Affiliation:
1Centre for Excellence in Molecular Biology and Regenerative Medicine, Department of Biochemistry, JSS Medical College, JSS Academy of Higher Education & Research, Mysore 570015, Karnataka, India
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0003-2236-4588
Affiliation:
2Cell Biology and Physiology, Translational Research Unit of Excellence CSIR, Indian Institute of Chemical Biology, Kolkata 700091, West Bengal, India
†These authors contributed equally to this work.
ORCID: https://orcid.org/0009-0003-2605-7806
Affiliation:
2Cell Biology and Physiology, Translational Research Unit of Excellence CSIR, Indian Institute of Chemical Biology, Kolkata 700091, West Bengal, India
ORCID: https://orcid.org/0000-0002-2547-1023
Affiliation:
1Centre for Excellence in Molecular Biology and Regenerative Medicine, Department of Biochemistry, JSS Medical College, JSS Academy of Higher Education & Research, Mysore 570015, Karnataka, India
ORCID: https://orcid.org/0000-0001-9167-9271
Affiliation:
1Centre for Excellence in Molecular Biology and Regenerative Medicine, Department of Biochemistry, JSS Medical College, JSS Academy of Higher Education & Research, Mysore 570015, Karnataka, India
ORCID: https://orcid.org/0000-0001-6050-1323
Affiliation:
3Department of Respiratory Medicine, JSS Medical College, JSS Academy of Higher Education & Research, Mysore 570015, Karnataka, India
Email: mahesh1971in@gmail.com; mahesh1971in@yahoo.com
ORCID: https://orcid.org/0000-0003-1632-5945
Explor Asthma Allergy. 2024;2:529–550 DOI: https://doi.org/10.37349/eaa.2024.00063
Received: June 18, 2024 Accepted: September 12, 2024 Published: October 22, 2024
Academic Editor: Ken Ohta, Japan Anti-Tuberculosis Association (JATA) Fukujuji Hospital, NHO Tokyo National Hospital, Japan
The article belongs to the special issue Molecules Involved in the Phenotypes of Asthma and/or Allergic Diseases
This review delves into the complex role of 12/15-lipoxygenase (12/15-LOX) in asthma pathogenesis, focusing on its contributions to mitochondrial dysfunction, oxidative stress, epithelial injury, and airway remodeling. We provide new insights into potential therapeutic strategies aimed at improving asthma management. Additionally, we examine the pro-inflammatory functions of interleukin-4 (IL-4) and its regulatory mechanisms that upregulate 12/15-LOX, leading to increased oxidative stress and airway remodeling. Key interventions such as vitamin E, esculetin, and baicalein are highlighted for their potential to inhibit 12/15-LOX activity, reduce oxidative stress, and restore mitochondrial function. Vitamin E suppresses IL-4 transcription, reducing 12/15-LOX expression and its inflammatory metabolites, while esculetin and baicalein directly inhibit 12/15-LOX, mitigating inflammation and oxidative damage. These antioxidants also promote mitochondrial biogenesis, protect mitochondrial DNA, and enhance respiratory efficiency, contributing to improved cellular metabolism and reduced apoptosis. This comprehensive approach emphasizes the therapeutic potential of targeting 12/15-LOX pathways to alleviate asthma symptoms and improve patient outcomes, paving the way for novel treatment strategies that significantly enhance asthma therapy.
Over the past decade, significant advancements have been made in understanding asthma’s complex biology. Progress in elucidating the intricate mechanisms underlying asthma inflammation has highlighted the heterogeneous nature of the disease, leading to the identification of numerous asthma phenotypes [1–3], each defined by distinct inflammatory pathways. Epithelial science has emerged as a pivotal frontier in asthma research, with biologists striving to delineate the essential role of the airway epithelium more precisely. However, numerous studies employing cellular, molecular, and animal models have revealed a plethora of interconnected cellular and immunological mechanisms underlying asthma pathogenesis [4–6]. These mechanisms prominently feature increased T helper 2 (Th2) cytokine levels in eosinophilic asthma and Th17 cytokine levels in refractory (neutrophilic) asthma, which recruit inflammatory immune cells to the airways [7, 8].
Despite these advancements, asthma remains a heterogeneous disease [9, 10], encompassing a diverse array of immune and structural cell types [11]. In the epithelial cells of asthmatic lungs, there is an increased synthesis of reactive oxygen species (ROS) associated with mitochondrial dysfunction [12–14]. The interplay between inflammatory cells, mitochondrial dysfunction, and ROS production culminates in epithelial damage, a common feature portraying conditions like asthma [13–16].
