Affiliation:
1Department of Otorhinolaryngology, Düsseldorf University Hospital (UKD), 40225 Düsseldorf, Germany
Email: martin.wagenmann@hhu.de
Affiliation:
2Department of Otorhinolaryngology – Head and Neck Surgery, University Hospital of Münster, 48149 Münster, Germany
3International Airway Research Center, First Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510080, Guangdong, China
Affiliation:
4Department of Otorhinolaryngology, King’s College London, SE1 9SP London, UK
ORCID: https://orcid.org/0000-0003-3993-1569
Affiliation:
6Global Health Economics and Value Assessment, Sanofi, 94250 Gentilly, France
ORCID: https://orcid.org/0000-0003-4733-5855
Affiliation:
7Medical Affairs, Regeneron Pharmaceuticals Inc., Tarrytown, NY 10591, USA
ORCID: https://orcid.org/0000-0001-9208-5280
Affiliation:
7Medical Affairs, Regeneron Pharmaceuticals Inc., Tarrytown, NY 10591, USA
ORCID: https://orcid.org/0009-0000-9136-4253
Affiliation:
5Global Medical Affairs, Sanofi, Bridgewater, NJ 08807, USA
ORCID: https://orcid.org/0000-0002-5372-8762
Explor Asthma Allergy. 2024;2:363–372 DOI: https://doi.org/10.37349/eaa.2024.00051
Received: December 18, 2023 Accepted: June 13, 2024 Published: July 30, 2024
Academic Editor: Giuseppe Guida, University of Torino, San Luigi Gonzaga University Hospital, Italy
The article belongs to the special issue Update on Chronic RhinoSinusitis
Aim: The impact of complete bilateral nasal obstruction [nasal polyp score (NPS) = 8/8] on treatment outcomes in chronic rhinosinusitis with nasal polyps (CRSwNP) is unclear. This post hoc analysis assessed disease burden and dupilumab efficacy in patients with severe CRSwNP and baseline NPS = 8 in SINUS-24/-52 (NCT02912468/NCT02898454).
Methods: Efficacy outcomes assessed: NPS, peak nasal inspiratory flow (PNIF), Lund-Mackay computed tomography (LMK-CT), nasal congestion/obstruction (NC), loss of smell (LoS), rhinosinusitis visual analog scale (rhino-VAS), University of Pennsylvania Smell Identification Test (UPSIT), 22-item Sinonasal Outcome Test (SNOT-22) in patients receiving dupilumab 300 mg or placebo every 2 weeks. Responder analyses evaluated clinically meaningful improvements [≥ 1 (NPS, NC, LoS); ≥ 5 (LMK-CT); ≥ 8 (UPSIT); ≥ 8.9 (SNOT-22); ≥ 20 L/min (PNIF)].
Results: Ninety-eight patients were included [59% prior NP surgery, 84% systemic corticosteroids (SCS) use in the previous 2 years, 60% coexisting asthma, 91% anosmic, 97% impaired nasal airflow]. Least squares (LS) mean differences [dupilumab vs. placebo (95% CI)] in change from baseline at week (W) 24: NPS, −2.04 (−2.67, −1.40); PNIF, 65.9 (39.4, 92.4) L/min; LMK-CT, −4.97 (−6.50, −3.44); NC, −1.30 (−1.72, −0.89); LoS, −0.96 (−1.39, −0.54); Rhino-VAS, −3.37 (−4.67, −2.07); UPSIT, 8.55 (4.91, 12.20); SNOT-22, −25.3 (−34.1, −16.4) (all P < 0.0001). For all outcomes, significantly greater proportions of dupilumab vs. placebo patients achieved clinically meaningful improvements at W24. Fewer dupilumab vs. placebo patients required SCS and/or surgery through W24 (11.8% vs. 36.7%; P = 0.0005). Efficacy outcomes were similar at W52 (SINUS-52; n = 39) with P values vs. placebo of < 0.0001 for NPS and NC; 0.0002 for LMK-CT; 0.0031 for LoS; 0.0014 for rhino-VAS; 0.0013 for UPSIT; 0.0012 for SNOT-22.
