Affiliation:
15th Department of Respiratory Medicine, Sotiria Thoracic Diseases General Hospital of Athens, 11527 Athens, Greece
2Laboratory of Molecular and Cellular Pneumonology, Medical School, University of Crete, 71003 Heraklion, Greece
3Respiratory Physiology Laboratory, Medical School, University of Cyprus, 2029 Nicosia, Cyprus
Email: matthaiou.andreas@gmail.com
ORCID: https://orcid.org/0000-0003-1941-0213
Affiliation:
15th Department of Respiratory Medicine, Sotiria Thoracic Diseases General Hospital of Athens, 11527 Athens, Greece
2Laboratory of Molecular and Cellular Pneumonology, Medical School, University of Crete, 71003 Heraklion, Greece
ORCID: https://orcid.org/0000-0001-5199-1519
Affiliation:
15th Department of Respiratory Medicine, Sotiria Thoracic Diseases General Hospital of Athens, 11527 Athens, Greece
ORCID: https://orcid.org/0000-0002-3456-6462
Affiliation:
15th Department of Respiratory Medicine, Sotiria Thoracic Diseases General Hospital of Athens, 11527 Athens, Greece
ORCID: https://orcid.org/0000-0002-1972-6683
Explor Asthma Allergy. 2024;2:502–510 DOI: https://doi.org/10.37349/eaa.2024.00061
Received: July 11, 2024 Accepted: August 22, 2024 Published: September 23, 2024
Academic Editor: Garry M. Walsh, University of Aberdeen, UK
Bronchiectasis is a heterogeneous chronic lung disease, characterised by irreversible dilatation of the airways and attributed to a wide spectrum of other underlying conditions, usually underdiagnosed and inadequately treated with a high burden for both the patients and the healthcare system. The way bronchiectasis is viewed by physicians has drastically changed over the last decades, with the topic of eosinophilia in the context of the disease being one of the substantially highlighted. Eosinophilia was traditionally considered as a means for distinguishing bronchiectasis from asthma, whereas bronchiectasis was primarily associated with neutrophilic inflammation. However, eosinophilic bronchiectasis is nowadays identified as a distinct disease endotype and is associated with a specific clinical course and response to treatment. Further research is needed to better characterise this endotype and improve its personalised investigation and management in daily clinical practice.
Bronchiectasis is a chronic lung disease characterised by irreversible dilatation of the airways and constitutes a heterogeneous nosological entity regarding its underlying causes, as a wide spectrum of other diseases eventually leads to its development. It is usually an underdiagnosed and inadequately treated condition with a high burden for both the patients and the healthcare system [1]. Eosinophilia was traditionally considered as a means for distinguishing bronchiectasis from asthma, whereas bronchiectasis was primarily associated with neutrophilic inflammation. Notably, in the 1960s, Strang [2] measured blood eosinophil counts (BEC) in 40 children with bronchiectasis and 43 with asthma and found that only the latter demonstrated peripheral eosinophilia [2].
In most cases of concomitant bronchiectasis and eosinophilia reported in the literature, bronchiectasis appeared as the result of another underlying disease involving eosinophilia. In the 1960s, Rab et al. [3] described two cases of tropical pulmonary eosinophilia that led to bronchiectasis as a direct complication [3]. Several decades later, Price et al. [4] reported the combination of eosinophilia and bronchiectasis in a patient with ulcerative colitis receiving mesalazine [4]. Bronchiectasis was also observed in the cases of two adolescents diagnosed with chronic eosinophilic pneumonia, suggesting bronchiectasis as a possible manifestation of the disease [5, 6]. Traction bronchiectasis was a common finding in four fatal cases of acute eosinophilic pneumonia in a small cohort of 41 patients, relating its presence to poor clinical outcomes [7]. Uncontrolled respiratory symptoms in the presence of bronchiectasis and airway microbial colonisation were also observed in a patient with eosinophilic granulomatosis with polyangiitis despite immunosuppressive therapy [8].