Recent advancements in exploring asthma pathogenesis have focused on the pivotal role of mitochondrial dysfunction [17–19]. This dysfunction exerts a profound influence on both bioenergetic metabolism and non-energetic pathogenesis [18]. The disruption of mitochondrial homeostasis results in cellular injury and apoptosis [20]. Key aspects of this dysfunction include the decreased activity of the crucial mitochondrial enzyme adenosine triphosphate (ATP) synthase [17], upregulated oxidative stress [17], initiation of apoptosis, and disrupted calcium homeostasis [17], all instrumental in driving pro-remodeling processes in the asthma-afflicted lung, such as fibrosis of the reticular basement membrane [17] thickening of the airway walls, damage to the epithelial cells, apoptosis of airway cells [17], enlargement and increased number of airway smooth muscle cells, overgrowth of mucus glands, and remodeling and regeneration of blood vessels [17, 21].
Studies have demonstrated an augmentation in Krebs cycle enzyme activity and a reduced reliance on glycolysis in the platelets of individuals diagnosed with asthma [22]. Riou et al. [16] reported that, in the setting of heightened oxidative stress, the oxidative capacity of platelets and peripheral blood mononuclear cells (PBMCs) is increased in individuals with asthma. Alongside these bioenergetic changes observed in platelets and PBMCs, patients have also displayed alterations in their mitochondrial DNA.
Oxidative stress is a significant factor contributing to the establishment and progression of asthma. During oxidative stress, ROS are released within the epithelium by activated inflammatory cells such as monocytes, macrophages, neutrophils, and eosinophils, as well as by resident cells like bronchial epithelial cells and airway smooth muscle cells. Exposure to antigens can potentially damage mitochondrial DNA, disrupt the Krebs cycle, and impair oxidative phosphorylation [13], since oxidative damage primarily occurs through the mitochondrial electron transport chain (ETC). Furthermore, asthma is associated with reduced levels of antioxidant defenses like glutathione, catalase, and superoxide dismutase (SOD) [23].
One of the main sources of superoxide anion (O2·–), a ROS, includes the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex, the mitochondrial ETC, and the xanthine oxidase system in the cytosol. O2·– enzymatically dismutates to hydrogen peroxide (H2O2) [23]. In biological systems, both O2·– and H2O2 interact with iron and other metal ions to form hydroxyl radicals (·OH) [24]. Inflammatory immune cells such as neutrophils, eosinophils, and monocytes possess peroxidases that facilitate the reaction between halides and H2O2, producing hypochlorous acid. Additionally, O2·– reacts with nitric oxide to form peroxynitrite, a potent reactive nitrogen species [24, 25], which nitrates tyrosine residues, potentially damaging functional and structural proteins and enzymes. Elevated numbers of eosinophils and enhanced release of eosinophil peroxidase (EPO) [26], along with protein oxidation through bromination [27] and subsequent tissue remodeling, result in increased 3-bromotyrosine levels in the bronchoalveolar lavage fluid (BALF) of asthmatic individuals compared to controls [28].
In asthma, membrane lipid peroxidation leads to isoprostane formation, biomarkers of oxidative stress [29]. The lipoxygenase (LOX) enzyme arachidonic acid 15-LOX (ALOX15) upregulated during mucociliary cell differentiation, converts arachidonic acid (AA) to 15-hydroxyeicosatetraenoic acid (15-HETE), a chemoattractant for eosinophils [30]. Interleukin-4 (IL-4) and IL-13 induce ALOX15 expression through Jak2 and Tyk2, promoting airway inflammation and remodeling [30, 31]. GWAS (genome-wide association studies) shows that the ALOX15 polymorphisms and epigenetic changes are linked to airway disease risk [30].
ALOX15 (human 15-LOX-1) exhibits both pro-inflammatory and anti-inflammatory roles. In asthma, it promotes inflammation, but studies show its anti-inflammatory functions in other diseases. For instance, Alox15 knockout mice experience skin issues and increased inflammation [32]. In colorectal cancer, ALOX15 expression is lost early, and its absence exacerbates carcinogenesis by disrupting key anti-inflammatory pathways [33]. This downregulation is also noted in cancers such as lung, breast, and pancreatic [34–36].