Conclusions: In patients with CRSwNP with complete bilateral nasal obstruction, dupilumab treatment resulted in clinically significant improvements in NPS, LMK-CT, PNIF, symptoms, and health-related quality of life.
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a predominantly type 2 inflammatory disease characterized by the presence of nasal polyps (NP) and symptoms of nasal obstruction, smell loss, and rhinorrhea [1]. The extent of nasal obstruction can be assessed using endoscopy and NP score (NPS; range 0–8), with bilateral NPS = 8 signifying complete obstruction [2]. Management of inadequately controlled CRSwNP, especially in patients with complete nasal obstruction, often requires sinonasal surgery followed by interventions including oral corticosteroids and biologics [3]. However, surgery does not control the underlying inflammation and carries a risk of complications, while recurrence is common [1]. There is therefore a need to assess the treatment algorithm for this group, including the potential earlier introduction of biologics to avoid or defer surgery.
Dupilumab blocks signaling by interleukin-4 and interleukin-13, key and central drivers of type 2 inflammation [4]. In the SINUS-24/-52 (NCT02912468/NCT02898454) trials in patients with severe CRSwNP, dupilumab significantly improved mean NPS [5] and increased the proportion of patients achieving clinically meaningful improvement in NPS [6]. Evidence is lacking on the impact of total nasal obstruction on treatment outcomes in CRSwNP. We conducted a post hoc analysis of the SINUS studies to assess dupilumab efficacy in this subgroup.
Details of the SINUS-24/-52 studies are published [5]. Briefly, eligible patients were 18 years or older with bilateral CRSwNP and symptoms despite the use of intranasal corticosteroids (INCS) and had received systemic corticosteroids (SCS) in the previous 2 years or had undergone prior sinonasal surgery. Patients had to have bilateral NPS ≥ 5 (≥ 2 each nostril) at screening and at least two of the following symptoms: nasal congestion/obstruction (NC) and either loss of smell (LoS) or nasal discharge (anterior or posterior). Patients received mometasone furoate nasal spray 100 mg in each nostril twice daily during the 28-day run-in before randomization and throughout the treatment period. Patients on background INCS were randomized to dupilumab (Regeneron Pharmaceuticals Inc./Sanofi) 300 mg or placebo every 2 weeks (q2w) for 24 weeks (SINUS-24) or to dupilumab 300 mg or placebo q2w for 52 weeks or dupilumab 300 mg q2w for 24 weeks, then every 4 weeks for 28 weeks (SINUS-52). This analysis included patients in the pooled intention-to-treat (ITT) population with NPS = 8 at randomization and compared patients who received placebo or dupilumab 300 mg q2w. NPS scores were averaged from two independent masked viewers of the endoscopy video.
Outcomes assessed were NPS, peak nasal inspiratory flow (PNIF), Lund-Mackay computed tomography (LMK-CT; range 0−24), NC (0−3), LoS (0−3), rhinosinusitis visual analog scale (rhino-VAS; 0−10), University of Pennsylvania Smell Identification Test (UPSIT; 0−40), and 22-item Sinonasal Outcome Test (SNOT-22; 0−110).
Least squares (LS) mean changes from baseline for patients treated with dupilumab 300 mg q2w or placebo were assessed at week (W) 24 (pooled SINUS-24/-52) and W52 (SINUS-52). Each of the imputed complete data was analyzed by fitting an analysis of covariance model with the corresponding baseline value, treatment group, asthma/nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD) status, prior NP surgery, regions, and the study (for the pooled SINUS-24/-52) as covariates. Analysis was based on the same imputed dataset using worst observation carried forward (WOCF)/multiple imputation (MI) from the primary analysis of the coprimary endpoints NPS and NC [5].
Responder analyses evaluated the proportion of patients with clinically meaningful improvements in each outcome [7–9]. Odds ratios (ORs) with 95% confidence intervals (CIs) for the comparison of the dupilumab and placebo groups were derived from the Mantel-Haenszel estimator; the treatment groups were compared via a Cochran-Mantel-Haenszel test performed on the association between the responder status and treatment group, stratified by asthma/NSAID-ERD status, prior NP surgery, regions, and study (for the pooled SINUS-24/-52).
