≥ 30 ng/mL (sufficient). Compared to sufficient D levels ≥ 30 ng/mL, vitamin D deficiency (< 10 ng/mL) was associated with 1.36 times higher odds of having a recent respiratory tract infection in children [170]. Interestingly, low vitamin D status was associated with 5.67 times higher odds of respiratory infection in pediatric patients with asthma than in peers with D levels ≥ 30 ng/mL [170].
In contrast, data from systematic reviews report inconsistent results. Jat and Khairwa [172], in a systematic analysis of 23 observational studies that included 13,160 subjects (aged 4–14 years), found that vitamin D deficiency [25(OH)D < 20 ng/mL] was common in children with asthma and prevailed in 28.5% of cases. The odds of vitamin D deficiency were 3.41 times higher among asthmatic children than in non-asthmatic peers. A positive association was noted between low 25(OH)D levels and asthma exacerbations in children and adolescents [172]. However, inconsistencies existed among studies regarding asthma prevalence, exacerbations, lung function, and severity. Most likely due to high heterogeneity among study designs, population differences, asthma definitions, and thresholds for vitamin D deficiency. In another systematic review of 23 observational studies conducted in children up to 18 years (n = 18 studies) and adults (n = 5) by Cassim et al. [173], vitamin D concentrations were found to be associated with a decreased risk of asthma exacerbations. However, corresponding with the review by Jat and Khairwa [172], there was limited evidence supporting the link between vitamin D levels and asthma prevalence, incidence, and severity. The authors concluded that clinical trials were needed to validate the prophylactic potential of vitamin D supplementation for asthma exacerbations.
From a therapeutic point of view, in vitro, the addition of vitamin D enhanced dexamethasone action in peripheral blood mononuclear cells from asthma patients more than dexamethasone alone and inhibited T-cell proliferation [166, 174]. Thus, implying that vitamin D supplementation may improve the therapeutic response to anti-inflammatory therapy in steroid-resistant asthma patients and consequently improve asthma control. Nonetheless, future studies are required to test whether normalization of suboptimal serum vitamin D concentrations in severe asthma patients who are vitamin D deficient via sun exposure and ingestion of vitamin D rich foods or supplements would have a synergistic effect on conventional glucocorticoid therapy, result in better management of asthma symptoms, and reduce the need for medication.
In this context, Kumar et al. [175], in a recent meta-analysis of pooled data from 18 RCTs that included 1,579 children and adolescents, did not find a prophylactic effect of vitamin D supplementation on asthma exacerbations, medication use, asthma severity, emergency visits, or hospitalization in children ≤ 18 years. However, in another meta-analysis of 14 RCTs (adults n = 9, children n = 5) that included 1,421 participants, Wang et al. [176] documented that vitamin D supplementation was associated with a 27% reduction in asthma exacerbations in adults only. Subgroup analysis showed that in adult patients with vitamin D insufficiency < 30 ng/mL, vitamin D supplementation was inversely associated with exacerbations and positively with lung function parameter FEV1%. No significant findings were observed for asthma control, bronchial inflammation biomarker [fractional exhaled nitric oxide (NO)], and IL-10 in both adults and children.
Based on these findings, it appears that the benefits of vitamin D supplementation with respect to asthma exacerbations and lung function are applicable to adult patients but not children. A feasible explanation for no significant outcome in children might be the small number of studies included in the meta-analysis, which would account for limited statistical power [176]. This is in agreement with other meta-analyses of clinical trials conducted in pediatric populations reporting no effect of vitamin D supplementation vs. asthma control, lung function, exacerbations, hospitalization, and acute care visits [175, 177]. Therefore, robust data supporting recommendations for vitamin D supplementation in childhood asthma is limited and inconclusive.
The main source of vitamin D in humans is produced endogenously after UV rays from sunlight activate vitamin D synthesis in the skin (Figure 2) [113]. Food provides only 10% of our needs for vitamin D, and rich sources include fortified dairy products and orange juice, cereals, fish liver, fatty fish (salmon, sardines, mackerel, and trout), red meat, liver, mushrooms, margarine, and egg yolk (Table 2) [113]. Sunshine exposure triggers the dermal synthesis of vitamin D3 (cholecalciferol), the inactive form of the vitamin from 7-dehydrocholesterol, a precursor intermediate from the cholesterol pathway [113]. UVB radiation from sunlight converts 7-dehydrocholesterol to the unstable pre-vitamin 25(OH)D3. After binding to the vitamin D binding protein (VDBP), it is transported to the liver, where it undergoes a series of hydroxylation reactions by vitamin D 25-hydroxylases to produce stable 25(OH)D3 or calcidiol, the storage form of vitamin D. Then, this metabolite returns to circulation via the VDBP and is transported to the kidneys, where it is converted to the biologically major active form 1,25(OH)2D3 or calcitriol, by the enzyme 1α-hydroxylase, and uptake occurs by tubular epithelial cells [113]. In the same way, dietary vitamin D is absorbed in the small intestine bound to chylomicrons where it is transported to the lymphatic system and enters the circulation bound to VDBP [113]. The active form 1,25(OH)2D3 is obtained via sequential hydroxylation steps in the liver and the kidneys.
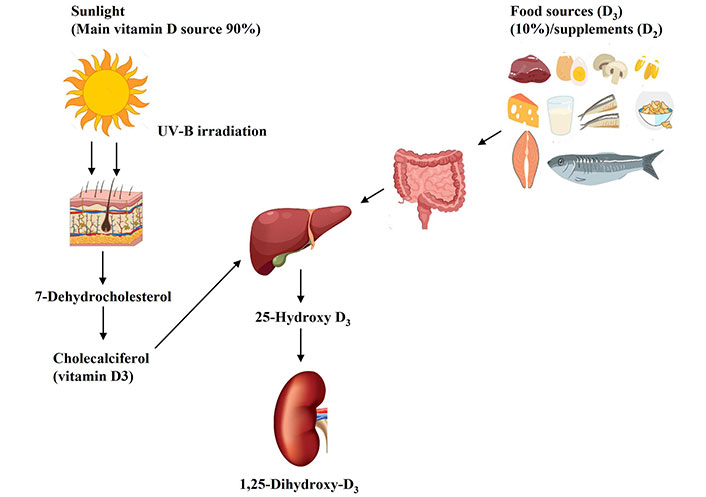
Vitamin D metabolism in the human body. This Figure was created by the authors using vectors from Freepik (https://www.freepik.com)
Vitamin D is synthesized in the skin from 7-dehydrocholesterol, an intermediate in cholesterol synthesis, by the action of UVB rays from sunlight into cholecalciferol (vitamin D3). In addition, vitamin D3 from dietary sources is absorbed by the intestine. Then D3 binds to the VDBP and is transported to the liver, where it is hydroxylated by the enzyme 25-hydroxylase to 25(OH)D3 (calcidiol), the storage and circulating form of vitamin D. In the kidneys, 25(OH)D3 is converted by 1α-hydroxylase to the biologically active form 1,25(OH)2D3 (calcitriol). Then, calcitriol binds to its receptor (VDR) forming a VDR complex. The VDR complex binds to specific gene sequences, regulating gene expression.