In asthma, mitochondrial dysfunction is closely linked to various metabolites, including polyunsaturated fatty acids (PUFAs) like AA, which is crucial in synthesizing eicosanoids and lipid mediators involved in asthma pathology [37]. Phospholipase A2 releases AA from cell membranes, which is then metabolized by cytochrome P450 monooxygenases, LOXs, and cyclooxygenases into different products, including hydroperoxides and prostaglandins [38]. Human ALOX15 (15-LOX) and mouse ALOX15 (12-LOX) convert AA into hydroperoxy derivatives and are involved in various inflammatory processes. Despite differing substrate specificities, they share substantial amino acid identity [39]. Studies reveal that activation of ALOX15 leads to mitochondrial breakdown and asthma symptoms, while its absence alleviates these symptoms [40]. Notably, IL-13 stimulates the production of 13-(S)-hydroxyoctadecadienoic acid [13-(S)-HODE] and 12-(S)-HETE by 12/15-LOX, leading to bronchial epithelial injury through oxidative stress and ROS production [40, 41]. In contrast, targeting 12/15-LOX with siRNA improves mitochondrial function and reduces epithelial injury in asthma models [40].
IL-4 exacerbates mitochondrial dysfunction in asthma by upregulating 12/15-LOX [20, 40]. This effect, mediated through signal transducer and activator of transcription-6 (STAT-6), leads to increased oxidative stress and damage to mitochondria [20, 42]. IL-4 monoclonal antibody treatment reduces oxidative damage and improves mitochondrial function [14]. IL-4 also influences asymmetric dimethylarginine (ADMA) metabolism, which is linked to oxidative stress and collagen deposition in asthma [43, 44]. Elevated ADMA levels contribute to nitrosative stress and enhanced oxidative responses in asthma [44] (Figure 1).
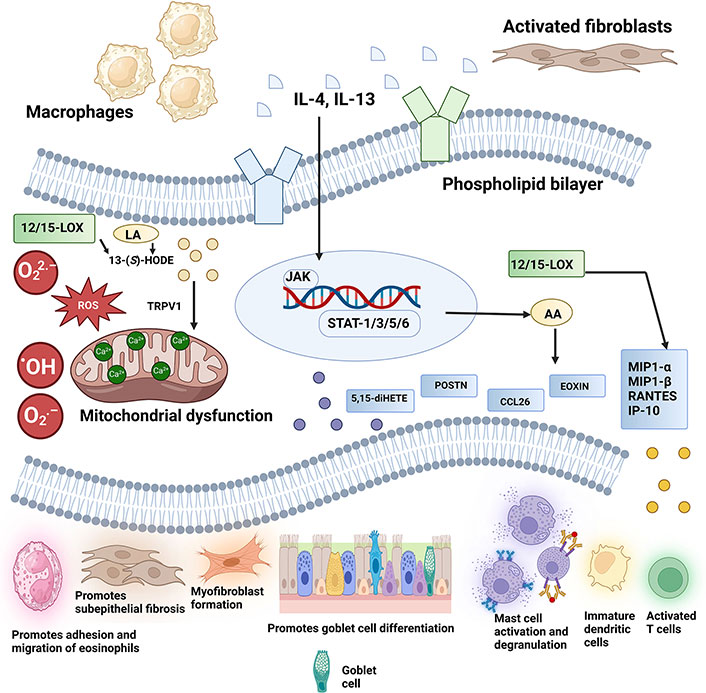
Scheme diagram to illustrate how 12/15-LOX induced airway inflammation and subsequent downstream signaling pathways lead to mitochondrial dysfunction by releasing metabolites such as 13-(S)-HODE. ·OH: hydroxyl radicals; 12/15-LOX: 12/15-lipoxygenase; 13-(S)-HODE: 13-(S)-hydroxyoctadecadienoic acid; 5,15-diHETE: 5(S),15(S)-dihydroxyeicosatetraenoic acid; AA: arachidonic acid; CCL26: chemokine ligand 26; EOXIN: 14,15-leukotrienes; IL-4: interleukin-4; IP-10: interferon-γ-inducible protein-10; JAK: Janus kinase; LA: linoleic acid; MIP: macrophage inflammatory protein; O2·–: superoxide anion; O22·–: peroxide anion; POSTN: periostin; RANTES: regulated upon activation, normal T cell expressed and secreted; ROS: reactive oxygen species; STAT-1: signal transducer and activator of transcription-1; TRPV1: transient receptor potential vanilloid-1. Created in BioRender. Baidya, A. (2024) BioRender.com/u37k639
IL-4 is central to asthma pathogenesis, as evidenced by elevated IL-4 levels in asthma patients, with plasma concentrations of 0.94 ± 0.05 pg/mL compared to 0.75 ± 0.03 pg/mL in healthy controls [45]. It drives inflammation by promoting the differentiation of Th0 cells into Th2 cells, facilitating eosinophilic asthma, and increasing immunoglobulin E (IgE)-mediated immune responses [46, 47]. IL-4 binds to IL-4 receptor α (IL-4Rα), activating signaling pathways involving STAT-6 and insulin receptor substrates, which enhance Th2 responses and mucus production, exacerbate airway obstruction, and induce eotaxin production [47]. It also prevents eosinophil apoptosis and promotes their migration through chemotaxis [47, 48]. Genetically, asthma is linked to the chromosomal region 5q31–33, which houses genes involved in Th2 cytokine synthesis, further implicating IL-4 in asthma pathogenesis [49, 50].