Comparisons of the time to NP surgery and/or SCS during treatment were performed using a Cox proportional hazard model, with treatment, asthma/NSAID-ERD status, prior NP surgery, regions, and study (for the pooled SINUS-24/-52) as covariates.
For all analyses, statistical significance was attributed at P-values < 0.05; all reported P-values are nominal.
Of the pooled ITT population (n = 724), 98 patients (13.5%) had NPS = 8 at randomization [30/286 placebo (10.5%); 68/438 dupilumab (15.5%)]. Approximately 59% of patients with NPS = 8 had prior NP surgery (of whom 24% had ≥ 3 previous surgeries), 84% had used SCS in the previous 2 years, and 60% had coexisting asthma (Table 1). Patients had a high symptom burden (mean [standard deviation (SD)] NC score 2.7 [0.4]/3.0 and LoS 2.9 [0.3]/3.0) and 91% were anosmic. Nasal airflow was severely impaired [mean (SD) PNIF 33.8 (45.1) L/min and 97% of patients below the normative value of 138.4 L/min [10]]. In SINUS-52 (n = 303), 17/153 (11.1%) placebo patients and 22/150 (14.7%) dupilumab patients had NPS = 8 at baseline.
Demographics and baseline characteristics [pooled SINUS-24/-52 studies, subgroup of patients with nasal polyp score (NPS) = 8 and intention-to-treat (ITT) population]
| Characteristic | Patients with NPS = 8(n = 98) | ITT population(n = 724) |
|---|---|---|
| Age, years | 53.8 (13.2) | 51.4 (12.8) |
| Male sex, n (%) | 55 (56) | 437 (60) |
| Region, n (%) | ||
| Asia | 8 (8) | 49 (7) |
| Latin America | 29 (30) | 137 (19) |
| East Europe | 22 (22) | 216 (30) |
| Western countries | 39 (40) | 322 (44) |
| Time since first NP diagnosis, years | 13.5 (10.9) | 11.0 (9.5) |
| Prior NP surgery, n (%) | 58 (59) | 459 (63) |
| Time since most recent surgery, years | 10.1 (6.9) | 7.2 (6.4) |
| Number of previous surgeries, n (%)* | ||
| 1 | 36 (62) | 254 (55) |
| 2 | 8 (14) | 94 (20) |
| ≥ 3 | 14 (24) | 111 (24) |
| SCS use in past 2 years, n (%) | 82 (84) | 538 (74) |
| Asthma, n (%) | 59 (60) | 428 (59) |
| NSAID-ERD, n (%) | 32 (33) | 204 (28) |
| NPS (0–8) | 8.0 (0.0) | 6.0 (1.3) |
| NC score (0–3) | 2.7 (0.4) | 2.4 (0.6) |
| LMK-CT total score (0–24) | 18.9 (3.9) | 18.4 (4.1) |
| LoS score (0–3) | 2.9 (0.3) | 2.7 (0.5) |