Vitamin D-rich foods [180]
| Food item | Vitamin D content | |
|---|---|---|
| IU per serving | Micrograms (μg) per serving | |
| Mushrooms (exposed to UV light, 100 g) | 2,300 | 57.5 |
| Halibut (Greenland, baked or broiled, 75 g) | 1,054 | 26.3 |
| Cod liver oil (10 mL) | 855 | 21.4 |
| Swordfish (baked or broiled, 75 g) | 761 | 19.0 |
| Mackerel (Pacific, salted or smoked, 75 g) | 754 | 19.0 |
| Sockeye salmon (canned, drained, without skin/boneless, 75 g) | 644 | 16.0 |
| Fish roe (baked or broiled, 75 g) | 465 | 11.6 |
| Pink salmon (canned, drained with bones, 75 g) | 435 | 10.9 |
| Mushrooms (maitake, raw, 115 g) | 409 | 10.2 |
| Herring (cisco, smoked, 75 g) | 397 | 10.0 |
| Snapper (baked or broiled, 75 g) | 392 | 9.8 |
| Whitefish (lake, smoked, 75 g) | 384 | 9.6 |
| Mackerel (Pacific, baked or broiled, 75 g) | 343 | 8.6 |
| Oysters (wild, 100 g) | 320 | 8.0 |
| Salmon (Atlantic, wild, baked or broiled, 75 g) | 245 | 6.2 |
| Mackerel (canned, drained 75 g) | 219 | 5.5 |
| Tuna bluefin (baked or broiled, 75 g) | 219 | 5.5 |
| Sea bass (baked or broiled 75 g) | 215 | 5.4 |
| Trout rainbow (wild, baked or broiled, 75 g) | 208 | 5.2 |
| Salmon (Atlantic, farmed, baked or broiled, 75 g) | 206 | 5.1 |
| Herring (Atlantic, pickled, 75 g) | 201 | 5.0 |
| Trout rainbow (farmed, baked or broiled, 75 g) | 192 | 4.8 |
| Herring (Atlantic, baked or broiled, 75 g) | 161 | 4.0 |
| Sardines (Pacific, canned in tomato sauce with bones, 75 g) | 145 | 3.6 |
| Halibut (Atlantic or Pacific, baked or broiled, 75 g) | 144 | 3.6 |
| Whitefish (lake, baked, 75 g) | 135 | 3.4 |
| Sea perch (baked or broiled, 75 g) | 113 | 2.8 |
| Tuna yellowfin (baked or broiled, 75 g) | 106 | 2.6 |
| Tuna albacore (baked or broiled, 75 g) | 106 | 2.6 |
| Sardines (Atlantic, canned in oil, drained with bones, 75 g) | 70 | 1.7 |
| Mullet (baked or broiled, 75 g) | 58 | 1.4 |
| Cod (Atlantic, baked or broiled, 75 g) | 34 | 0.9 |
| Beef liver (braised, 75 g) | 37 | 0.9 |
| Egg yolk (2 yolks, cooked, 34 g) | 64 | 1.6 |
| Egg (2 large whole, cooked or fried, 92 g) | 81 | 2.0 |
| Breakfast cereal (ready to eat, 30 g) | 113 | 2.8 |
| Swiss cheese (Emmental, 100 g) | 20 | 0.5 |
| Monterey cheese (100 g) | 22 | 0.6 |
| Milk (condensed whole or skim, canned, undiluted, 125 mL) | 106 | 2.7 |
| Cow’s milk (whole, reduced, skim, 250 mL) | 103 | 2.6 |
| Orange juice (250 mL) | 100 | 2.5 |
| Milk (goat, enriched, whole, 250 mL) | 100 | 2.5 |
| Soy milk (250 mL) | 86–120 | 2.5–3.0 |
| Margarine (non-hydrogenated canola oil, plant sterols calorie-reduced, 10 g) | 83 | 2.8 |
| Margarine (palm, soybean oils, 10 g) | 72 | 1.8 |
| Buttermilk (125 mL) | 67 | 1.7 |
| Margarine (hydrogenated canola oil, 10 g) | 66 | 1.6 |
| Yogurt (plain, sweetened 185 g) | 60 | 1.5 |
| Margarine (hydrogenated soybean oil, 10 g) | 59 | 1.5 |
1 IU = 0.025 μg or 1 μg = 40 IU. IU: International Units
Notably, the biologically active metabolite of vitamin D, 1,25(OH)2D3 circulates in the blood, and its regulatory function is activated by binding to VDR [178]. The 1,25(OH)2D-VDR complex enables the molecule to act as a transcription factor and regulate transcription in over 900 genes [179]. Animal and human studies demonstrate that 1α-hydroxylase and VDRs are abundant in cells constituting the immune system, such as macrophages, monocytes, respiratory alveolar macrophages, dendritic cells (DCs), T and B cells, natural killer (NK) T cells as well as in lung fibroblasts, airway smooth muscle, and airway epithelial cells [178]. In other words, having high levels of 1α-hydroxylase at these locations enables local hydroxylation of 25(OH)D into the active 1,25(OH)2D3. High concentrations of active vitamin D would increase the expression of vitamin D-regulated genes related to immune function [180]. Therefore, the presence of VDR and 1α-hydroxylase in these specific sites explains the local effects of vitamin D in the respiratory system and its involvement in the immune and inflammatory responses relevant to upper respiratory infections and asthma.