IL-4 significantly influences epithelial cell differentiation and upregulates transcripts like mucin 5AC (MUC5AC), periostin (POSTN), and inducible nitric oxide synthase (INOS) in stromal cells through IL-4Rα signaling, correlating with asthma severity [51, 52]. Dupilumab, an IL-4Rα monoclonal antibody, demonstrates the clinical impact of IL-4Rα-triggered pathways in severe Th2-high asthma [53, 54]. Historically, ALOX15 incubation has been shown to impair mitochondrial function [55, 56]. IL-4-induced peroxidation of lipids, including cardiolipin, disrupts mitochondrial membranes and respiratory enzyme activity [52]. IL-4 and IL-13 upregulate 15-LOX (ALOX15) in human cells and 12-LOX (ALOX15) in murine systems, with IL-4’s effect being STAT-6 dependent [42]. Thus far, the upregulation of human 15-LOX (15-LOX or ALOX15) by IL-4 and IL-13 has been observed solely in vitro [57–60]. In vivo studies with IL-4 transgenic mice show increased 12-LOX activity, linked to STAT-6 activity, suggesting that IL-4 stimulates LOX expression via STAT-6 [42]. This upregulation of LOX enzymes underlines their role in asthma pathogenesis by contributing to mitochondrial dysfunction and inflammation.
Mitochondria, vital for energy production and cellular functions, are integral to immune responses, cellular differentiation, and adaptation to hypoxic stress [61]. Dysfunctional mitochondria contribute to disease progression by increasing ROS, compromising bioenergetics, and impairing mitochondrial biogenesis and mitophagy, leading to epithelial vulnerability and inflammation [62]. In asthma, this dysfunction is marked by reduced ATP synthase activity, elevated oxidative stress, and disrupted calcium balance [62]. In asthmatic lungs, mitochondrial dysfunction manifests through several key disruptions: a decrease in the activity of complex IV of the mitochondrial ETC, an overload of calcium within the mitochondria, a reduction in mitochondrial membrane potential, and an increase in oxidative stress within the mitochondria [14, 40] (Figure 2).
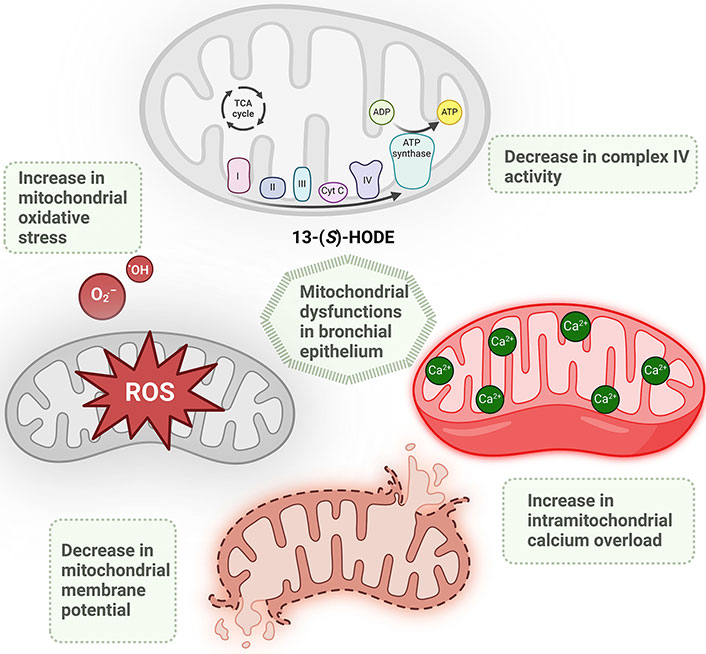
The schematic illustrates the mitochondrial dysfunctions linked to 12/15-lipoxygenase (12/15-LOX) metabolites, specifically 13-(S)-HODE. Mitochondrial dysfunction in asthmatic lung is associated with a reduction in the activity of complex IV of the mitochondrial electron transport chain (ETC), increased intramitochondrial calcium overload, decreased mitochondrial membrane potential, and heightened mitochondrial oxidative stress. ·OH: hydroxyl radicals; 13-(S)-HODE: 13-(S)-hydroxyoctadecadienoic acid; ATP: adenosine triphosphate; Cyt: cytochrome; O2·–: superoxide anion; ROS: reactive oxygen species; TCA: tricarboxylic acid. Created in BioRender. Baidya, A. (2024) BioRender.com/z57o609
The mitochondrial genome haplogroup “U” correlates with elevated IgE levels and allergic asthma [63]. Asthmatic airway epithelial cells often show mitochondrial swelling, disintegration, and oxidative damage, affecting airway smooth muscle and bronchial epithelium [64, 65]. These abnormalities impact airway contractility, cell proliferation, and calcium regulation, contributing to fibrosis, oxidative stress, and apoptosis [64–67].
