Affiliation:
1Centre of Excellence in Treatable Traits, College of Health, Medicine and Wellbeing, The University of Newcastle, New Lambton Heights, NSW 2305, Australia
2Clinical Research Centre for Allergy and Rheumatology, National Hospital Organization Sagamihara National Hospital, Sagamihara 252-0392, Japan
ORCID: https://orcid.org/0000-0002-3234-652X
Affiliation:
1Centre of Excellence in Treatable Traits, College of Health, Medicine and Wellbeing, The University of Newcastle, New Lambton Heights, NSW 2305, Australia
3School of Nursing and Midwifery, College of Health, Medicine and Wellbeing, The University of Newcastle, Newcastle, NSW 2305, Australia
ORCID: https://orcid.org/0000-0002-9566-5181
Affiliation:
1Centre of Excellence in Treatable Traits, College of Health, Medicine and Wellbeing, The University of Newcastle, New Lambton Heights, NSW 2305, Australia
4Department of Respiratory and Sleep Medicine, John Hunter Hospital, New Lambton Heights, NSW 2305, Australia
Email: Peter.Gibson@newcastle.edu.au
ORCID: https://orcid.org/0000-0001-5865-489X
Explor Asthma Allergy. 2024;2:287–300 DOI: https://doi.org/10.37349/eaa.2024.00045
Received: October 13, 2023 Accepted: March 28, 2024 Published: June 27, 2024
Academic Editor: Karl-Christian Bergmann, Charité – Universitätsmedizin Berlin, Freie Universität Berlin, Germany
The article belongs to the special issue The Global Picture of Asthma after Guideline Changes and the COVID Pandemics
As a novel respiratory viral infection, coronavirus disease 2019 (COVID-19) has influenced asthma in unpredictable ways. In the post-COVID era, there is a need to review asthma care and the new challenges and opportunities that are presented. Long COVID is a new and complex syndrome that has arisen. Treatable traits (TTs) have already been developed to address complex asthma and can be adapted to manage long COVID. Consumers are seeking more information on and answers to what to expect with a dual diagnosis of asthma and COVID-19. People with asthma identify a strong need for research into COVID and asthma. Completion of a national survey (n = 593) resulted in a list of research themes. From these, participants prioritized 10 asthma research themes. Among the top 10 asthma research priorities, the theme of COVID and asthma was ranked as the second priority in the overall rank list. Addressing these issues has the potential to improve global asthma health.
The coronavirus disease 2019 (COVID-19) pandemic has had a major impact on the lives of people across the world [1, 2]. In addition to the large disease burden from COVID-19, there have been major changes in healthcare delivery [3, 4]. With case numbers reducing, and effective treatment and vaccination available, it is timely to reflect on health care in the post-COVID era. Models of care have changed, with greater attention to telehealth and research priorities have also changed [2]. In addition, new problems have emerged, such as long COVID.
Asthma is an important condition in the post-COVID era. It is a common chronic inflammatory airway disease that occurs in all of the world’s populations, but where there is great geographic variation in prevalence [5]. Since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a respiratory virus, and respiratory viruses are a major cause of asthma attacks, it was anticipated that viral-induced asthma attacks would increase during the pandemic. Unexpectedly, the opposite occurred [6, 7], and this provided insight into the mechanisms of COVID-19. SARS-CoV-2 enters lung cells via angiotensin-converting enzyme 2 (ACE2) receptor. Several studies suggest that interleukin-13, an important cytokine expressed during the type 2 (T2) inflammation that characterizes asthma, reduces ACE2 receptor expression [8–10], and inhaled corticosteroid, a fundamental asthma therapy, also reduces ACE2 receptor expression [11]. These observations provide a plausible reason why asthma did not emerge as a significant risk factor for the development of COVID-19. In addition, public health measures were implemented to reduce the community spread of SARS-CoV-2, and these measures likely reduced the transmission of other respiratory viruses, reducing viral-induced asthma attacks [12]. Together these factors meant that asthma morbidity was not increased during the pandemic [13]. In fact, studies indicate that the seasonal rhythms in asthma and chronic obstructive pulmonary disease (COPD) attacks were suppressed during the lockdown. However, now we are in a post-COVID era and attacks have now returned after the lockdown. In some cases, the rebound was greater than before the pandemic, especially in younger age groups [14].