COVID-19 and asthma are characterized by high levels of inflammatory markers, including pro-inflammatory cytokines and chemokines. Vitamin D is a pleiotropic hormone regulating the immune response and plays a critical role in response to viral infections such as SARS-CoV-2 infection as well as in the development of asthma exacerbations [144]. Its therapeutic effects are due to the diversity of extra-skeletal functions, namely anti-inflammatory, anti-viral, anti-oxidant, and immunomodulating effects [144]. The mode of action pertaining to these two conditions can be explained through a variety of plausible mechanisms, both genomic and non-genomic, that include effects on inflammation, immunomodulation, airway smooth muscle, genetic activation, the renin-angiotensin system (RAS), and interacting with corticosteroid therapy.
Vitamin D plays a key role in maintaining pulmonary barrier integrity, in the production of antimicrobial peptides, and in the upregulation of neutrophil activity, enhancing the innate response [144, 178]. It inhibits the cytokine storm by inducing a skewing of the adaptive immune response from a Th1 and Th17 phenotype to an anti-inflammatory Th2 through increased production of Th2 cells and differentiation of naive T cells to Th2 [178]. To add further, vitamin D stimulates the synthesis of T regulatory cells (Tregs), which are important in preserving immune homeostasis and tolerance as well as in limiting Th2-mediated inflammation (such as eosinophils, mucus hypersecretion, and airway hyper-responsiveness) by suppressing T and B cell proliferation along with pro-inflammatory cytokine production and nuclear factor kappa B (NF-kB) expression [181]. Noteworthy, vitamin D deficiency and low VDR concentrations and function have been linked to glucocorticosteroid resistance, the mainstay of asthma treatment [166, 174, 182].
In response to the inflammatory cascade, vitamin D reduces the production of Th1-derived pro-inflammatory cytokines (IL-6, IL-8, IL-9, IL-12), TNF-α, and IFN-γ, as well as inhibiting NF-kB pathways [178]. On the other hand, increased production of Th2-derived anti-inflammatory cytokines (IL-4, IL-5, and IL-10) [178]. Sequelae of events enhancing the anti-inflammatory response in both SARS-CoV-2 infection and asthma exacerbations.
With respect to respiratory infection, vitamin D may reduce COVID-19 risk by activating the transcription of genes coding for the anti-microbial peptides cathelicidin and defensin which possess anti-viral replication properties and promote chemotaxis of macrophages and differentiation of monocytes and epithelial cells, triggering their expression at multiple sites of inflammation, including airways [178]. Cathelicidin enhances the clearance of bacteria from various sites by binding to and neutralizing the lipopolysaccharide (LPS) cell membranes of invading pathogens causing autophagy, phagosome maturation, preventing the biological activity of their endotoxin and inevitably the intracellular destruction of pathogens [178]. Likewise, defensin produced by neutrophils and epithelial cells, possesses the ability to induce the chemotaxis of immune cells, reduce inflammation, and assist in wound repair [178]. Regarding the lungs, a deficiency of VDRs in the respiratory epithelial barrier weakens its defense, prompting severe LPS-induced lung injury [183]. Vitamin D deficiency and low VDBP concentrations are common in patients who develop ARDS [183]. In fact, the odds of ARDS were 3.5-fold higher in patients with 25(OH)D3 < 20 nmol/L as compared to those with sufficient vitamin D levels (≥ 20 nmol/L) [183]. In the same study, human alveolar cells treated with 100 nmol/L of 25(OH)D3 for 24 h activated genes regulating cell proliferation and wound repair and inhibited soluble Fas ligand (sFasL)-mediated cell death [183]. Vitamin D attenuated LPS-induced lung injury and maintained alveolar barrier function [183]. Therefore, vitamin D may be a potential therapeutic strategy for acute lung injury and ARDS.
Airway remodeling, a consequence of chronic airway inflammation, is a feature of asthma pathogenesis [184]. This condition refers to structural changes that occur in the central and peripheral airways that contribute to airway hyperresponsiveness and deficits in lung function in both mild and severe asthma patients [184]. Airway remodeling is characterized by thickening of airway smooth muscle caused by hypertrophy and hyperplasia [185], deposition of extracellular matrix in the subepithelial layer (resulting in subepithelial fibrosis), neovascularization within the airway wall, and airway epithelial alterations that subsequently lead to mucus hypersecretion, edema, and airway narrowing along with bronchial hyper-responsiveness [184]. Even slight changes in airway diameter will disrupt the pulmonary barrier, reduce airflow caliber, and eventually cause irreversible airway obstruction and increased disease severity [184]. The development of airway remodeling has been confirmed in early childhood during the preschool years in pediatric patients suffering from wheeze [186], even before an asthma diagnosis [187]. Therefore, in clinical practice, monitoring and early detection of changes in airway smooth muscle in infants and young children presenting with “wheeze” could signify the onset of childhood asthma development. Intervention in this time period could modify the natural course of childhood asthma. However, to date, asthma treatments have focused on decreasing airway inflammation and exacerbations but not on airway remodeling. Salameh et al. [188] performed a comprehensive systematic review of nine in vitro exploratory studies to investigate the role of vitamin D supplementation on airway remodeling. Qualitative analysis of the data suggests that vitamin D possesses the ability to inhibit airway smooth muscle cell contraction and remodeling, reduce inflammation, and downregulate collagen and fibroblast synthesis in airways [188]. In particular, two studies suggested that vitamin D may have anti-inflammatory and anti-fibrotic effects in human smooth muscle cells [189, 190]. Song et al. [189] reported that 1,25(OH)2D3 attenuated airway inflammation and collagen synthesis via inhibition of NF-kB in human sensitized airway smooth muscle cells. Hence, it appears that vitamin D suppressed the transcription of pro-inflammatory cytokines, IL-6 and IL-8. Jin et al. [190] found that 1,25(OH)2D3 treatment of human lung fibroblasts reduced the expression of collagen type I and the activity of metalloproteinases [such as protein arginine methyltransferase 1 (PRMT1)] involved in collagen synthesis. Collectively, these studies imply that vitamin D may be involved in the tissue remodeling pathway by regulating the processes of bronchial airway muscle activation and extracellular matrix deposition by fibroblasts.
Hence, vitamin D supplementation could be useful in preventing airway remodeling and reducing asthma severity in patients. Future studies are recommended to substantiate this theory.