Mitochondrial signaling includes anterograde (nuclear control of mitochondrial activity) and retrograde (mitochondria-induced nuclear transcriptional reprogramming) pathways [62–68]. Retrograde signaling involves ABI4- and WRKY-type transcription factors and is linked to ATP generation and cellular proliferation [62, 69]. Increased cytosolic Ca2+ enhances mitochondrial function and contributes to airway hyperresponsiveness (AHR) in asthma [66, 70]. Rowlands [66] and Agarwal et al. [71] emphasize the broader impact of mitochondrial dysfunction in asthma, highlighting the role of ubiquinol-cytochrome c reductase complex core protein 2 (UQCRC2) in maintaining mitochondrial function and its deficiency in exacerbating airway inflammation and hyperresponsiveness. Specialized therapies like mitochondrial transfer techniques including microinjection, gap junctions, and tunneling nanotubes (TNTs) are efficacious in alleviating respiratory damage. Mesenchymal stem cells (MSCs), known for their regenerative properties, can transfer mitochondria via TNTs to lung epithelial cells [72–74]. This process has shown promise in treating respiratory conditions like asthma and acute respiratory distress syndrome (ARDS) [72–74]. Studies indicate MSCs enhance cell viability and restore mitochondrial function in injured lung cells. Miro1, a GTPase, regulates this mitochondrial transfer, improving treatment efficacy in asthma models [72].
Mabalirajan et al. [40] showed that 12/15-LOX OE (overexpressed) mice demonstrated elevated caspase 3 activity, decreased cytochrome c oxidase (COX) activity, reduced activity of complex IV, lowered membrane potential of lung mitochondria, heightened lung cytosolic cytochrome c, and an increased number of apoptotic bronchial epithelial cells. These changes, along with mitochondrial architectural modifications like the disappearance of cristae and swollen epithelium of the bronchi, were associated with upregulated oxidative stress and inflammation, ultimately contributing to the pathogenesis of asthma in the 12/15-LOX OE mice [40] (Figure 3).
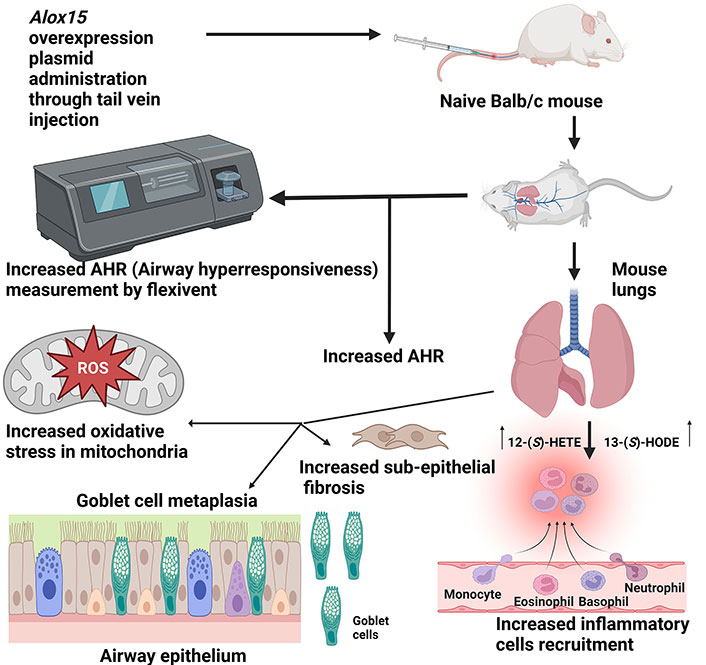
The schematic diagram depicts the impact of Alox15 overexpression in the lungs of Balb/c mice following intravenous administration of an Alox15 overexpression plasmid. Overexpression resulted in elevated levels of 12-(S)-HETE and 13-(S)-HODE in the mice, leading to several pathological changes: increased AHR, heightened oxidative stress in the mitochondria of bronchial epithelial cells, goblet cell metaplasia, increased sub-epithelial fibrosis, and augmented recruitment of inflammatory cells to the bronchovascular regions. 12-(S)-HETE: 12-(S)-hydroxyeicosatetraenoic acid; 13-(S)-HODE: 13-(S)-hydroxyoctadecadienoic acid; ROS: reactive oxygen species. Created in BioRender. Baidya, A. (2024) BioRender.com/m57a691
A promising therapeutic approach for asthma involves evaluating 12/15-LOX inhibitors as potential treatments. 12/15-LOX amplifies oxidative stress, leading to mitochondrial damage, which further exacerbates oxidative stress and cellular injury [75]. Studies have shown that OE of 12/15-LOX leads to mitochondrial dysfunction and bronchial epithelial injury in naive mice [40]. Conversely, knockout or knockdown of 12/15-LOX reduces airway epithelial damage in allergic mice, indicating that 12/15-LOX contributes to such injuries [40]. Notably, OE of 12/15-LOX results in bronchospasm even without allergen exposure, underscoring its role in promoting airway epithelial injury and asthma pathogenesis.