Ongoing research throughout the last three decades has questioned whether neutrophilic inflammation is a hallmark of bronchiectasis and highlighted that eosinophils have also a key role in the pathogenesis of the disease. Subsequently, eosinophilic inflammation in bronchiectasis remains a wide-open field for future research with potential clinical importance, as this distinct disease endotype seems to reflect clinical outcomes, such as clinical manifestations, disease severity, and response to particular treatment modalities, and thus it plays a critical role in decision-making by the physician during the management of the condition in the context of precision medicine.
Evidence of eosinophilic activation in bronchiectasis was reported as early as 1998 by Kroegel et al. [9], who investigated patients with bronchiectasis unrelated to cystic fibrosis (CF) or bronchopulmonary aspergillosis and compared them with healthy individuals and patients with allergic bronchial asthma or chronic obstructive pulmonary disease (COPD). Patients with bronchiectasis did not show increased BEC, in contrast to patients with asthma, but showed increased serum levels of eosinophil cationic protein, further highlighting it as a more sensitive marker to measure local eosinophil involvement [9]. Also, the presence of bronchiectasis in severe asthma was found to be associated with airway eosinophilia, more extensive eosinophil degranulation, and higher mRNA expression of Charcot-Leyden crystal galectin, indicating higher eosinophilic activity in the coexistence of asthma and bronchiectasis [10].
In patients with bronchiectasis with or without chronic rhinosinusitis (CRS), sputum eosinophilia is significantly higher in the presence of CRS and is correlated with BEC, whereas methacholine tests are negative in the vast majority of the patients, identifying bronchiectasis with CRS as an eosinophilic airway disease and distinguishing it from asthma. Eosinophilic bronchiectasis with CRS also encompasses an altered interactome [11]. In bronchiectasis-COPD overlap syndrome, peripheral eosinophilia is associated with higher exacerbation and hospitalisation rates, whereas peripheral eosinopenia (BEC less than 100 cells/μL) is associated with higher infectious outcomes, including pneumonia episodes and chronic airway infection. Furthermore, treatment with inhaled corticosteroids (ICS) in bronchiectasis-COPD overlap syndrome patients with increased BEC leads to improvement of exacerbation rate without any negative impact on infectious events [12]. Figure 1 illustrates the highlight features of the coexistence of eosinophilic bronchiectasis with other respiratory diseases based on the evidence in previous studies.
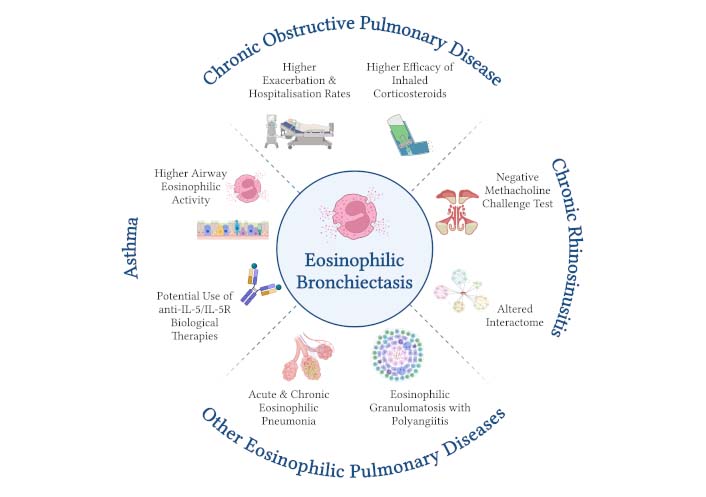
Eosinophilic bronchiectasis in association with other respiratory diseases. Eosinophilic bronchiectasis is commonly associated with other concurrent respiratory diseases, including upper airway, lower airway, and lung parenchymal diseases. The presence of bronchiectasis in severe asthma is associated with higher eosinophilic activity in the airways, while the presence of asthma in eosinophilic bronchiectasis offers the therapeutic option of biologics targeting the type 2 inflammatory pathway. The coexistence of eosinophilic bronchiectasis and chronic obstructive pulmonary disease encompasses higher exacerbation and hospitalisation rates and higher efficacy of inhaled corticosteroids compared to the absence of eosinophilia. Eosinophilic bronchiectasis with chronic rhinosinusitis is characterised by altered interactome and negative bronchoprovocation test, thus distinguishing it from asthma. The association of bronchiectasis with acute and chronic eosinophilic pneumonia and eosinophilic granulomatosis with polyangiitis has also been reported. Created in BioRender. Matthaiou, A. (2024) BioRender.com/r72s873
Nowadays, there is an emerging number of studies pointing towards the identification of eosinophilic bronchiectasis as a distinct disease endotype, demonstrating a constellation of specific clinical, laboratory, pulmonary functional, and chest imaging features, as well as response to specific treatment modalities and prognostic indices. However, although BEC has been associated with several clinical parameters, its baseline value significantly fluctuates in patients with bronchiectasis over time, indicating that its monitoring is crucial for its use in daily clinical practice [13].