One more outstanding function is the potential of vitamin D as an adjunct to conventional asthma pharmacotherapy. Glucocorticoids are the first-line anti-inflammatory treatment for asthma exacerbations [30]. Their main mode of action is the inhibition of Th2-derived cytokine synthesis and enhancement of IL-10 production from activated T-cells [174]. IL-10 is a pleiotropic cytokine produced from Th2 cells [191] that plays a critical role in the resolution of airway inflammation and disease control [191]. IL-10 is a potent anti-inflammatory cytokine that exhibits immune suppressive effects by inducing allergen tolerance, inhibiting antigen-presentation by DCs, macrophage activation, and infiltration into the lungs, as well as attenuating pro-inflammatory cytokine expression, resulting in inhibition of the Th1 cell-mediated immune response [191]. Furthermore, IL-10 has the capacity to suppress NO production [192] and downregulate and inactivate neutrophils, eosinophils, and mast cell function [191]. This is important because mast cells release histamine and secrete a broad spectrum of Th2-cytokines and lipid mediators (leukotrienes, prostaglandins, TNF-α) that drive inflammation [193].
Taken together, a series of events that signify the onset of asthma pathogenesis as marked by bronchoconstriction, edema, mucus production, hyper responsiveness, and increased vascular permeability [193], features that lead to airflow obstruction and characteristic symptoms of asthma. Of note, lower levels of IL-10 were observed in the lungs of asthma patients than in healthy controls [194]. In fact, the expression of IL-10 was 10-fold lower in severe asthma patients than in mild asthma counterparts and healthy controls [195]. However, approximately 10% of asthma patients suffer from severe steroid-resistant asthma, which fails to respond to glucocorticoid treatment [196]. In an earlier study, Xystrakis et al. [174] demonstrated that administration of the active form of vitamin D calcitriol 1,25(OH)2D3 in cultures of human steroid-resistant CD4+ T cells restored responsiveness to dexamethasone in steroid-resistant adult asthma patients by up regulating the expression and production of anti-inflammatory IL-10-secreting Tregs, which inhibited proliferation and cytokine production by CD4+ T cells. This study highlighted that vitamin D was able to overcome impaired IL-10 production by CD4+ T cells from steroid-resistant asthma patients. From a clinical point of view, vitamin D was able to reverse steroid-resistance in severe asthma patients to a state of steroid-sensitive. Even though both glucocorticoids and vitamin D3 induced IL-10 synthesis in vivo, when combined, the effect was additive.
Coinciding with this study, Jirapongsananuruk et al. [197] demonstrated that administration of 1,25(OH)2D3 in combination therapy with glucocorticoids modestly improved medication response in steroid-resistant asthma by acting synergistically to decrease pro-inflammatory Th1-driven cytokines (IFN-γ) and increase anti-inflammatory Th2 cytokines (IL-5, IL-13) as compared to glucocorticoid therapy alone.
In this context, given that both steroid therapy and vitamin D are known to elevate endogenous IL-10 levels, one might speculate that vitamin D supplementation alone or as an add-on therapy with glucocorticoids would enhance IL-10 production, lead to inhibition of eosinophilia, production of inflammatory cytokines, suppression of NO production in airways, and indirectly reduce lung inflammation [192]. Therefore, high levels of IL-10 might be indicative of the anti-inflammatory and inflammatory-resolving activity of IL-10 in severe asthma patients. Thus, suggesting that further study of vitamin D therapy and IL-10 as targets for treatment of airway inflammatory diseases, including asthma and COVID-19, is warranted.
Another novel function of vitamin D mediated by its VDR and related steroid-molecule lumisterol (a stereoisomer of ergosterol) is that they participate via non-genomic activity [198]. Lumisterol is formed from the photoisomerization of previtamin D3 after prolonged skin exposure to high doses of UV radiation from sunlight [199]. Qayyum et al. [199] demonstrated that these two molecules were potent inhibitors of SARS-CoV-2 host replication and reduced disease severity. More specifically, vitamin D and lumisterol caused a reduction in viral protease Mpro activity by 10–19% and RNA polymerase RdRP activity by 50–60% [199]. Thus, suggesting that vitamin D3, lumisterol, and 7-dehydrocholesterol analogs might have an active role in combating SARS-CoV-2 infection, attenuating COVID-19 progression and severity in patients. This is important given the high morbidity and mortality rate associated with COVID-19 infection in high-risk patients suffering from respiratory disease. Indeed, this observation is remarkable and indicates the prospect of vitamin D3 and lumisterol derivatives as strong candidates for antiviral research. A plausible mechanism for the anti-viral activity includes inhibition of fusion between viral and cell membranes (and therefore entry into the host cell), suppression of membranous web formation, and inducing anti-viral genes [200]. The ability of vitamin D3 metabolites in inactivating the activity of other human CoVs such as MERS-CoV remains to be elucidated in future experimental studies.
On the subject of natural metabolites as therapeutic agents to treat COVID-19 infection, oxysterols are molecules derived from cholesterol oxidation that contain a hydroxyl, epoxide, or ketone group in the sterol nucleus and/or a hydroxyl group in the side chain [201]. These molecules are implicated in cellular signaling pathways [202]. Oxysterol, 27-hydroxycholesterol is a metabolite produced from the precursor of vitamin D3, 7-dehydrocholesterol, by the action of sterol 27-hydroxylase [cytochrome P450 family 27 subfamily A member 1 (CYP27A1)] [202]. Marcello et al. [203] reported low serum concentrations of 27-hydroxycholesterol in patients detected positive for SARS-CoV-2 infection as compared to healthy controls. Furthermore, a 50% decrease in 27-hydroxycholesterol was observed in severe COVID-19 patients, and serum levels of 7-ketocholesterol and 7β-hydroxycholesterol were elevated, indicating an oxidative imbalance in tissues. In the same study, Marcello et al. [203] observed that 27-hydroxycholesterol exerted inhibitory activity against SARS-CoV-2 replication. This is remarkable. The antiviral activity of 27-hydroxycholesterol against herpes virus, rhinovirus, rotavirus, and papillomavirus infections has been established previously [201]. These findings add the bactericidal activity of 27-hydroxycholesterol against COVID-19 and SARS-CoV-2 diseases to the broad spectrum of human viral pathogens.
Comparably, Zu et al. [200] established in an in vivo murine model that intragastric administration of 100 mg/kg of the oxysterol 25-hydroxycholesterol per day reduced viral RNA load in the lung and trachea of mice. Notably, 25-hydroxycholesterol supplementation at 1,000 mg/kg for a period of 2 weeks did not induce adverse effects, supporting the safety of this molecule.
In summary, vitamin D3 and its derivatives are natural human metabolites. Based on the anti-viral potency outlined in these studies, collectively, they support further clinical development for COVID-19 treatment as a monotherapy or as an add-on adjunct to conventional drug therapy.