Given that 12/15-LOX induces oxidative stress, which exacerbates inflammatory responses and promotes AHR, antioxidants can play a crucial role in alleviating oxidative stress in asthma by neutralizing oxidants and minimizing lung damage [76]. Antioxidants help prevent radical formation, repair oxidative damage, and remove damaged molecules, thereby protecting lung tissues and improving respiratory health [77].
ROS in asthmatic airways originates from various sources, including environmental oxidants, inflammatory cell infiltration, metabolic disorders, and decreased cellular antioxidant levels [78]. Oxidative stress in the airway has been linked to worsening disease status, poor lung function, and epigenetic changes. Growing evidence suggests that antioxidant treatments, including vitamins (VITs) and dietary supplements, can alleviate oxidative stress, improve symptoms, and reduce asthma severity [79]. Recent studies provide evidence that natural antioxidants can protect asthma patients from attacks [80].
VIT-D has been extensively studied for its protective effects against asthma. It reduces oxidative stress and inflammation in the airways by enhancing the expression of antioxidant enzymes and modulating immune responses, which can help reduce asthma exacerbations and improve overall lung function. Glutathione, an antioxidant critical for maintaining redox balance in cells, is found in high concentrations in the lung’s airway surface liquid. Studies indicate that glutathione supplementation can reduce oxidative stress and improve asthma symptoms by restoring the balance between oxidants and antioxidants [81]. A study on the impact of diet on childhood asthma found that a higher intake of antioxidants such as VITs C and E, β-carotene, and selenium is associated with better asthma control and reduced risk of asthma exacerbations [82]. These antioxidants help mitigate oxidative damage and inflammation in the respiratory tract. These findings suggest that integrating natural antioxidants through diet or supplements could be a beneficial strategy for managing asthma.
Inhibiting 12/15-LOX shows promise as a strategy to alleviate inflammation and enhance asthma symptoms by targeting the production of lipid mediators [83]. Inhibitors of 12/15-LOX impede its enzymatic function, halting the conversion of AA into inflammatory lipid messengers like 12-HETE and 15-HETE. These inhibitors effectively reduce the production of lipid mediators that promote inflammation in the airways, potentially leading to decreased airway inflammation, which is a hallmark of asthma. This reduction in airway inflammation can translate into improvements in asthma symptoms.
However, the role of 12/15-LOX and its products in asthma is complex. ALOX15 orthologs have been implicated in the biosynthesis of both pro-inflammatory and anti-inflammatory mediators, such as lipoxins, resolvins, protectins, and maresins [84–87]. Specifically, 12- and 15-hydroperoxyeicosatetraenoic acid (15-HpETE) are metabolic intermediates involved in the biosynthesis of specialized pro-resolving mediators (SPMs), which play a crucial role in resolving inflammation and promoting tissue repair.
In bronchial asthma, the balance between pro-inflammatory and PMs is critical. SPMs, such as lipoxins and resolvins [84–90], have protective effects by reducing AHR, decreasing leukocyte infiltration, and promoting the resolution of inflammation. Therefore, inhibition of the ALOX15 pathway could potentially disrupt the production of these beneficial SPMs, leading to an exacerbation of asthmatic inflammation.
