Affiliation:
1Department of Pediatrics, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Tehran 1916893813, Iran
ORCID: https://orcid.org/0000-0003-1399-8351
Affiliation:
1Department of Pediatrics, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Tehran 1916893813, Iran
ORCID: https://orcid.org/0000-0001-6318-4458
Affiliation:
2Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Tehran 1916893813, Iran
Email: pantea.savodji@yahoo.com
ORCID: https://orcid.org/0009-0005-8276-223X
Explor Asthma Allergy. 2024;2:473–484 DOI: https://doi.org/10.37349/eaa.2024.00059
Received: December 28, 2023 Accepted: August 07, 2024 Published: September 19, 2024
Academic Editor: Diego Bagnasco, University of Genoa, IRCCS Policlinic San Martino, Italy
The article belongs to the special issue Update on Chronic RhinoSinusitis
Chronic rhinosinusitis (CRS) is an inflammatory disorder of the paranasal sinuses and the nasal cavity lasting longer than 12 weeks. This disease is a common clinical syndrome with significant monetary burden due to the high costs of medical visits, diagnostic tests, medications, and surgical therapies. CRS without nasal polyposis (CRSsNP) is the most common subtype of CRS, accounting for about 70% of all patients. Other subtypes include CRS with nasal polyposis (CRSwNP) and allergic fungal rhinosinusitis (AFRS). CRSwNP has the worldwide prevalence of 2% to 4% and is often accompanied by type 2 inflammation and asthma as a comorbid condition. Pediatric chronic sinusitis is distinct from adult chronic sinusitis and is currently considered an infectious process, characterized by persistent inflammation representing an exaggerated immune response to an external stimulus. The medical and surgical management of CRS has been remarkably modified in the past two decades. The aim of this study was to present an update on CRS based on the recent years’ literature.
Chronic rhinosinusitis (CRS) is generally defined as inflammation of paranasal sinuses and nasal cavity, lasting for more than 12 weeks, with certain clinical manifestations along with objective evidence of sinonasal involvement, such as direct visualization or an imaging study [1, 2]. This disease is a common clinical syndrome with significant financial burden due to the high costs of medical visits, diagnostic tests, medications, and surgical therapies [3]. Frequent absences from school, decreased work productivity, and detrimental influences on physical and emotional health are other adverse impacts of this disease [3]. This heterogeneous disorder, the diagnosis of which requires objective evidence of mucosal inflammation, may result in significant morbidity.
CRS without nasal polyposis (CRSsNP) is the most common subtype of CRS, accounting for about 70% of all patients. Other subtypes include CRS with nasal polyposis (CRSwNP) [4] and allergic fungal rhinosinusitis (AFRS). CRSwNP has the worldwide prevalence of 2% to 4% and is often accompanied by type 2 (T2) inflammation and asthma as a comorbid condition [4] in approximately 50% of the total patients [5]. The cardinal features of CRSwNP, particularly during exacerbations, include nasal congestion or blockage, complete or partial loss of smell, anterior or posterior drainage, and facial pressure and pain [4].
Pediatric chronic sinusitis is different from adult chronic sinusitis and is recognized as an infectious process [6], characterized by persistent inflammation representing an excessive immune response to an external stimulus [7]. According to the clinical consensus statement, published in 2015 by the American Academy of Otolaryngology-Head and Neck Surgery Foundation, CRS in children is defined as at least 90 consecutive days with two or more symptoms of cough, nasal blockage, purulent nasal discharge, or facial pressure/pain along with either endoscopic signs of mucosal edema, nasal polyps, or purulent drainage, and/or CT scan imaging indicating mucosal alterations within the ostiomeatal complex, and/or sinuses [7].
The medical and surgical management of CRS has undergone significant changes during the past two decades. The recent application of biological, monoclonal therapies which target specific mediators has the potential to change the course of the disease, yet the patients’ exact CRS endotype must be first determined appropriately so that the biological, monoclonal treatments could be applied accordingly. The aim of this study was to present an update on CRS based on the recent years’ literature.