In this review, we address some of the key issues that have arisen in asthma care in the post-COVID era, with a particular focus on treatable traits (TTs) as a model of care and their relevance in the post-COVID era [15]. It is important to evaluate this model of care in a post-pandemic era. New syndromes have emerged after the COVID pandemic. Collectively labelled “long COVID”, these presentations affect up to 20% of people infected by SARS-CoV-2 [16, 17], they may be more prevalent in people with asthma, and they are both complex and heterogeneous in their manifestations. This presents a challenge for clinical care. TTs have been designed to manage the complexity and heterogeneity of asthma and have the potential to be applied to long COVID.
A TT is a recognizable phenotypic or endotypic characteristic that can be assessed and successfully targeted by therapy to improve a clinical outcome in a patient with airway disease [18]. TTs must share three characteristics: (1) clinical relevance, which means that they are linked to a relevant clinical outcome such as exacerbations, quality of life (QOL), or lung function decline; (2) detectable either “phenotypically” (i.e., clinically; e.g., presence of emphysema on computed tomography) and/or based on a deep understanding of critical causal pathways (“endotypes”) through validated “trait identification markers (TIMs)” (e.g., circulating eosinophils, see below); and, (3) treatable, meaning that effective treatment is available and accessible, and when implemented leads to a clinically important outcome for patients with airway diseases [5]. Eosinophilic airway inflammation is well established as a TT [19], that is underpinned by the endotype of T2 inflammatory response mediated by interleukin-5 [20, 21], indicating that this trait is clinically relevant. Eosinophilic inflammation can be detected via blood eosinophil count (TIM) and can be treated via inhaled corticosteroids, oral corticosteroids, or T2-directed monoclonal antibody therapy [22]. The endotype of eosinophilic airway inflammation meets each of the three criteria named above for designation as a TT because it is relevant, detectable, and treatable.
The TTs strategy proposes to investigate in each individual patient with asthma the number and type of TTs present and to treat each of them according to guideline recommendations [18]. This strategy can be applied to any patient with airway disease, and to asthma of all severities and subtypes. In clinical practice, when using a TT approach the traditional diagnostic label of asthma is viewed as a starting point for management and diagnostic processes, and not its end. Therefore, after establishing that a patient has asthma, it is then necessary to continue the assessment process and understand the clinical and biological complexity of each patient using a TT strategy. This then permits a compound diagnostic label that represents the key traits present in the individual’s asthma. For example, after a TT assessment, a patient may receive a compound label of “eosinophilic asthma with nasal polyposis, high body mass index (BMI), and poor asthma self-management skills”. Each of these traits is clinically important and amenable to treatment. A TTs-based treatment plan for such a patient might recommend: inhaled corticosteroid and long-acting beta-agonist for the airway traits of T2 inflammation and variable airflow obstruction; nasal corticosteroid, saline nasal lavage and surgical assessment for nasal polyposis; weight reduction methods for high BMI; and asthma self-management education for poor self-management skills. Examples are shown on several referenced websites [23–25].
The TT strategy is supported by several different types of evidence. Each TIM and treatment recommendation is supported by a specific level of evidence, usually level 1 or 2, as detailed in Duszyk et al. [26]. Systematic reviews have shown that the TT strategy is effective in severe asthma and COPD [27, 28]. In a randomized controlled trial (RCT), McDonald et al. [29] showed that TTs gave superior clinical outcomes for patients with severe asthma. These reports demonstrate the considerable evidence supporting a TT strategy for asthma.
At its core, TT is a model of care. This means that it is a way to manage patients with complex and heterogeneous airway diseases including asthma. The model is modified based on the clinical circumstances, patient preferences, available resources, and priorities. For example, the model can be modified to prioritize 6 or 7 traits of high impact that respond well to treatment, so-called “supertraits” [30, 31]. Similarly, in primary care or low-middle-income countries, modifications to the strategy demonstrate how to adjust the TT strategy to the situation [32]. New clinical challenges have manifested because of the COVID pandemic, and it is appropriate to consider how the TT strategy can be adapted in the post-COVID era.