Given this dual role, the therapeutic targeting of 12/15-LOX in asthma must be approached with caution. While inhibiting the production of pro-inflammatory lipid mediators can reduce airway inflammation, it is essential to consider the potential impact on SPMs and the overall inflammatory balance. Further research is needed to fully understand the implications of 12/15-LOX inhibition in asthma and to develop strategies that selectively modulate its activity to maximize anti-inflammatory benefits while preserving or enhancing the production of PMs.
Preclinical studies have often assessed these improvements in asthma models treated with 12/15-LOX inhibitors, demonstrating their potential therapeutic benefits [83, 91]. However, more studies are required to see its impact on human asthma patients.
VIT-E acts as a protective antioxidant, while ALOX15 (12/15-LOX) contributes to inflammation and oxidative stress [92] (Table 1). IL-4 induces 12/15-LOX, promoting epithelial apoptosis and exacerbating asthma [92]. Notably, 12/15-LOX is predominantly found in bronchial epithelial cells and is implicated in mitochondrial degradation. VIT-E complexes inhibit the activity of 12/15-LOX [92].
Highlighting the potential functions of the three nutraceuticals: vitamin E (its derivatives), esculetin, and baicalein in mitigating asthmatic features
| Source/component | Derivatives | Potential functions/mechanisms in alleviating asthmatic features | References |
|---|---|---|---|
| Vitamin E | α-13’-Carboxychromanol and amplexichromanol |
| [93] |
| [93] | ||
| [93] | ||
| [93] | ||
| Vitamin E | α-Tocopherol |
| [94] |
| [92] | ||
| [92] | ||
| [92] | ||
| [92] | ||
| [95] | ||
| [96] | ||
| [96] | ||
| [97, 98] | ||
| [99] | ||
| [100] | ||
| [101] | ||
| Coumarin | Esculetin |
| [102] |
| [102] | ||
| [103] | ||
| [103] | ||
| [103] | ||
| [103] | ||
| [103] | ||
| [103] | ||
| Bioflavone | Baicalein |
| [104] |
| [104] | ||
| [105] | ||
| [91] | ||
| [91] | ||
| [105] | ||
| [106] | ||
| [106] | ||
| [106] |
12/15-LOX: 12/15-lipoxygenase; AP-1: activator protein 1; COXETC: cytochrome c oxidase of electron transport chain; IgE: immunoglobulin E; IL-4: interleukin-4; NF-κB: nuclear factor kappa B; NRF2: nuclear factor erythroid 2-related factor 2; OVA: ovalbumin; PDE4: phosphodiesterase 4; PKC: protein kinase C; ROS: reactive oxygen species; SOD: superoxide dismutase; TGF-β1: transforming growth factor-β1; Th2: T helper 2; TNF-α: tumour necrosis factor-α
Maternal intake of VIT-E during pregnancy has been consistently associated with a lower incidence of childhood asthma, inversely correlated with allergen sensitization and total serum IgE levels [107]. VIT-E supplementation reduces airway inflammation and hyperresponsiveness, although the exact mechanisms remain uncertain [92]. Antioxidant deficiency, particularly of VIT-E, leads to mitochondrial dysfunction in neurological and muscular diseases, including asthma [108]. VIT-E derivatives, such as tocopheryl succinate, show promise in combating diseases associated with mitochondrial-derived oxidative stress [4]. Notably, orally administered α-tocopherol predominantly localizes in mitochondrial fractions, with α-tocopherol being the most biologically significant form among natural VIT-E analogs, crucial for maintaining mitochondrial health [109].
Given these findings, it is hypothesized that VIT-E could alleviate mitochondrial dysfunction in asthma. α-Tocopherol’s effects on mitochondrial dysfunction were evaluated using a mouse model of asthma, which mirrors various aspects of human asthma pathology such as goblet cell metaplasia and sub-epithelial fibrosis [92] (Table 1).
VIT-E plays a crucial role in mitigating 12/15-LOX expression in asthma, significantly contributing to the alleviation of asthma symptoms [110]. VIT-E exerts its effects on asthma pathophysiology through multiple mechanisms. By suppressing the transcription of IL-4, VIT-E inhibits nuclear factor kappa B (NF-κB) binding to the IL-4 promoter [111]. Since IL-4 plays a pivotal role in activating 12/15-LOX, a reduction in IL-4 levels leads to decreased expression of 12/15-LOX [92] (Table 1). Furthermore, VIT-E directly diminishes the expression of 12/15-LOX, an enzyme responsible for metabolizing linoleic acid and AA into HODE and HETE, respectively [92].