| Anterior rhinorrhea (0–3) | 2.4 (0.6) | 2.1 (0.7) |
| Posterior rhinorrhea (0–3) | 2.3 (0.8) | 2.0 (0.8) |
| TSS (0–9) | 8.0 (1.2) | 7.2 (1.4) |
| Rhinosinusitis VAS (0–10) | 8.3 (2.2) | 7.9 (2.1) |
| UPSIT score (0–40) | 11.5 (6.3) | 14.0 (8.2) |
| UPSIT anosmia†, n (%) | 88 (91)‡ | 551 (78)‡ |
| SNOT-22 total score (0–110) | 56.9 (21.6) | 50.9 (20.7) |
| PNIF (L/min) | 33.8 (45.1) | 87.1 (56.1) |
| PNIF group (L/min), n (%) | ||
| < 97.5 | 93 (94.9) | 453 (62.6) |
| ≥ 97.5–< 138.4 | 2 (2.0) | 150 (20.7) |
| ≥ 138.4 | 3 (3.1) | 121 (16.7) |
All data are mean (standard deviation) unless otherwise stated. * Percentages calculated using number of patients with surgery as denominator; † UPSIT < 18; ‡ n = 97 (NPS = 8 population), n = 710 (ITT population). SCS: systemic corticosteroids; NSAID-ERD: nonsteroidal anti-inflammatory drug-exacerbated respiratory disease; NC: nasal congestion/obstruction; LMK-CT: Lund-Mackay computed tomography; LoS: loss of smell; TSS: total symptom score; VAS: visual analog scale; UPSIT: University of Pennsylvania Smell Identification Test; SNOT-22: 22-item Sinonasal Outcome Test; PNIF: peak nasal inspiratory flow
At W24, 38/273 placebo patients (13.9%) and 16/420 dupilumab patients (3.8%) had NPS = 8 (Figure 1). In patients with NPS = 8 at baseline, 16.7% of placebo patients and 78.1% of dupilumab patients had NPS < 8 at W24, and all outcomes significantly improved with dupilumab vs. placebo (P < 0.0001; Figure 2). Similar statistically significant improvements were observed at W52 in SINUS-52 [P < 0.0001 for NPS and NC; P = 0.0002 for LMK-CT; P = 0.0031 for LoS; P = 0.0014 for rhino-VAS; P = 0.0013 for UPSIT; and P = 0.0012 for SNOT-22 (Figure S1)].
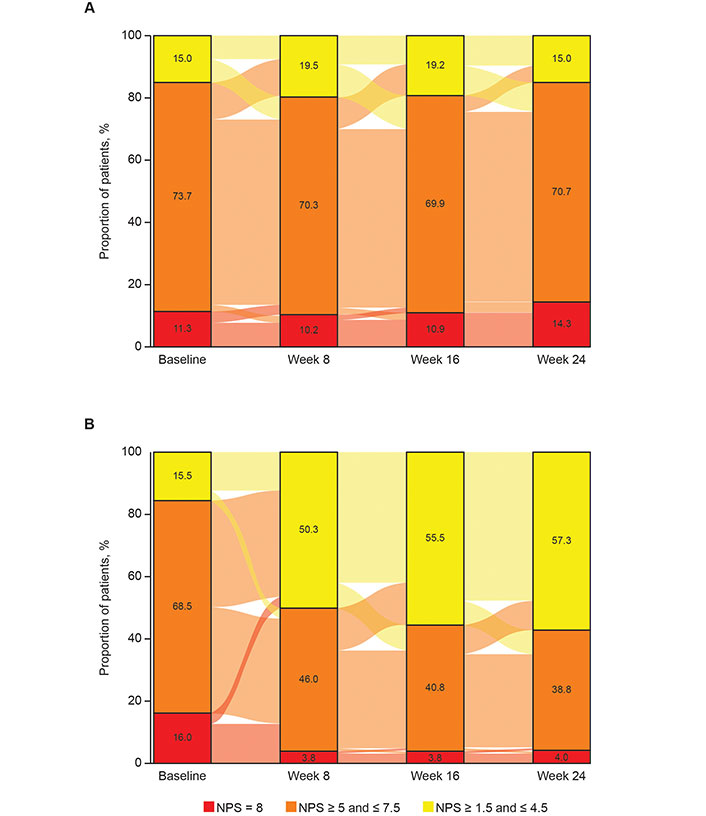
Sankey plots showing the distribution of nasal polyp score (NPS) categories from baseline to week 24 in patients treated with (A) placebo or (B) dupilumab 300 mg every 2 weeks (q2w; pooled SINUS-24/-52 studies)*. Although the eligibility criteria for the SINUS-24 and SINUS-52 studies included a requirement for NPS > 5 at the screening visit, some patients (15.0% and 15.5% of those randomized to placebo and dupilumab 300 mg q2w, respectively) had an NPS below this value (NPS ≥ 1.5 and ≤ 4.5; shown in yellow) at baseline, following the 28-day run-in period when all patients received mometasone furoate nasal spray 100 mg in each nostril twice daily. * Percentages were computed based on the total numbers of patients with a value at each visit. The percentages may not sum to 100.0 due to rounding