From another angle, CoV infection generates a considerable risk of complications and mortality in elderly hypertensive patients with heart or respiratory disease [204]. Having these conditions was associated with almost four-fold higher odds of respiratory complications, hospitalization, and mortality than in healthy subjects [204]. Antihypertensive drugs ACE1 and ARBs (angiotensin II receptor blockers) block the RAS and stimulate increased production of ACE2 [205]. ACE and ACE2 are homologues with different key functions in the RAS system. ACE cleaves angiotensin I to yield angiotensin II, while ACE2 inactivates angiotensin II and is a negative regulator of the system. ACE2 is expressed in the lungs [205]. High ACE2 levels exert a protective effect on the lung parenchyma and are relevant in the defense against respiratory viral infections [206]. This is significant because ACE2 is the receptor to which the S1 domain of the SARS-CoV-2 S protein attaches, gains entry into the host cell, and causes a reduction in intracellular levels of ACE2 [207]. The mechanism by which ACE2 confers a protective effect against infection might be by reducing the production of cytokines associated with the inflammatory response that leads to lung impairment and severe respiratory complications [206, 208]. Animal studies have illustrated that the ACE-inhibitor lisinopril and the ARB losartan increased mRNA expression of cardiac ACE2 by 5-fold and 3-fold, respectively, and that the latter significantly increased cardiac ACE2 activity [209]. Therefore, in order to preserve the protective effect of increased ACE2 in lungs, adherence to anti-hypertensive therapy in COVID-19 patients, is vital [210].
Another less invasive approach to increasing ACE2 expression is via vitamin D. Vitamin D is a critical regulator of the RAS, mediated by ACE2, the entry point into host cells that is used by the SARS-CoV-2 virus [43]. There is ample evidence from experimental models that administration of vitamin D diminishes RAS activity at both the tissue and intracellular levels via upregulation of ACE2 synthesis and activity, and downregulation of ACE (ACE1) activity [211]. More specifically, calcitriol impaired the effect of LPSs (a potent pro-inflammatory molecule) on the expression of ACE and ACE2 in rodent pulmonary microvascular endothelial cells, preventing acute lung injury [211]. It seems that restoration of the ACE-ACE2 balance facilitated by vitamin D is critical to providing a prophylactic effect in the lungs and reducing respiratory events [211]. These studies provide novel targets for the prevention and future treatment of CoV-induced lung injury using vitamin D interventions. The utility of vitamin D and its metabolites in high-risk patients infected with CoV is worth consideration in future clinical trials. The proposed mechanisms by which vitamin D exerts beneficial effects in combating SARS-CoV-2 infection are summarized in Figure 3.
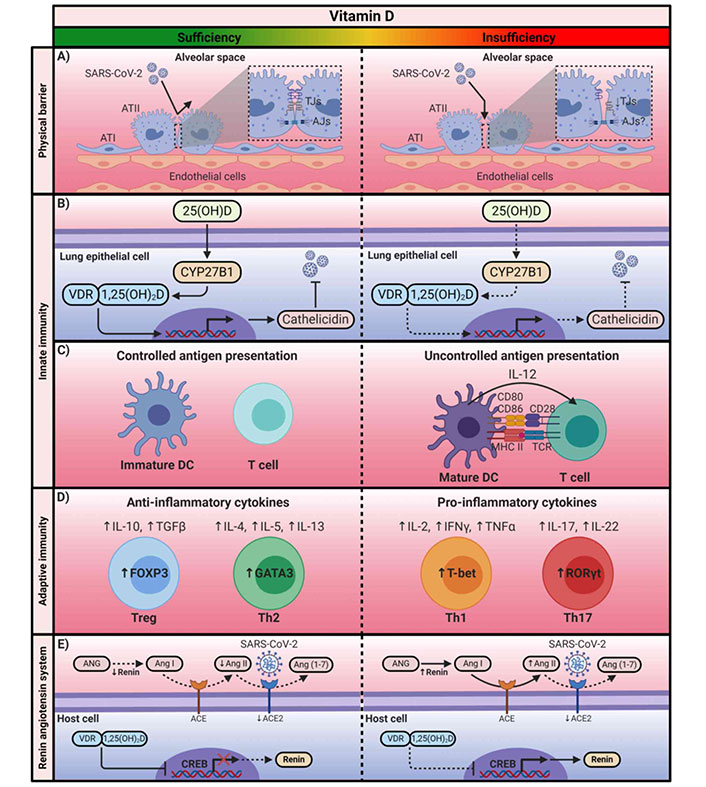
Proposed mechanism of vitamin D antiviral activity in lung epithelial cells exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen. ATI: alveolar type I; TJs: tight junctions; AJs: adherens junctions; 25(OH)D: 25-hydroxy-D; VDR: vitamin D receptor; 1,25(OH)2D: 1,25-dihydroxyvitamin D; CYP27B1: cytochrome P450 family 27 subfamily B member 1; DC: dendritic cell; IL-12: interleukin 12; TCR: T cell receptor; FOXP3: forkhead box P3; GATA3: GATA binding protein 3; Treg: T regulatory cell; Th2: T helper cell type-2; IFNγ: interferon γ; TNFα: tumor necrosis factor α; T-bet: T box transcription factor TBX21; RORγt: retinoic acid-related orphan receptor γt; ANG: angiotensinogen; Ang: angiotensin; ACE: Ang-converting enzyme; CREB: cAMP response element binding protein; MHC II: major histocompatibility complex class II; TGFβ: transforming growth factor β. ↑: upregulation
Note. Reprinted with permission from “Potential immunomodulatory effects of vitamin D in the prevention of severe coronavirus disease 2019: An ally for Latin America (Review)” by Turrubiates-Hernández FJ, Sánchez-Zuno GA, González-Estevez G, Hernández-Bello J, Macedo-Ojeda G, Muñoz-Valle JF. Int J Mol Med. 2021;47:32 (https://www.spandidos-publications.com/ijmm/47/4/32). CC BY-NC-ND.