CRS can affect both children and adults, yet quite differently [8]. Due to the lack of a gold standard, CRS diagnosis is challenging [9]. Since there are different diagnostic criteria, including symptom-based diagnoses, rhinoscopy-based objectives, or imaging findings, the estimation of CRS prevalence may vary significantly in different parts of the world, ranging from 5% to 12% of the general population in total [9], with higher rates in the Middle East [10]. The exact prevalence of rhinosinusitis in children is unknown because of the low percentage of referrals to physicians. It has been estimated that 0.5% to 13% of upper respiratory infections may lead to bacterial rhinosinusitis, and 6% to 13% of children may experience at least one episode of rhinosinusitis before 3 years of age [6]. Factors leading to predisposition towards CRS include anatomic and inflammatory elements, resulting in CRS. Septal deviation, NP, lateral wall anomalies, and nasal foreign bodies are examples of the anatomic causes, while allergic rhinitis, allergic fungal sinusitis, gastroesophageal reflux, tobacco smoke, and occupational exposure to gases, fumes, and dust are examples of the inflammatory causes [6, 11]. Studies have shown that the development of CRS could be attributed to high levels of NO2 [11]. Smokers and those working as house cleaners have also shown a higher prevalence of CRS [11]. In addition, a slightly higher rate of association with male gender has been noted in the prevalence of CRSwNP [12].
In one study, CRS was described as an interaction at the site of the interface between the host immune system and the environmental factors such as microbial agents, toxins, and allergens [13]. In another study, CRS pathophysiology was mainly defined based on T cells [14], where three types of immune responses were proposed in accordance with the immune polarization induced by T cell cytokine production [15].
Many studies have suggested “endotypic” correlations with histopathologic and clinical findings in CRS patients [16, 17]. The most common histopathologic findings in this regard have been neutrophilia, eosinophilia, fibrosis, glandular hypertrophy, and epithelial dysmorphosis [18]. However, more recent studies are seeking to define CRS endotypes based on specific molecular mechanisms, with biomarkers as a defining characteristic of each pathway, which appear to have strong associations with phenotypes and therapy responses [19].
CRS patients are categorized according to distinct endotype subsets, presenting with T1 endotype [interferon-γ (IFN-γ) expression], T2 endotype (eosinophil cationic protein and charcot-leyden crystal galectin), and T3 endotype (IL-17A expression), or a mix of two. The importance of these 3 types of immune responses lies in the definition of certain endotypes, which may greatly affect the clinical manifestations [14].
T1 immune responses include T1 innate lymphoid cells (ILC1s) as well as Tc1 and Th1 cells, with a pivotal role in dealing with intracellular microbes, protozoa, and viruses [20]. Stimulation of these cells results in IFN-γ and TNF-α production, leading to the activation of mononuclear phagocytes [15]. ICL2, Tc2, and Th2 cells are known to be implicated in T2 immune responses, which play an important role in the pathogenesis of allergic diseases through the involvement of eosinophils, IgE production, and goblet cell hyperplasia [15, 21]. T3 immune responses are induced by IL-3, Tc17 cells, and Th17 cells, with a role in fighting extracellular bacteria and fungi [15]. CRSwNP and CRSsNP are both linked to inflammatory patterns produced by the above-mentioned immune responses, which may vary across patients from various geographical places and racial backgrounds [4, 14].
CRSwNP is considered a diffuse inflammatory process with downregulation of Tregs, while CRSsNP occurs due to up-regulation of Tregs and the resultant sinus outflow tract obstruction, leading to secondary inflammation and infection [18]. Tumor growth factor (TGF)-beta is the major product of Tregs, which has a potential role in tissue remodeling in CRSsNP. Tissue remodeling results from the function of TGF-beta in induction and proliferation of fibroblasts and extra-cellular matrix synthesis [14]. Some reports suggest that CRSsNP is more common (two-thirds of cases) and mostly exhibits T1 immune response [3]. This type of CRS is often characterized by frequent infections and therefore colonization of the sinonasal cavity with organisms such as Pseudomonas aeruginosa, Haemophilus influenzae, and Streptococcus pneumoniae, which produce ciliotoxic or ciliostatic toxins [22]. This may result in mucociliary function impairment, leading to biofilm formation, which subsequently creates a vicious cycle [21].