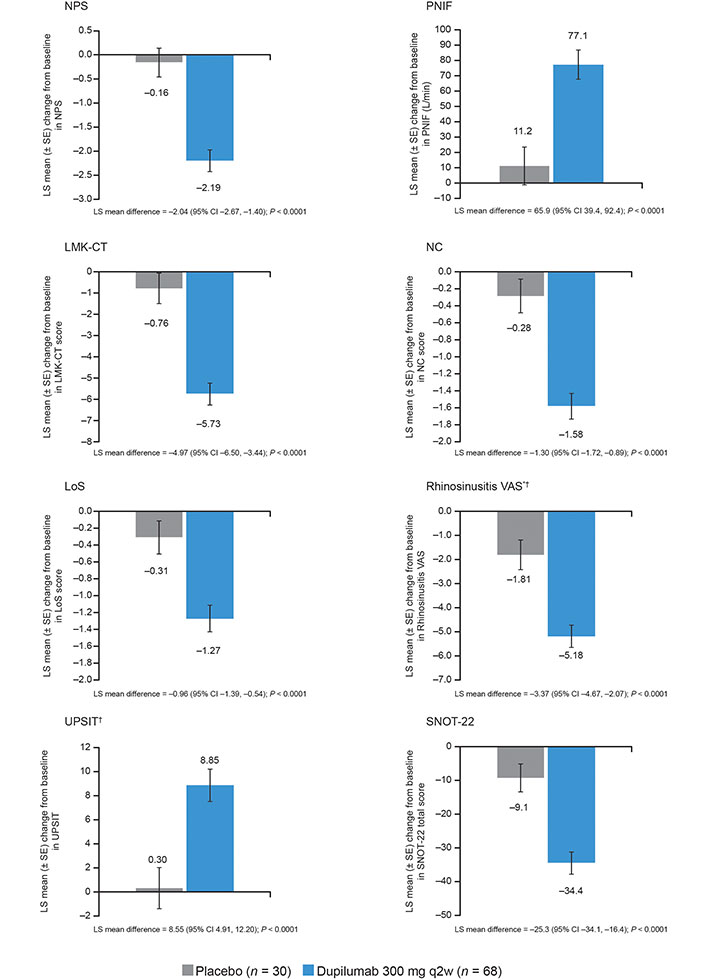
Least squares (LS) mean change from baseline in chronic rhinosinusitis with nasal polyps (CRSwNP) outcomes for placebo and dupilumab 300 mg every 2 weeks (q2w) at week 24 [pooled SINUS-24/-52 studies, subgroup of patients with nasal polyp score (NPS) = 8]. Each of the imputed complete data was analyzed by fitting an analysis of covariance (ANCOVA) model with the corresponding baseline value, treatment group, asthma/nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD) status, prior NP surgery, regions, and the study as covariates. Analysis was based on the same imputed dataset using worst observation carried forward (WOCF)/multiple imputation (MI) from primary analysis of the coprimary endpoints. * Placebo n = 29; † dupilumab n = 67. SE: standard error; CI: confidence interval; PNIF: peak nasal inspiratory flow; LMK-CT: Lund-Mackay computed tomography; NC: nasal congestion/obstruction; LoS: loss of smell; VAS: visual analog scale; UPSIT: University of Pennsylvania Smell Identification Test; SNOT-22: 22-item Sinonasal Outcome Test
At W24 in the pooled population with NPS = 8 at baseline, 69.1% of dupilumab patients had achieved a clinically meaningful improvement in NPS vs. 10.0% of placebo patients [OR (95% CI) 25.1 (4.3, 145.5), P < 0.0001; Figure 3]. Over half (55.9%) of dupilumab-treated patients achieved an NPS response at the first post-baseline assessment (W4). The proportions of patients with clinically meaningful improvements in the other outcomes were also significantly greater with dupilumab vs. placebo at W24 [P < 0.0001 for NPS, LMK-CT, NC, and LoS; P = 0.0002 for PNIF; P = 0.0005 for UPSIT; and P = 0.0010 for SNOT-22 (Figure 3)]. Similar results were seen at W52 (Figure S2), statistically significant for all outcomes (P = 0.0013 for NPS; P = 0.0018 for LMK-CT; P = 0.0034 for NC; P = 0.0013 for LoS; and P = 0.0089 for UPSIT) except SNOT-22 (P = 0.2569).