The scope of this study was to clarify the therapeutic potential of vitamin D on lung health in the context of asthma and in patients infected with COVID-19 (Figure 4). We herein present an extensive review of the literature regarding the pathophysiology of asthma and COVID-19 infection, the step(s) of viral replication inhibited by specific vitamin D3 and its metabolites, and their underlying molecular mechanisms of action. Meta-analyses provide promising data on the utility of vitamin D in COVID-19 prevention and treatment [145, 146, 149, 150], in enhancing recovery [156], and with a synergistic effect on anti-inflammatory therapy [174]. Due to the importance of early diagnosis of this COVID-19 disease, identifying biochemical parameters as possible biomarkers of its severity could prevent, control, and mitigate the spread of this condition, ensure a better outcome and a quick recovery, reduce patient burden, and allow patients to return to routine daily life pre-COVID-19 years.
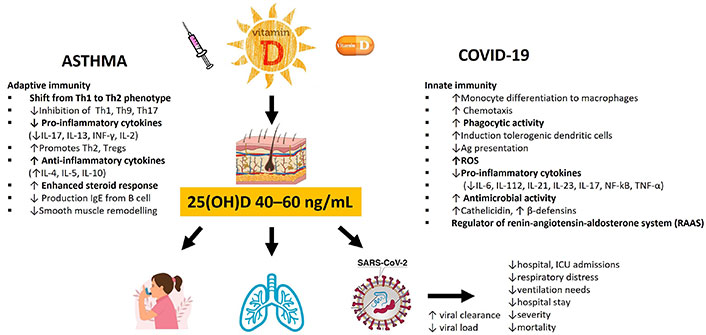
Overview of the therapeutic role of vitamin D in the immune response associated with asthma and coronavirus disease 2019 (COVID-19). Th1: T helper cell type-1; IL-17: interleukin 17; INF-γ: interferon-γ; Tregs: T regulatory cells; ROS: reactive oxygen species; TNF-α: tumor necrosis factor α; ICU: intensive care unit; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; 25(OH)D: 25-hydroxy-D; NF-kB: nuclear factor kappa B. ↑: upregulation; ↓: downregulation
In light of the evidence outlined, suboptimal levels of vitamin D appear to be characteristic of COVID-19 [109, 145, 146, 152, 158, 212] and asthma [163–165, 172]. One might speculate that these conditions favor the attachment and infiltration of pulmonary pathogens such as CoV and the progressive initiation of pro-inflammatory Th1-derived cytokine storm, TNF-α and IFN-γ which constitute the innate immune response to viral infection and lung damage in the late phase of SARS-CoV-2 [43]. Given that the SARS-CoV-2 virus is able to bypass the body’s natural immune response in high-risk individuals, investigating interventions that optimize the host immune system is worth consideration.
Experimental studies implicate that vitamin D is a potent regulator of the innate and adaptive immune systems [144, 178]. Specifically, the VDR is expressed in a diversity of cells and organs, including the lungs [144, 178], and is known to regulate hundreds of genes [179]. In this context, vitamin D has the ability to act synergistically on the immune response to acute systemic inflammation associated with respiratory infection, lung epithelial function [183], muscle function, and metabolism [43, 213]. In terms of SARS-CoV-2 infection, vitamin D insufficiency or deficiency leads to a dysregulated immune response, the development of chronic inflammation, and aggravation of clinical symptoms [214]. Then, appropriately, vitamin D status has been proposed as a potentially modifiable risk factor for respiratory infections and asthma.
Based on the outstanding findings of studies summarized in this review, an intricate relationship exists between viral infections and vitamin D, which includes genomic and non-genomic factors: induction of antiviral status, immunoregulatory functional characteristics, interaction with cellular and viral factors, activation of autophagy and apoptosis, resolution of inflammation, stimulation of gene expression, and epigenetic alterations [178]. Overall, these functions indicate that vitamin D is able to interrupt viral intracellular signaling pathways, causing a modulating effect on viral gene transcription and suppression of replication [178]. It is through these mechanisms that vitamin D reduces the viral load in the airways, diminishes inflammation, and ultimately minimizes disease severity and burden.
Even though the recent data on the possible antiviral effects of vitamin D in COVID-19 infection is compelling, gaps remain in our understanding of the complex mechanisms. Nonetheless, this literature review has opened up new horizons for further investigation addressing the role of vitamin D status and supplementation in the primary prevention of COVID-19, including the prophylactic potential for deterring complications in populations at very high risk of severe COVID-19.
To our knowledge, there is no vitamin D supplementation protocol to guide the dose, frequency, or mode of administration (oral, intravenous, bolus or daily). Numerous studies have reported that daily or weekly supplementation of vitamin D decreases disease severity and mortality risk in both pediatric and adult populations [148, 152, 215, 216]. In children, Zurita-Cruz et al. [152] demonstrated that a daily dose of 1,000–2,000 IU of vitamin D was sufficient to decrease the severity and mortality of vitamin D deficient (< 20 ng/mL) pediatric patients hospitalized for COVID-19. Consistent with this study, daily vitamin D supplementation ranging from 400–1,000 IU per day prevented acute respiratory infections in children and adolescents [154] and up to 1,600 IU per day in adults [217]. With respect to adults, Nogues et al. [216] found that high daily doses ranging from 10,810–21,620 IU decreased ICU admission and mortality in hospitalized patients with vitamin D-deficient < 20 ng/mL (30–80 years) and COVID-19.
Sánchez-Zuno et al. [212] reported that outpatients with vitamin D insufficiency (< 30 ng/mL) presented with more than one symptom of COVID-19 as compared to those D sufficient. Administration of 10,000 IU of vitamin D per day for 14 days adequately raised serum levels above 30 ng/mL and resulted in fewer symptoms than in the non-supplemented group [212]. Likewise, Rastogi et al. [218] documented that high dose supplementation of 60,000 IU of cholecalciferol daily for 7 days in vitamin D deficient (< 20 ng/mL) patients asymptomatic or manifesting mild COVID-19 symptoms resulted in a reduction in viral load as reflected by SARS-CoV-2 RNA negativity. In contrast, Murai et al. [215] assigned a single megadose of 200,000 IU of vitamin D3 to vitamin D-deficient adults (mean: vitamin D 20.9 ng/mL, age 56 years) diagnosed with COVID-19, which yielded no beneficial effect on hospital length of stay, in-hospital mortality, ICU admission, or the need for mechanical ventilation. Overall, inferring that a daily dose schedule is therapeutically superior to large bolus doses and that the protective immunosuppressive effects of vitamin D might be obtained at high doses in hospitalized D-deficient (< 20 ng/mL) COVID-19 patients.