When it comes to CRSwNP, insufficient or exaggerated immune response to external stimuli may progress to persistent mucosal inflammation with morphological alterations in respiratory epithelium of nasal polyps [23]. An example of such an event is localized allergic hyper-responsiveness to Staphylococcus aureus enterotoxin, which may act as a superantigen, broadly activating T lymphocytes [24]. Therefore, dysfunction of the airway epithelium may lead to an altered mucociliary clearance as well as altered maintenance barrier function in the epithelial cells of NP [23].
Other studies demonstrate that about 80% of patients with CRSwNP in the western developed countries predominantly show a T2 response, including expression of IL-4, IL-5, and IL-13 with elevated levels of IgE. Clinically, T2 response may also lead to asthma, which can even result in more severe courses of various comorbidities. On the other hand, there are fewer reports of patients with T2 cytokine expression, eosinophilic inflammation, and asthma comorbidity in East Asia [12, 23, 25]. These Asian patients predominantly show neutrophil-biased responses [14]. Considering the contribution of neutrophils to the pathogenesis of NP in the western world, it is confirmed that neutrophils are not good biomarkers for endotype differentiation in these patients [18].
High-levels of IFN-γ and IL-17A expressions have been reported in NP of patients with cystic fibrosis, indicating T3 immune response. In T1 and T3 immune responses, epithelial cells are stimulated by environmental triggers in order to secrete osteopontin, thus triggering dendritic cells to activate Th1 and Th17 cells [14]. Tc1, Tc17, Th1, and Th17 cells collectively stimulate non-eosinophilic inflammation and produce IFN-γ, IL-17A, and IL-22 [14]. This subset of inflammation is clinically associated with purulent nasal discharge [26].
Using different markers for each endotype, Kato et al. [18] conducted a study to find associations between endotypes and phenotypes. In their study, CRS patients with T2 endotype suffered from NP, asthma comorbidity, anosmia, and allergic mucin, while those with T3 endotype had pus [19]. Another study emphasized the role of neutrophils and their proinflammatory cytokines in older adults with CRS. These patients showed a higher likelihood of neutrophilic inflammation, a better treatment response to macrolides, and no response to corticosteroids and biologics [19]. In his study, Bachert et al. [19] observed elevated local IgE in nasal polyp tissue in patients with CRSwNP, which was indicative of IgE against Staphylococcus aureus enterotoxin in nasal polyp tissue and also systemic circulation, revealing a more severe sinonasal disease.
In a recent study, some patients with CRSsNP showed increased levels of IL-4, IL-5, eosinophilic cationic protein, total serum IgE, and anti-Staphylococcus aureus enterotoxin specific-IgE, indicating a T2 immune response [27]. Despite the significantly increased recurrence of CRSsNP and asthma occurrence in patients with T2 CRSsNP, only 3% developed NP within the next 12 years [26]. This shows the same T2 immune response in CRSsNP as those in CRSwNP, yet less prominent. Klingler et al. [28] compared gene expression levels in samples taken from three groups of patients; without sinonasal disease, with CRSsNP, and with CRSwNP. The gene expression patterns in this study showed a close similarity between the ethmoid tissue and nasal polyp samples of the CRSwNP patients and the T2 patterns in the ethmoid tissue of T2 subset of the CRSsNP patients [28].
Considering CRS to be an inflammatory sinonasal disease, it is generally classified into eosinophilic inflammation versus non-eosinophilic inflammation with three distinct patterns, namely allergic, eosinophilic, and non-eosinophilic CRS [14]. Most of the patients with eosinophilic inflammation are immunocompetent adults from 30 years to 50 years of age [29] with CRSwNP and increased concentrations of T2 cytokines. The eosinophilic inflammation leads to extensive sinus disease with higher rates of polyp recurrence after surgery and without sufficient post-operative quality of life improvement. These patients may also show late-onset asthma comorbidities [14]. Their hallmark symptoms include rhinorrhea, nasal congestion and blockage, hyposmia or anosmia, postnasal drip, and headache [30], yet fever and excessive facial pain are not common.