A large unicentre retrospective analysis in China showed that eosinophilic bronchiectasis was associated with a stronger inflammatory response, a longer duration of hospital stay, and a higher hospitalisation cost [14]. Also, in a study of 169 patients with non-CF bronchiectasis hospitalised for acute exacerbation, those with eosinophilic bronchiectasis based on BEC demonstrated sinusitis, worse airway obstruction, sputum eosinophilia, higher serum immunoglobulin E, higher BSI (bronchiectasis severity index) and E-FACED (exacerbations, forced expiratory volume in one second, age, chronic colonisation, extension, and dyspnoea) scores, as well as higher hospitalisation cost [15].
Importantly, in another cross-sectional study, the authors defined type 2 inflammation in patients with bronchiectasis as the presence of either BEC ≥ 300 cells/µL or fractional exhaled nitric oxide (FeNO) ≥ 25 ppb. While 88 patients were consistent with this definition, only 19 had both markers increased based on the cutoffs. BSI scores were higher in patients with type 2 inflammation, irrespective of whether both markers or one of them were elevated [16]. On the other hand, a study of 164 patients with non-CF bronchiectasis investigating the utility of type 2 inflammatory markers, i.e. BEC and FeNO, revealed that the subgroup of 41 patients with low BEC and high FeNO demonstrated better pulmonary function, less involvement of lung lobes, lower dyspnoea prevalence, and less disease severity [17]. In another retrospective cohort study of 318 patients with bronchiectasis, the patients with BEC below 250 cells/μL were at increased risk for an exacerbation that led to hospitalisation [18]. However, these observations should be treated with caution by taking into consideration the small numbers of patients in each subgroup, the large range of FeNO levels (from 27 ppb to 43 ppb), and the relatively high BEC used as cutoffs.
In a large Spanish study of 928 patients with bronchiectasis subdivided into four groups based on BEC, i.e. eosinopenic (< 100 cells/μL), low BEC (51–100 cells/μL), normal BEC (101–300 cells/μL), and eosinophilic (> 300 cells/μL) bronchiectasis, BEC, and disease severity, defined by exacerbation and hospitalisation rates, showed a significant U-shaped behavior. More specifically, at the extremes of the curve, eosinopenic and eosinophilic patients presented higher levels of the severity indices, while the patients with low and normal BEC presented lower levels of the severity indices and thus milder disease. Interestingly, when comparing patients receiving ICS with all patients, a response to treatment was only present in eosinophilic patients leading to a decrease in exacerbation and hospitalisation rates. This observation indicates the importance of recognising this subgroup of patients and taking eosinophilia into consideration when deciding the therapeutic approach in a patient with bronchiectasis [19].
In the study by Shoemark et al. [20] the authors extracted data from different bronchiectasis cohorts from five countries to correlate the BEC with the sputum eosinophil counts, suggesting that BEC could be used as a surrogate of lung eosinophil counts in the same way as in asthma and COPD, as blood and sputum eosinophils seemed to correlate in two cohorts. Patients with high BEC tended to have Streptococcus- and Pseudomonas-predominated microbiome profiles. Also, moderately or highly elevated BEC was associated with more frequent exacerbations [20]. Furthermore, stratification of the data from the FRIENDS cohort based on BEC (< 100 cells/μL, 100–299 cells/μL, and > 300 cells/μL) was associated with sputum microbiome, with Haemophilus- and Moraxella-predominant microbiomes observed in patients with BEC < 100 cells/μL and Streptococcus-predominant microbiome in patients with BEC > 300 cells/μL, whereas Pseudomonas airway infection was found in both groups [21].