COVID-19, caused by the SARS-CoV-2, has had a significant global impact on morbidity and mortality since its emergence in December 2019 [1, 33]. Long COVID, alternatively referred to as “ongoing symptomatic COVID-19,” “post-COVID syndrome,” “post-COVID-19 condition,” “long-haul COVID-19,” or “post-COVID conditions,” is characterized by persistent symptoms that develop during or after the acute phase of SARS-CoV-2-induced COVID-19 and cannot be explained by an alternative diagnosis [34–36]. In this paper, we use the term long COVID, which is often used by people experiencing persistent symptoms after acute COVID-19 [37]. The estimated prevalence of long COVID ranges up to 20% [16, 17], depending on the method used to assess the outcomes and the time point since acute infection [38–41]. The symptoms of long COVID manifest across multiple organs, encompassing fatigue, dyspnea, cough, myalgia, joint pain, headache, sleep disorders, depression, and cognitive alterations [42–46] (Figure 1). In particular, dyspnea, chest pain, and cough have emerged as significant respiratory symptoms in patients with long COVID [42, 43, 47, 48]. Patients who experience long COVID symptoms, including fatigue, dyspnea, and cough, have been found to report a significant reduction in their QOL [42, 47, 49].
The pathogenesis underlying the chronic symptoms of long COVID is not yet fully understood, although several hypotheses have been suggested, including persistent reservoirs of SARS-CoV-2 in tissues, immune dysregulation, gut microbiota dysbiosis, and autoimmunity, as potential mechanism for its development [50, 53]. Currently, there are no established therapies that are specific for long COVID; however, it has been observed that vaccines against COVID-19 can contribute to reducing its prevalence [54–59]. A recommended approach to the management of long COVID involves a multidisciplinary approach, including rehabilitation, as the complex impairments in patients with long COVID encompass various domains such as physical, cognitive, and mental health [60–63]. To untangle the complexities of this intricate disease, a multidisciplinary approach utilizing TTs can be employed.
Risk factors associated with the development of long COVID include increasing age, female gender, the severity of the acute COVID-19 infection, and the presence of comorbidities such as obesity, diabetes, and depression [44, 64]. Furthermore, a recent meta-analysis has shown that individuals with asthma can have an increased risk of long COVID [64], while the association of a specific asthma phenotype with long COVID remains unknown. People with asthma had a higher risk of experiencing respiratory symptoms, characterized by cough, shortness of breath, bronchospasm, and wheezing, during the post-acute period of COVID-19 compared to those without asthma [65]. People with asthma experiencing long COVID also reported increased dyspnea compared to those without long COVID [66]. Therefore, they may have a higher risk of developing long COVID due to persistent respiratory symptoms, including cough and difficulty breathing. A careful clinical assessment is required to diagnose long COVID as there can be symptom overlap between asthma and long COVID, leading to the possibility of symptom misattribution. The TT approach is able to address these issues.
Cough is one of the most common persistent symptoms in acute COVID-19 and long COVID [42–44, 46, 67]. The prevalence of cough symptoms can decrease after acute COVID-19 [46, 67]. Nevertheless, the prevalence of post-COVID cough ranges up to 25%, depending on the study selection criteria [42, 43, 46, 68–70]. Notably, the characteristics of post-COVID persistent cough could resemble those of chronic cough [71]. Neuroinflammation leading to a condition of laryngeal and cough hypersensitivity has been proposed as a potential mechanism of persistent cough in long COVID, although the exact mechanism of post-COVID cough remains unknown [70]. The treatment for this condition is currently based on available guidelines and treatments for chronic cough [70, 71].
In contrast, more cases of new-onset asthma may exist among patients experiencing post-COVID cough. The recent study conducted by Lee et al. [72] revealed a significant association between COVID-19 infection and the development of new-onset asthma. Notably, among the participants in the study, those with new-onset asthma suffered from persistent coughs following acute COVID-19 [72]. Furthermore, Hastie et al. [73, 74] reported an increased prevalence of late-onset dry and productive cough after 6 months of acute SARS-CoV-2 infection, although the association between late-onset cough and new-onset asthma remains unknown. The implications of post-COVID cough on asthma remain unclear, warranting further research in the post-COVID era.
Dysfunctional breathing (DB) is a term used to describe a group of conditions characterized by a disrupted biomechanical breathing pattern, leading to dyspnea and non-respiratory symptoms that cannot be fully explained by disease pathophysiology [75–77]. Other terms, such as DB disorder, breathing pattern disorder, and behavioural breathing, have also been utilized to describe DB [76, 77]. Common symptoms of DB include respiratory symptoms, such as breathlessness, chest tightness, and chest pain, while individuals may also experience general symptoms, such as dizziness, paraesthesia, headache, and light headedness [76–78]. The clinical diagnosis of DB is often based on the Nijmegen questionnaire, cardiopulmonary exercise testing, and the Breathing Pattern Assessment Tool; however, there are currently no gold standard criteria to diagnose DB [75, 79–81].