Their clinical examination is based on evaluating nasal airway patency and presence of polyps. Small to large polyps protruding from the middle meatus to the nasal passage with tenacious mucin, shown at endoscopy, may further reveal the T2 inflammation nature of the disease [14]. A pan-sinus opacification with evidence of secondary obstruction on CT imaging and neo-osteogenesis changes are other radiological findings which can establish the existence of NP and/or any concomitant anatomical cause of nasal obstruction [14]. Plain X-rays are not recommended anymore due to their insufficient sensitivity or specificity to assess CRS.
CRSsNP is a heterogeneous condition, which may occur with allergic and non-allergic rhinitis, structural abnormalities, and/or immunodeficiencies. These patients typically have persistent symptoms with periodic exacerbations, yet usually without fever. In a subset of these patients, recurrent acute rhinosinusitis occurs with good response to antibiotic therapy [31].
A unique subset of CRSwNP is AFRS, which typically affects adults below 30 years of age. These immunocompetent individuals with atopy and T1 hypersensitivity to fungi may have asthma with a lower incidence (24%) compared to other types of CRSwNP (where asthma may coexist in 50% of cases). The involved sinuses often sustain expansile changes, sometimes leading to the erosion of medial orbital wall or anterior skull base with resultant facial or orbital deformities, although with disproportionate occurrences of nasal obstruction, hyposmia, or drainage due to the slow progression of disease over years [32]. Fever is not a common symptom in these patients. The presence of allergic mucin containing viable fungal hyphae with evidence of IgE-mediated allergy differentiates AFRS from CRSwNP. NP and allergic mucin without fungal hyphae and allergy are mainly attributed to eosinophilic mucin rhinosinusitis, with significant refractory or recurrent NP. Cocaine abuse and esthesioneuroblastoma are two rare conditions mimicking CRS signs and symptoms at the onset [33, 34].
The cornerstone of the treatment of CRS patients, whether with or without NP, is medical management [5]. CRSsNP represents the most common subtype of CRS patients, most of whom cannot be cured, yet can achieve some degrees of symptoms control with long-term management. The first step in the treatment of the patients with CRSsNP is to make sure that the danger signs do not exist. These signs, including high fever, severe headache, meningeal signs, significant epistaxis, and orbital signs and symptoms, may suggest the presence of complications, necessitating more caution.
The primary goal of the therapy should be the improvement of the quality of life by individualizing the treatments for each patient. Intranasal saline irrigation has long been recommended as the initial intervention [35]. The proposed mechanism of this treatment focuses on water transport through the mucosal epithelial membrane so that the nasal mucosa becomes hydrated and moisturized, which in turn leads to increased mucociliary clearance [28]. It seems that saline irrigation may improve symptoms and quality of life provided that water or hypotonic saline is avoided as they may cause nasal irritation. These patients should also avoid any nose blows within 15 min after irrigation to prevent further auto-insufflation of the solution into eustachian tubes.
According to various guidelines and consensus statements, topical intranasal corticosteroids (INCS) have long been recommended as the first-line pharmacotherapy in the management of CRSsNP [1, 36, 37]. The major underlying mechanism is to reduce mucus production and mucosal edema in order to improve drainage. The patients who still show inadequate response to saline irrigation and INCS after several months of treatment and suffer from both infection and inflammation simultaneously are advised to receive a combination of oral antibiotics and glucocorticoids for 10 days to 14 days [38, 39]. However, in the case of infection alone, particularly with mucopurulent discharge, antibiotics are normally the treatment modality, and in the presence of only thickened secretions, which are not clearly purulent, glucocorticoid alone is the choice. One challenging issue is the differentiation between infection and inflammation as in most cases the two conditions cannot be distinguished from each other [1, 38].
Acute exacerbations of CRS may be caused by several factors, including infections, allergen or irritant exposures, and nonadherence to medications [40]. When these exacerbations are accompanied by purulent secretions, they can be simply treated with oral antibiotics as acute uncomplicated bacterial sinusitis.
The most important difference between the management of CRS in children and adults is the role of adenoidal hypertrophy in children with CRS unresponsive to medical therapy. In these children, adenoidectomy may be beneficial [41].
In patients with severe nasal blockage or severe symptoms of nasal congestion or anosmia, a short course of oral glucocorticoids may effectively shrink nasal polyps [40, 41]. A substantial benefit of oral glucocorticoids is reported in the first 2 weeks or 3 weeks after therapy, consisting of reduced size of polyps as well as improved sense of smell and sinonasal symptoms, although these improvements are not sustained until 10–12 weeks after treatment [42–44].