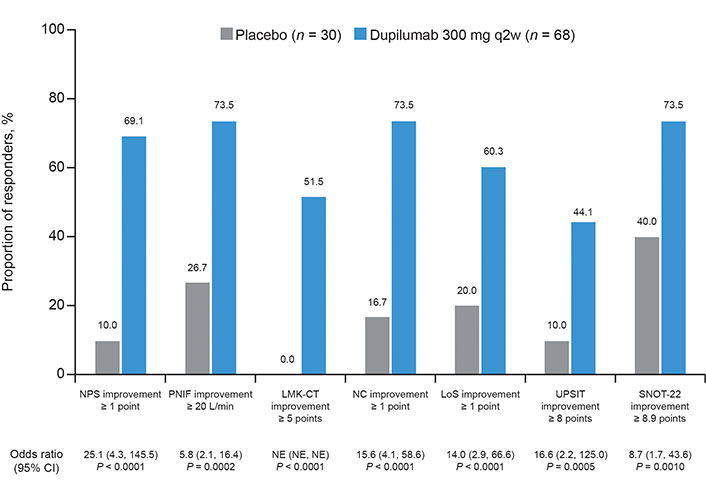
Proportion of responders for placebo and dupilumab 300 mg every 2 weeks (q2w) at week 24 [pooled SINUS-24/-52 studies, subgroup of patients with nasal polyp score (NPS) = 8]. Odds ratios (OR) were derived from the Mantel-Haenszel estimator, with Cochran-Mantel-Haenszel test performed on the association between the responder status and treatment group (dupilumab vs. placebo), stratified by asthma/nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD) status, prior surgery history, study identifier, and region. PNIF: peak nasal inspiratory flow; LMK-CT: Lund-Mackay computed tomography; NC: nasal congestion/obstruction; LoS: loss of smell; UPSIT: University of Pennsylvania Smell Identification Test; SNOT-22: 22-item Sinonasal Outcome Test; CI: confidence interval; NE: not evaluable
Among patients with baseline PNIF < 138.4 L/min, 23/66 (34.8%) dupilumab vs. 0/29 (0%) placebo patients achieved PNIF ≥ 138.4 L/min at W24 (P = 0.004).
Fewer dupilumab than placebo patients required SCS and/or NP surgery during the treatment period to W24 (11.8% vs. 36.7%; Cox proportional hazard model P = 0.0005); 0/68 dupilumab vs. 2/30 placebo patients had NP surgery. SCS were used by 8/68 (11.8%) dupilumab and 11/30 (36.7%) placebo patients.
This analysis focused on patients with severe CRSwNP and complete nasal obstruction despite ongoing standard treatment with INCS and, in most cases, previous surgery. Alongside complete nasal obstruction, patients had high symptom burden at baseline (severe nasal congestion, smell loss, severely reduced nasal airflow) and reduced health-related quality of life (HRQoL). An NPS = 8 would also likely prohibit adequate dispersion of INCS. These characteristics emphasize the need for effective treatment in this subgroup. A standard treatment option for these patients is sinonasal surgery, although we are unaware of any study that has examined the benefit of surgery specifically in patients with NPS = 8. While surgery can provide disease control for extended periods, it does not control the underlying inflammation and polyps may recur. The results of the current analysis suggest that targeting type 2 inflammation with dupilumab may provide a viable alternative to surgery in patients with complete nasal obstruction. These results do not allow comparison of biologic therapy with surgery in this group of patients but future studies addressing this question, or addressing the possibility of a combination of biologic with surgery, may be informative. The relatively small sample size precluded stratification of patients according to surgical status, as it might have been informative to compare outcomes between surgery-naive patients and those with prior surgery. Another important point to consider is the relative public health impact of conventional treatments vs. newer approaches such as biologic therapy. While the evidence is limited, recent studies suggest that patients receiving biologics use more drug-related services but have a reduced need for procedures including nasal endoscopy and sinus CT scans compared with those receiving conventional treatments, and require fewer outpatient visits compared with patients who underwent surgery alone [11, 12]. However, we are unaware of any such data for patients with NPS = 8.