DB is recognized as a common comorbidity of asthma with a prevalence of up to 30% in people with asthma. Its presence can have a detrimental impact on asthma outcomes, including asthma control, exacerbations, and QOL [57, 63–65]. In contrast, DB has been associated with dyspnea in patients experiencing long COVID [61, 66, 67]. One of the mechanisms contributing to dyspnea and exertional breathlessness in long COVID may involve DB, including hyperventilation; however, the pathophysiology of DB in long COVID is not fully understood [68, 69].
The management of DB in asthma and long COVID includes pulmonary rehabilitation programs, such as breath retraining and exercise programs [57, 70]. Telerehabilitation may also contribute to improving dyspnea and QOL in long COVID [71–73], although further studies will be needed to establish the effectiveness of rehabilitation interventions conclusively.
Long COVID is a new and complex chronic health condition that has been recognized in patients who recover from COVID-19. Long COVID involves multiple symptoms that cross different organ systems and fluctuate over time. Not all patients exhibit the same symptom profile or organ system involvement, demonstrating the heterogeneity of long COVID. In addition, multiple symptom profiles can be present at the same time in any one patient, indicating the heterogeneity of long COVID. Because of this complexity and heterogeneity, a one-size-fits-all approach is neither appropriate nor relevant for the assessment and treatment of long COVID. This presents a problem of how to deal with long COVID. The TT approach has the ability to address both complexity and heterogeneity in patient problems and appears to be well-suited as a model of care for long COVID management. Lewthwaite et al. [82] assessed the range of different domains of long COVID and proposed a TTs model for assessing and managing TTs in long COVID (Figure 2). The varied long COVID symptoms could be grouped into eight long COVID TT clusters: neurological, chest, psychological, pain, fatigue, sleep impairment, functional impairment, and others. A systematic review of RCTs identified that for long COVID management, current evidence supports exercise training or respiratory muscle training for long COVID TTs in the chest and functional impairment trait clusters (Figure 2). For an intervention targeting chest TTs, Romanet et al. [83] conducted an RCT involving 60 people with COVID-19-related acute respiratory distress syndrome. They found that a 3-month course of exercise training rehabilitation led to a significant reduction in dyspnea, although standard physiotherapy did not reduce dyspnea. Furthermore, Del Corral et al. [84] and McNarry et al. [85] found respiratory muscle training increased respiratory muscle strength and dyspnea in RCTs in people with long COVID.