The next step in therapy is deciding between consultation with an otolaryngologist for functional endoscopic sinus surgery (FESS) and available biologic therapies. Persistent improvements in signs and symptoms are reported in many studies over the next 5 years to 10 years after surgery [45, 46]. In patients suffering from both asthma and CRS, evidence suggests better long-term control of asthma along with FESS and medical therapy compared to medical therapy alone [47]. FESS involves removing the tissue around the ostia so that the outflow tracts could be widened in order to facilitate sinus drainage and ventilation. In patients with NP, the polypoid material and inflammatory debris are also removed. Mucosal preservation is the accepted technique to avoid excessive scarring and maintain normal mucociliary clearance. FESS is safe in children but is usually performed only on the maxillary sinuses. After surgery, some surgeons apply adjunct therapy with steroid eluting stents and dressings. These are bioabsorbable sinus implants eluting 370 mcg of mometasone furoate over 30 days. The aim of this adjunctive therapy is to maintain the patency of the sinus opening after surgery [40].
Balloon ostial dilation is another procedure using a balloon catheter in order to dilate the frontal, sphenoid, or maxillary sinus ostium. However, one limitation of this method is lack of surgical tissue removal, making it less effective compared to FESS. It is also noteworthy that this method cannot be used for ethmoid sinus as an area of considerable inflammation [48–50]. In 2018, this technique was suggested as an adjunct procedure to FESS for patients with CRSsNP as well as a way to widen stenosed sinus openings in previously operated patients [51].
Generally, in most long-term follow-up studies after FESS, between 15% and 20% of patients, mostly those with NP, required repeat surgery [46]. This may happen due to numerous reasons, including inconsistent adherence to maintenance therapies, improper sinus surgery, and unrecognized comorbid conditions such as allergic rhinitis and primary immunodeficiencies.
Miglani et al. [52] have recently conducted a study to compare the therapeutic effects of endoscopic sinus surgery versus biologics for the treatment of CRSwNP. In this cohort study, the efficacy of FESS was compared with three biological therapies (including dupilumab, omalizumab, and mepolizumab), the results of which showed superiority of FESS over these three agents [52]. However, in another study, the polyp recurrence rate of up to 60% was reported as the main drawback of FESS [53]. The continuous need to administer biological therapies in timely intervals in order to control the symptoms is the reason why they are not the first priority [52, 54–57]. On the other hand, biologics are preferred in CRS patients due to their application in the treatment of sinonasal disease, the presence of contraindications to surgery, and CRS coexisting with poorly controlled asthma.
Some of the major criteria for surgery are failure of medical therapies, ineffective use of topical medications due to the presence of polyps, and extension of the disease beyond sinuses, yet determining surgical indications in most patients with CRSwNP is still challenging [58, 59]. Although FESS has shown more significant polyp burden improvement in comparison to biological therapies, these agents have a greater impact on controlling T2 inflammation, subjective symptoms, and therefore the patient’s overall quality of life [60].
Dupilumab, omalizumab, and mepolizumab are three respiratory biologics, which are approved for the treatment of moderate to severe asthma as well as CRSwNP. Among these three biologics, the first one has been identified to be the most effective in several studies [61–64]. When a patient has dual indications for biological therapy, the second indication may aid in selecting one of the three biologics [18, 64]. Dupilumab is a monoclonal antibody which targets IL-4 receptor alpha, and therefore inhibits the signaling of the two most important drivers of T2 inflammation, namely IL-4 and IL-3, along with key roles both in asthma and CRSwNP [65, 66]. Omalizumab is effective in reducing the local IgE present in nasal polyp tissue and can also improve the sinonasal symptoms in CRSwNP. Mepolizumab targets IL-5, which is known to have a key role in survival and activation of eosinophils. Previous studies have shown that eosinophils increase in nasal polyp tissue and may cause an underlying inflammation in CRSwNP [67]. Table 1 summarizes the modes of action, efficacy, and adverse effects of these biologics.