Regarding the critically ill, only one RCT study (VITdAL-ICU) conducted by Amrein et al. [219] reported that vitamin D supplementation lowered hospital mortality in patients with severe vitamin D deficiency ≤ 12 ng/mL. More specifically, 492 critically ill adult patients (> 18 years) with vitamin D levels ≤ 20 ng/mL were randomized to intervention (n = 249) vs. placebo (n = 243). The intervention group received a single high bolus dose of oral vitamin D (540,000 IU) administered nasogastrically followed by 90,000 IU per month for 5 months, and the placebo group 45 mL of oleum arachidic. Results showed that among vitamin D-deficient patients, administration of high-dose vitamin D3 compared with placebo did not reduce hospital length of stay, hospital mortality, or 6-month mortality. However, in the severe D-deficient group, hospital mortality was lower in the intervention group as compared to the placebo (28/98 vs. 47/102, respectively; hazard ratio: 0.56, 95% CI: 0.35–0.90; P interaction = 0.04) but not for 6-month mortality (P interaction = 0.12) [219]. Currently, the results of an ongoing double-blind placebo-controlled RCT (VITDALIZE trial) performed by the same investigators will conclude whether vitamin D replenishment is beneficial for 28-day all-cause mortality in severely vitamin D-deficient critically ill patients [220].
Future studies are desperately needed to corroborate these observations, controlling for possible confounding factors such as baseline serum vitamin D concentrations pre-intervention and hypocalcemia, which has been recently identified as a potential biomarker for COVID-19 disease severity and prognosis in patients with SARS-CoV-2 infection [221–223].
Novel to this review, we reported for the first time that vitamin D and a range of vitamin D3-related metabolites, naturally occurring in humans, such as 7-dehydrocholesterol and L3 hydroxy-derivatives, displayed anti-SARS-CoV-2 activities, and this provides possible targets for direct action.
Despite the promising evidence reported from systematic reviews and meta-analyses [148–151, 153], it suffers from inherent limitations, and these results should be interpreted with caution. The high heterogeneity and limited published literature are factors that are worth consideration [148–151, 153]. Plausible sources of bias include diversity among study designs, the small number of studies included in the analyses along with sample size, method of SARS-CoV-2 diagnosis (self-report vs. laboratory), differences in endpoints assessed, statistical heterogeneity (non-reporting of adjusted estimates, accounting for confounding factors), differences in population age, absence of baseline serum 25(OH)D measurements and vitamin D metabolites supplemented [224]. Regarding the lack of effect of vitamin D supplementation on the prevention of COVID-19, this could be based on differences in the dosing regimens and duration of vitamin D intervention among trials [148–153]. Most studies failed to report the time of vitamin D supplementation after COVID-19 symptom onset. One would expect that early supplementation during the course of the disease and in patients presenting with vitamin D deficiency would provide the most beneficial effect [152, 155]. Another drawback is that studies did not mention the degree of increase in serum levels of vitamin D after supplementation, which is useful in determining the level at which vitamin D exerts its immunomodulatory effects.
To date, there is no universal consensus on the level of 25(OH)2D providing health benefits or optimal respiratory function [133]. Inconsistency in the threshold level renders accurate comparisons of reported observations a difficult task. It has been suggested that different circulating levels of 25(OH)D are required for optimized outcomes based on the type of disease [225–229]. Martineau et al. [226], in a meta-analysis of 25 RCTs (n = 11, 321 participants), established that vitamin D supplementation was associated with 12% lower odds of developing acute respiratory tract infection compared with placebo. In subjects with 25(OH)D levels < 25 nmol/L (10 ng/mL), receiving daily or weekly supplementation resulted in 70% reduced risk as compared to those with higher D levels. On the other hand, a prospective cohort study of healthy adults revealed a two-fold decrease in the risk of developing an acute respiratory tract infection in individuals with serum 25(OH)D concentrations of ≥ 38 ng/mL (95 nmol/L) [230]. In a cross-sectional study of hospital records of 235 COVID-19 patients presenting to the emergency ward, vitamin D sufficiency [25(OH)D ≥ 30 ng/mL] was associated with a significantly lower risk of progressing to severe-critical disease, unconsciousness, and hypoxia, as well as lower concentrations of the inflammatory biomarker CRP and a higher lymphocyte count [147]. A lower mortality rate was observed in those with serum levels ≥ 30 ng/mL and ≥ 40 ng/mL. Furthermore, from the literature reviewed, vitamin D deficiency (< 20 ng/mL) or insufficiency (< 30 ng/mL) was associated with an increased risk of SARS-CoV-2 infection [109, 128, 145, 146, 158, 212], severity [128, 158, 212], ICU admission [128], and consequent mortality [128]. So, based on convincing evidence, serum vitamin D concentrations within the 40–60 ng/mL (100–150 nmol/L) range seem to be necessary to optimize lung functioning [138], confer immunomodulatory [147, 231], and COVID-19 protective effects [147, 231], including overall health benefits [147, 231]. The amount of vitamin D intake required to achieve mean blood concentrations of the desirable 40–60 ng/mL is 4,000–6,000 IU daily [231], which exceeds the recommended dietary allowance (RDA) for both the young and elderly of 600 IU daily [113]. Given the low cost, the safety, and the demonstrated benefit of higher 25(OH)D concentrations, vitamin D supplementation should become a public health priority to ameliorate common costly respiratory ailments like COVID-19 and asthma. Perhaps it is time for the current RDA of vitamin D to be updated.
Another shortcoming to be considered is the fact that 40–70% of patients in the ICU suffer from vitamin D deficiency [232, 233]. This suggests that the phenomenon of hypovitaminosis D observed in critically ill COVID-19 patients requiring ICU hospitalization could be attributed to reverse causation, where deficiency is caused by dysregulation of vitamin D metabolism due to hepatic dysfunction, including down regulation of VDBP synthesis along with fluid resuscitation, renal wasting of vitamin D, decreased renal conversion to calcitriol, and increased tissue conversion of 25(OH)D3 to calcitriol [232]. Furthermore, in critically ill patients exhibiting low 25(OH)D levels, response to vitamin D supplementation [234] is poor, possibly due to the conversion of vitamin D to alternative D-related metabolites [235].
One more source of bias, the possibility of selection bias or confounding in observational studies, and the cross-sectional nature of other studies [149–151] cannot explain the causal relationship between vitamin D levels and COVID-19. More prospective studies are necessary to clarify whether vitamin D intake indeed improves clinical symptoms in all COVID-19 variants. In the interim, sensible sun exposure when weather conditions are amicable is a healthy, non-toxic source of vitamin D, offering overall health benefits for the young and old.