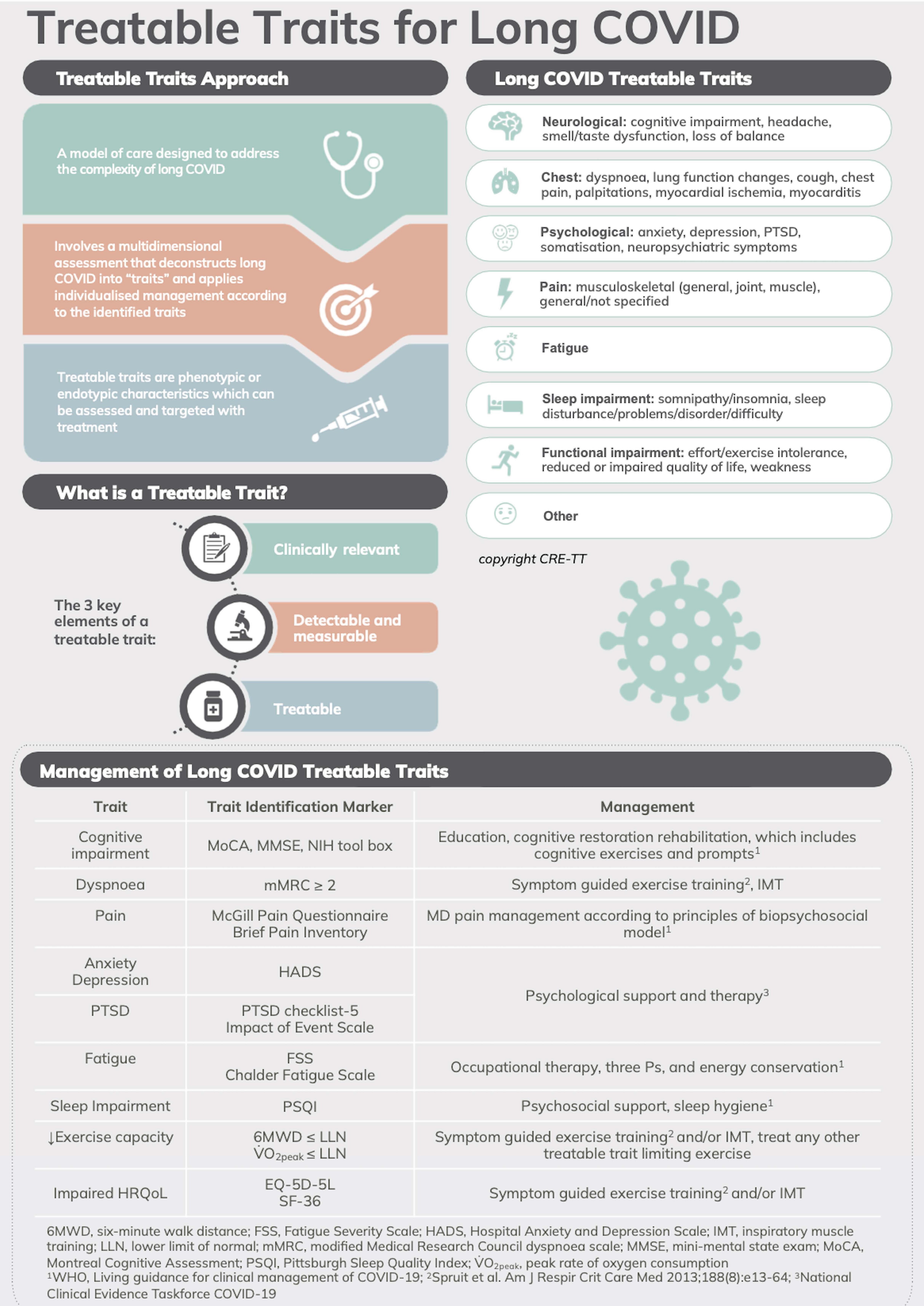
TTs model of care for long COVID. PTSD: post-traumatic stress disorder; NIH: National Institutes of Health; HRQoL: health-related quality of life; MD: multidisciplinary. Content has been reproduced with permission from the Centre of Excellence in Treatable Traits, originally developed as part of the Centre of Excellence in Treatable Traits (https://treatabletraits.org.au)
What are the research priorities of patients with asthma around COVID? With the emergence of an increased disease burden, and new clinical syndromes, there is a need to provide direction for research in asthma in a post-COVID era. A research priority setting exercise was conducted to identify the research priorities for asthma for the end-users of research: consumers and health care professionals. This work identified research into COVID and asthma as a high priority for consumers. Using the modified James Lind Alliance (JLA) methodology, Majellano et al. [86] engaged people with asthma (n = 491), their carers (n = 50), and other stakeholders (n = 52) to identify the top 10 asthma research priorities. The themes of COVID-19 and asthma, asthma care, and self-management were ranked second and third, respectively in the overall top 10 list of asthma research priorities (Figure 3). Under this theme, consumers wanted more knowledge about the long- and short-term impacts of COVID-19 on people with asthma. Consumers needed answers to whether COVID-19 can cause asthma and the impact of masks on people with asthma. Consumers also wanted more understanding of the unique experience of people with asthma who have COVID. Using a frequency count approach, three specific research questions were identified in the theme COVID-19 and asthma including: (1) What are the short-term and long-term health impacts of COVID-19 in people with asthma? (2) Why do people with asthma experience breathing difficulty while wearing a mask, and how can it be managed? (3) What are the symptoms experienced by people with asthma who develop COVID-19 infection? Does COVID-19 trigger asthma symptoms? Moreover, consumers emphasized the importance of timely access to asthma care irrespective of geographical factors.
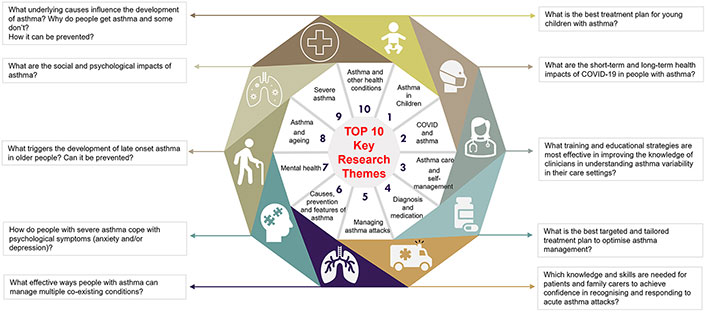
COVID and asthma as a research priority: the top key research themes as prioritized in the priority setting workshops with the highest frequency research questions within themes as revealed in the content analysis
Note. Reprinted from “Identifying the asthma research priorities of people with asthma, their carers and other stakeholders” by Majellano EC, Bell RL, Flynn AW, Mckenzie A, Sivamalai S, Goldman M, et al. Respirology. 2023;28:636–48 (https://onlinelibrary.wiley.com/doi/10.1111/resp.14492). CC BY.