A comparison between efficacy and adverse effects of three approved respiratory biologics
| Biologics | Efficacy | Adverse effects |
|---|---|---|
| Dupilumab | Reduces:
Improves: | |
| Omalizumab | Improves:
|
|
| Mepolizumab | Improves:
|
|
FESS: functional endoscopic sinus surgery; NP: nasal polyposis
Benralizumab is a recently approved biological therapy for severe asthma by the US FDA, yet it is still under investigation for CRSwNP [66, 68]. Benralizumab is a monoclonal antibody which binds with eosinophils via IL-5 receptor-alpha and attracts natural killer cells to induce eosinophil apoptosis, resulting in a rapid and significant depletion of blood and tissue eosinophils. Therefore, a favorable response to benralizumab is anticipated in patients with NP [69].
Depemokimab is another, yet in clinical development, biological therapy for the treatment of asthma and rhinosinusitis with NP in adults. This is a monoclonal antibody which reduces the number of eosinophils by blocking IL-5 through binding with its cognate receptor and then inhibiting its action. IL-5 not only mediates the growth and differentiation of eosinophils in the bone marrow but also has a crucial role in their recruitment and activation within other tissues [70]. Therefore, the inactivation of IL-5 through depemokimab may cause a rapid reduction in the circulating population of eosinophils as well as their counts in nasal polyp tissues [71]. Depemokimab is reported to have an acceptable safety and efficacy profile with the potential, long-acting suppression of IL-5 with one subcutaneous injection every 6 months [72, 73].
Figure 1 illustrates the therapeutic approach in patients with CRS.
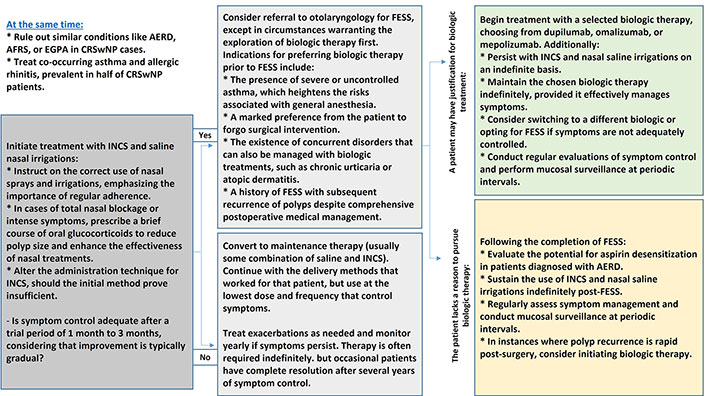
The therapeutic approach in patients with CRS. AERD: aspirin-exacerbated respiratory disease; AFRS: allergic fungal rhinosinusitis; CRS: chronic rhinosinusitis; CRSwNP: CRS with nasal polyposis; EGPA: eosinophilic granulomatosis with polyangiitis; FESS: functional endoscopic sinus surgery; INCS: intranasal corticosteroids
Disease-endotyping may help the better understanding of the natural history, pathophysiology, and therefore treatment of CRS. Individualization of treatments based on predicted endotypes may lead to more favorable outcomes. Treatment success, thus, depends on the underlying pathogenesis and disease-provoking factors. As a result, identifying the patients who may benefit from new classes of biotherapeutics based on their endotypes is currently deemed to be essential. Any improvement in the clinical endotyping methods of CRS may significantly increase the use of biological therapies, which can target patients’ specific endotypes. However, the clinical practice of such endotyping should be defined more comprehensively to guide personalized therapeutic guidelines.
AFRS: allergic fungal rhinosinusitis
CRS: chronic rhinosinusitis
CRSsNP: chronic rhinosinusitis without nasal polyposis
CRSwNP: chronic rhinosinusitis with nasal polyposis
FESS: functional endoscopic sinus surgery
IFN-γ: interferon-γ
T2: type 2
The authors would like to thank Mr. Ramin Kordi for English editing of the text.
SD: Conceptualization, Investigation, Writing—original draft, Supervision. MH: Investigation, Writing—review & editing. PBS: Investigation, Writing—review & editing, Validation. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Ludger Klimek ... Claus Bachert
Ayşegül Verim ... Tuğba Çelik
Martin Wagenmann ... Juby A. Jacob-Nara
Edyta Jura-Szołtys ... Radosław Gawlik