Sunshine is the main source of vitamin D in humans. Increased awareness within the public sector is needed to realize and appreciate the role of vitamin D in disease pathogenesis. The modern lifestyle, hectic work, and school programs have contributed to families spending more time indoors. Together with sunscreen use, and the COVID-19 preventive measures enforced in most countries recommending social distancing, remote work, and schooling. These are factors that have contributed to the widespread prevalence of hypovitaminosis D across all ages.
Asthma and COVID-19 are major worldwide public health problems affecting populations of all ages throughout the world. Implementing strategies to reduce the prevalence and severity of both respiratory disorders should be a priority of the 21st century. COVID-19 is a hypercatabolic disease, and the consequent deterioration of nutritional status would lead to a worse clinical prognosis. Unequivocally, vaccines against SARS-CoV-2 are clearly the cornerstone of controlling COVID-19 infection and minimizing disease morbidity. The anti-viral potency of vitamin D and D-related analogues as strong therapeutic candidates support further clinical development for COVID-19 treatment as a monotherapy or as an add-on to conventional corticosteroid therapy or vaccination. The possibility of vitamin D as a prophylactic agent against COVID-19 infection is indeed attractive and supports its role as a safe adjunct to pharmacotherapy intervention, especially in severe nosocomial patients presenting with vitamin D deficiency. One other pending area of interest worth consideration is the role of vitamin D intervention in boosting the action of corticosteroids in steroid-resistant asthma patients. This is important in minimizing the dosage and duration of treatment, as well as the possibility of drug side effects.
A protocol to guide the nutritional care of COVID-19 patients, both non-critically ill and critically ill, is desperately needed. In clinical practice, evaluation of serum vitamin D concentration in patients could be an effective strategy for distinguishing high-risk from low-risk susceptibility to severe COVID-19 infection, respiratory distress, and poor outcome or death. Hypovitaminosis D is a modifiable environmental factor. In sunny regions across the globe, adopting simple lifestyle changes of 10–15 min per day of sun exposure (equivalent to 1,000 IU) [236] and during the winter months or in Nordic countries where sunshine is limited, food fortification and vitamin D supplementation are effective strategies for maintaining body levels of 25(OH)D within the recommended range of > 30 ng/mL and ideally 40–60 ng/mL [113] could provide prophylaxis against or dampen the severity of acute respiratory diseases such as COVID-19 and asthma.
With respect to patients presenting with severe vitamin D deficiency, different dosing regimens (bolus vs. daily or weekly) and time intervals of vitamin D supplementation may have different effects on clinical outcomes [148, 152, 155, 215, 216]. A daily dose would lead to the stable availability of a wide range of vitamin D metabolites and analogs [233]. Furthermore, the type of D metabolites chosen for supplementation (cholecalciferol, calcifediol, and calcitriol) will determine absorption and metabolism, stability, efficacy, half-life, and risk of toxicity [224]. To date, the optimal level for vitamin D supplementation is a matter of debate, and the dose and duration of supplementation required to confer respiratory benefits in COVID-19 patients, are yet to be established. Recommendations vary across countries, ranging from 400 to 10,000 IU daily depending on the medical authority, IOM, or Endocrine Society guidelines [113, 237]. Vitamin D supplementation offers a cheap natural alternative to pharmacotherapy and could be useful for subjects objecting to vaccination, especially children. Considering the significant impact of COVID-19 on long-term health and healthcare expenditure, vitamin D supplementation might effectively reduce disease spread, mortality rate, oxygen support, medication needs, and hospital stay in high-risk patients with co-morbidities, thereby reducing the financial burden of this condition. From this standpoint, high-quality clinical trials with a large sample size in both nosocomial and non-hospitalized patients are urgently needed to substantiate the promising findings and elucidate the best vitamin D metabolite, quantity, dose, and method of supplementation required to reduce the risk of infection and symptom severity in high-risk populations of all ages, including the ICU. Meanwhile, clinicians’ attention should be drawn to vitamin D insufficiency or deficiency in COVID-19 patients as a marker of disease severity and worse prognosis.
In terms of halting the worldwide spread of COVID-19, prevention is the key. Hypovitaminosis D (< 30 ng/mL) may be a distinctive biochemical feature of COVID-19, potentially impacting disease clinical severity and the worse prognosis, representing a novel possible treatment target worth consideration in the clinical setting. Serum concentrations of ≥ 40 ng/mL may be required for vitamin D’s prophylactic immunomodulatory effect. This review supports the guidelines recommended by the Endocrine Society, to achieve a serum 25(OH)D concentration of at least 30 ng/mL, and ideally, 40–60 ng/mL to provide immune-beneficial effects in children and adults, potentially reduce the risk of contracting COVID-19 infection, and prevent progression to severe and fatal COVID-19. In a public health effort to prevent vitamin D deficiency, policymaking bodies should consider the cost-effectiveness of making recommendations for routine testing and supplementation for vitamin D-deficient individuals in the near future.
1,25(OH)2D: 1,25-dihydroxyvitamin D
25(OH)D: 25-hydroxy-D
ACE2: angiotensin-converting enzyme 2
ARDS: acute respiratory distress syndrome
COPD: chronic obstructive pulmonary disease
CoV: coronavirus
COVID-19: coronavirus disease 2019
CRP: C-reactive protein
ECMO: extracorporeal membrane oxygenation
FDA: Food and Drug Administration
ICU: intensive care unit
IFN: interferon
IL: interleukin
IU: International Units
LPS: lipopolysaccharide
NF-kB: nuclear factor kappa B
NIH: National Institutes of Health
NO: nitric oxide
RAS: renin-angiotensin system
RCT: randomized controlled trial
S: spike
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
Th1: T helper cell type-1
TNF-α: tumor necrosis factor-alpha
US: United States
VDBP: vitamin D binding protein
VDR: vitamin D receptor
WHO: World Health Organization
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100944_sup_1.pdf.
MMP: Conceptualization, Investigation, Writing—original draft. CK: Writing—review & editing, Supervision. Both authors have read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3079
Download: 24
Times Cited: 0
Riyad Allehebi, Hamdan AL-Jahdali
Yasmeen Othman ... Asmaa Ali
Yuto Hamada ... Peter Gerard Gibson
Andrea Giovanni Ledda ... Stefano Del Giacco
Teresa Garriga-Baraut ... Margarita Tomás-Pérez