Other researchers have also evaluated the research priorities for asthma and COVID. Two studies sought to identify research priorities regarding the long-term sequelae of COVID-19. Adeloye et al. [87] adopted the Child Health and Nutrition Research Initiative (CHNRI) priority-setting framework to identify research priorities for long COVID in the context of airways disease. Experts’ scores (n = 59) and input from patients (n = 61) were utilized to generate a list of the top 20 research ideas. The four highest-ranked research priorities centred on comparing the health outcomes of COVID-19 survivors with and without pre-existing conditions. Overall, their priority research exercise revealed an important level of concordance between experts and patients in the 20 highest-ranking research topics when the criteria were weighted based on patient input. Using a modified JLA framework, Houchen-Wolloff et al. [88] sought to determine the top 10 research priority questions for COVID-19 hospitalization survivors. Their final list of top 10 priority questions includes understanding the fundamental processes of protracted COVID-19, identifying diagnostic and prognostic tools, and pharmacological and non-pharmacological therapies in the treatment of symptoms.
These papers identify that COVID and asthma-related research is a high priority in the post-pandemic era, and provide clear research questions to be addressed.
Access to care for people with COVID and asthma is crucial for managing their health effectively. Pursuing health equity is a challenge as access to healthcare is dependent on socio-demographic factors or health characteristics, resulting in disparities in outcomes for population subgroups [89]. However, improved care can be achieved using patient perspectives on healthcare access. In a cross-sectional patient-oriented study conducted by Collins-Fairclough et al. [90], they engaged people with asthma and COPD to assess the impact of the COVID-19 pandemic (second wave) on their access to asthma and COPD healthcare. The authors found that patients experienced decreased access to care amid the second wave of the COVID-19 pandemic, which was linked with lower self-assessed financial capability. Furthermore, specialty care services such as specialist appointments, exercise programs, and educational sessions, which are vital for self-management support, were particularly impacted. The need to re-evaluate this disparity post-pandemic is important to restoring routine healthcare services for asthma and COPD. Importantly, understanding the perspectives of people living with asthma and COPD on what matters most to them is necessary and fundamental to achieving person-centred care. Addressing barriers to access to care is essential for optimizing health outcomes for individuals with both COVID-19 and asthma.
The post-COVID era presents new challenges and provides new solutions for asthma health care. Consumers with asthma identify COVID-19 and its consequences as an important research area. People with asthma can have a higher risk of developing long COVID. TTs have emerged as a useful model of care that has been adapted to long COVID, as well as chronic airway diseases, although TTs for long COVID have not been tested. It is a personalised medicine strategy based on the treatment of each of identified traits. Considering the needs and values of individual patients, as well as their biological and pathophysiological characteristics, is necessary to deliver personalized care using TT approaches [91]. Addressing these issues will help the welfare of people with asthma globally.
ACE2: angiotensin-converting enzyme 2
COPD: chronic obstructive pulmonary disease
COVID-19: coronavirus disease 2019
DB: dysfunctional breathing
QOL: quality of life
RCT: randomized controlled trial
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
T2: type 2 (inflammation)
TIMs: trait identification markers
TTs: treatable traits
The authors thank Dr Janet Bristow and Dr Vanessa Clark for assistance with manuscript preparation and the Japanese Respiratory Society for their support of Yuto Hamada through their Fellowship Grants.
YH, ECM, and PGG: Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
Peter Gerard Gibson reports personal fees from AstraZeneca, GlaxoSmithKline, and Novartis, personal fees from Chiesi, and Sanofi, and grants from AstraZeneca, and GlaxoSmithKline, outside the submitted work. Yuto Hamada reports personal fees from AstraZeneca and Kyorin, outside the submitted work. Eleanor C. Majellano reports no conflicts.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Peter Gerard Gibson is supported by the current NHMRC Practitioner Fellowship [APP1155810]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 8630
Download: 41
Times Cited: 0
Riyad Allehebi, Hamdan AL-Jahdali
Yasmeen Othman ... Asmaa Ali
Maria Michelle Papamichael, Charis Katsardis
Andrea Giovanni Ledda ... Stefano Del Giacco
Teresa Garriga-Baraut ... Margarita Tomás-Pérez