Findings of most studies collectively indicate that neither too low nor too high BEC is in favour of the patients with bronchiectasis, but a state characterised by normal BEC shows better disease outcomes. Thus, defining the distinct “eosinophilic bronchiectasis” endotype as well as recognising specific therapeutic options beneficial for these patients is of clinical relevance, as described further below. Figure 2 summarises the major differences between bronchiectasis with low- and high-eosinophilic inflammation.
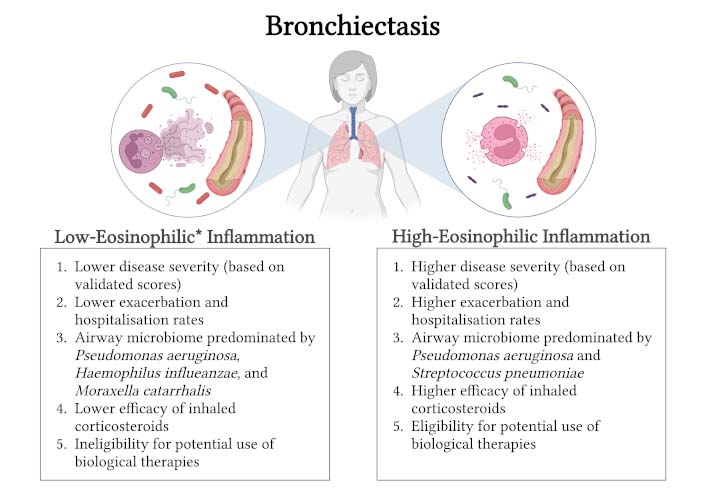
Major differences between bronchiectasis with low- and high-eosinophilic inflammation. Bronchiectasis encompassing high-eosinophilic inflammation is characterised by higher disease severity, higher exacerbation and hospitalisation rates, distinct airway microbiome, higher efficacy of inhaled corticosteroids, and eligibility for potential use of biological therapies targeting type 2 inflammatory mediators. * Bronchiectasis with extremely low blood eosinophil counts, i.e. eosinopenia, also demonstrates higher disease severity and higher exacerbation and hospitalisation rates. Neutrophils constitute the predominant immune cell population in bronchiectasis with low-eosinophilic inflammation and are known to contribute to the inflammatory process in several ways, including the formation of neutrophil extracellular traps. Created in BioRender. Matthaiou, A. (2024) BioRender.com/a70s161
A significant degree of discrepancy between the studies can be observed. This is attributed: i) to the limited available data due to the small number of relevant studies and small sample sizes, ii) the non-harmonised protocols, including the diverse cutoffs of type 2 inflammatory markers, such as BEC and FeNO, between the studies, and iii) the possible confounding factors, including comorbidities or other conditions, such as severe eosinophilic asthma (SEA) and obesity, lifestyle habits, such as smoking, and treatment modalities, such as corticosteroids, affecting the same markers. Large-scale multicenter studies with harmonised definitions and cutoffs of type 2 inflammatory markers are needed to draw robust conclusions.
Although current evidence is limited, the so-far experience regarding the response to different treatment modalities in bronchiectasis supports the use of ICS in patients with prominent eosinophilia. However, future clinical trials are needed to provide robust data on their clinical benefits, and they should be undertaken with caution to avoid potential adverse clinical outcomes, especially an increased risk of infections [22]. Martinez-Garcia [23–25] conducted a useful pooled post-hoc analysis of two randomised clinical trials (RCT) on the use of ICS in bronchiectasis, which failed to show adequate clinical benefit. However, the authors hypothesised that the subgroup of the eosinophilic endotype might respond differently than the neutrophilic endotype, and they succeeded in proving statistically significantly reduced exacerbations upon treatment with ICS in those demonstrating peripheral eosinophilia compared to eosinopenia [23–25]. Moreover, in a retrospective study of 140 patients with non-CF bronchiectasis, BEC of at least 3.5% was found to be the best predictor of the benefit of ICS in significantly reducing the exacerbation rate [26]. Finally, in a large study of 928 patients with bronchiectasis, a clinical benefit from the use of ICS in decreasing the rate and severity of exacerbations was only seen in eosinophilic bronchiectasis, i.e. with a BEC of > 300 cells/μL [19].