The rapidity of the dupilumab response, with over half of patients responding by W4, is important as the rapid benefit is considered an advantage of surgery. The magnitude of efficacy improvements was similar to those previously reported for the ITT population [5, 6]. The results also add to existing evidence of dupilumab efficacy in patients with multiple prior surgeries [13]. Dupilumab treatment significantly reduced the requirement for SCS, thus lowering the risk of steroid-associated adverse effects [14].
Limitations of this analysis include its post hoc nature, the relatively small sample size, the lack of data on long-term outcomes beyond 52 weeks, and questions regarding generalizability to the wider population of patients with CRSwNP. The relatively small sample size also precluded subdividing the groups according to surgical status.
In patients with CRSwNP with complete bilateral nasal obstruction, dupilumab treatment resulted in clinically significant improvements in NPS, LMK-CT, nasal inspiratory flow, symptoms, and HRQoL.
CI: confidence interval
CRSwNP: chronic rhinosinusitis with nasal polyps
INCS: intranasal corticosteroids
ITT: intention-to-treat
LMK-CT: Lund-Mackay computed tomography
LoS: loss of smell
NC: nasal congestion/obstruction
NP: nasal polyps
NPS: nasal polyp score
NSAID-ERD: nonsteroidal anti-inflammatory drug-exacerbated respiratory disease
PNIF: peak nasal inspiratory flow
q2w: every 2 weeks
rhino-VAS: rhinosinusitis visual analog scale
SCS: systemic corticosteroids
SNOT-22: 22-item Sinonasal Outcome Test
UPSIT: University of Pennsylvania Smell Identification Test
W: week
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100951_sup_1.pdf.
The authors thank Asif H. Khan (Sanofi) for insights and guidance, and Carole Mercier and Christine Taniou (Axial Group) for their support with statistical analyses, which was funded by Sanofi and Regeneron Pharmaceuticals Inc. Medical writing/editorial assistance was provided by Olympia Gianfrancesco, PhD, of Adelphi Group, funded by Sanofi and Regeneron Pharmaceuticals Inc.
MW, CB, CH, MC, JM, SN, YD, PJR, HS, and JAJN: Conceptualization, Writing—original draft, Writing—review & editing. JM: Formal analysis. All authors contributed to manuscript revision, read, and approved the submitted version.
MW: ALK-Abelló, AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, Stallergenes Greer, Takeda—advisory board member, lecture fees, and research grants. MC, JM, PJR, and JAJN: Sanofi—employees, may hold stock and/or stock options in the company. CB: ALK, AstraZeneca, GlaxoSmithKline, Mylan, Novartis, Sanofi, Stallergenes Greer—advisory board member and speakers’ fees. CH: AstraZeneca, Dianosic, GlaxoSmithKline, Sanofi—advisory board member. SN, YD, and HS: Regeneron Pharmaceuticals Inc.—employees, may hold stock and/or stock options in the company.
The SINUS-24 and SINUS-52 studies were conducted according to the principles laid down in the Declaration of Helsinki. Institutional review boards and independent ethics committees reviewed and approved the protocols, informed consent forms, and patient information before study initiation. A full list of institutional review boards and independent ethics committees is shown in Table S1.
All patients provided signed written informed consent prior to study participation.
Not applicable.
Qualified researchers may request access to data and related study documents. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial patients. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
The SINUS trials and the analyses contained within this manuscript were sponsored by Sanofi and Regeneron Pharmaceuticals Inc. ClinicalTrials.gov Identifiers: NCT02912468 (SINUS-24) and NCT02898454 (SINUS-52). Sanofi and Regeneron Pharmaceuticals Inc. designed the SINUS-24 and SINUS-52 studies, collected the data, and, in collaboration with the authors, contributed to analysis, data interpretation, manuscript preparation, and the decision to publish.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Ludger Klimek ... Claus Bachert
Ayşegül Verim ... Tuğba Çelik
Sepideh Darougar ... Pantea Bozorg Savoji
Edyta Jura-Szołtys ... Radosław Gawlik