No targeted therapies are currently indicated for the management of bronchiectasis. However, novel biological drugs targeting the type 2 immune response hold a central role in the treatment of SEA, which is occasionally followed by the development of bronchiectasis. These include mepolizumab, a monoclonal antibody targeting interleukin 5 (IL-5), benralizumab and reslizumab targeting IL-5 receptor (IL-5R), and dupilumab targeting IL-4R. Several studies have shown that treatment with these agents can lead to clinical remission and fewer exacerbations per year, decreased use of oral corticosteroids (OCS), improvement in symptoms and pulmonary function, and better quality of life in SEA [27].
Previous experience with the use of biological therapies in the coexistence of bronchiectasis and SEA sheds light on their efficacy and safety in this condition and opens the path for the investigation of their potential benefit in eosinophilic bronchiectasis in the absence of asthma. A previous real-life retrospective study of patients with SEA treated with mepolizumab aimed to investigate whether the presence of bronchiectasis interferes with the effects of the treatment. They concluded that the efficacy of mepolizumab did not differ between patients with and without bronchiectasis, nor was it affected by the severity of bronchiectasis [28]. On the other hand, evidence regarding the use of benralizumab in the coexistence of SEA and bronchiectasis is contradictory. Presence of bronchiectasis in SEA constitutes a negative predictor for the effectiveness of anti-IL-5R therapy with benralizumab, as these patients show less sparing of OCS and improvement of pulmonary function [29]. However, another two different studies with concurrent SEA and bronchiectasis, i.e. a real-world study by Bendien et al. [27] including 97 patients with computed tomography-confirmed bronchiectasis and a case series by Oriano et al. [16] including four patients with idiopathic bronchiectasis and one patient with allergic bronchopulmonary aspergillosis, revealed that anti-IL-5/anti-IL-5R therapy with mepolizumab, benralizumab, and reslizumab is capable of reducing exacerbation rate and use of OCS, suggesting that comorbid bronchiectasis should not constitute an exclusion criterion for the initiation of these targeted treatment modalities [16, 27].
Biological therapy in eosinophilic bronchiectasis with or without overlap with other eosinophilic diseases, including asthma, asthma-COPD overlap syndrome, allergic bronchopulmonary aspergillosis, and eosinophilic granulomatosis with polyangiitis, was previously evaluated by Rademacher et al. [30] in a small cohort of 21 patients. Importantly, patients with primary ciliary dyskinesia-related and idiopathic eosinophilic bronchiectasis were also included in the study. The use of mepolizumab and benralizumab was initiated due to the presence of chronic symptoms refractory to the standard-of-care treatment. The results of these add-on therapies were promising, as they were well-tolerated and led to reduced exacerbations and improved symptoms, pulmonary function, and overall quality of life [30]. The available evidence from previous studies collectively supports the potential benefit of biological therapy in clinically significant eosinophilic bronchiectasis and points towards the necessity of an RCT for the assessment of the use of anti-eosinophil monoclonal antibodies in this endotype.
The way bronchiectasis is viewed by physicians has drastically changed over the last decades, with the topic of eosinophilia in the context of the disease being one of the substantially highlighted. Eosinophilic bronchiectasis is nowadays identified as a distinct disease endotype and is associated with a specific clinical course and response to treatment. Further research is needed to better characterise this endotype and improve its personalised investigation and management in daily clinical practice.
BEC: blood eosinophil counts
CF: cystic fibrosis
COPD: chronic obstructive pulmonary disease
CRS: chronic rhinosinusitis
FeNO: fractional exhaled nitric oxide
ICS: inhaled corticosteroids
IL-5: interleukin 5
IL-5R: interleukin 5 receptor
OCS: oral corticosteroids
SEA: severe eosinophilic asthma
AMΜ: Conceptualisation, Writing—original draft. NB: Writing—original draft. GH: Conceptualisation, Writing—review & editing. AL: Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported by a Grant from the Hellenic Thoracic Society. The Grant was awarded to A.M.M. as a Clinical Research Award from the Hellenic Thoracic Society in 2022. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Authors 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3122
Download: 72
Times Cited: 0