However, the induction of ALOX15 expression by IL-4 in human monocytes requires IL-4 concentrations significantly higher than those typically found in the plasma of atopic patients. According to Marbach-Breitrück et al. [45], the effective induction of ALOX15 in human monocytes requires IL-4 concentrations at least 100-fold higher than the IL-4 plasma concentrations observed in atopic patients. This suggests that while IL-4 can activate 12/15-LOX, the IL-4 concentrations in the lungs of asthma patients may not be sufficiently high to induce significant ALOX15 expression. Additionally, IL-13 can also contribute to the activation of 12/15-LOX, as both IL-4 and IL-13 utilize the common receptor IL-4Rα [112–114]. The combined effects of IL-4 and IL-13 may have a more significant role in inducing 12/15-LOX expression [114], though this hypothesis requires further investigation. Thus, while IL-4 and IL-13 are pivotal activators of 12/15-LOX, their impact in the context of asthma might be limited by the actual concentration levels achievable in the lung environment. The therapeutic modulation of IL-4 and IL-13 levels or direct inhibition of 12/15-LOX by agents such as VIT-E could still offer benefits, but the effectiveness of these interventions must consider the realistic concentrations of these cytokines and the specific cellular contexts within the lung. By diminishing 12/15-LOX expression and its inflammatory metabolites, VIT-E aids in reducing AHR, inflammation, and remodeling, all hallmark features of asthma [85]. This includes a reduction in goblet cell metaplasia and sub-epithelial fibrosis [92]. VIT-E lowers levels of ovalbumin (OVA)-specific IgE, which are associated with allergic reactions in asthma. This reduction is partly due to decreased IL-4 levels, which stimulate IgE production [115]. IL-4 activates STAT-6, a signaling molecule that induces IgE synthesis and 12/15-LOX activation. VIT-E enhances the antioxidant defense system by decreasing lipid peroxidation and nitric oxide metabolites, which are markers of oxidative stress [116]. VIT-E plays a crucial role in mitigating structural changes in the asthmatic airway by reducing the levels of transforming growth factor-β1 (TGF-β1), a growth factor that causes airway remodeling and fibrosis [85]. Additionally, VIT-E helps alleviate the expression of 12/15-LOX and its inflammatory metabolites in asthma through various mechanisms. By inhibiting IL-4 and STAT-6, VIT-E reduces Th2 cytokine responses [92]. Moreover, it enhances antioxidant defenses, thereby decreasing oxidative stress and inflammation in the airways [92]. These combined effects contribute to the suppression of 12/15-LOX expression and its detrimental metabolites, aiding in the management of asthma symptoms and progression. These actions collectively help to reduce AHR, inflammation, and remodeling in asthma [92].
VIT-E plays a significant role in restoring mitochondrial function and attenuating asthma features through various mechanisms. VIT-E reduces lipid peroxidation and nitric oxide metabolites, which are key indicators of oxidative stress in asthma [116]. This reduction helps to protect mitochondrial integrity and function. By neutralizing oxygen and nitrogen free radicals, VIT-E prevents oxidative damage that impairs mitochondrial function [117]. VIT-E boosts the antioxidant defense system by replenishing levels of α-tocopherol and other enzymatic and non-enzymatic antioxidants, which are often depleted in asthmatic conditions due to repeated allergen exposures [92]. VIT-E helps restore COX activity, which is vital for maintaining efficient ATP production and overall mitochondrial function [92]. The reduction of lipid peroxidation and nitric oxide, both of which inhibit COX, further supports this restoration. VIT-E plays a crucial role in restoring the 3rd subunit of mitochondrial COXETC in airway epithelial cells, essential for the proper assembly and function of the ETC [92]. This restoration helps maintain mitochondrial integrity by reducing the release of cytochrome c into the lung cytosol, thus preventing apoptosis [92]. By lowering IL-4 levels and other Th2 cytokines such as IL-5, IL-13, and eotaxin, VIT-E diminishes inflammation, particularly eosinophilia, leading to a reduction in airway inflammation [92]. Moreover, VIT-E’s inhibition of 12/15-LOX and its metabolites, including HODE and HETE, further contributes to this anti-inflammatory effect [92]. This decrease in pro-inflammatory cytokines and oxidative stress might be attributable to a reduction in AHR, a characteristic feature of asthma [92]. Furthermore, VIT-E supplementation not only decreases mucus hypersecretion, airway fibrosis, and levels of TGF-β1—key factors in the structural changes observed in the airways of asthma patients but also reduces the Th2 response. This includes a reduction in IL-4, IL-5, IL-13, and OVA-specific IgE, along with a reduction in the expression and metabolites of 12/15-LOX in lung cytosol [92]. Therefore, while IL-4 and IL-13 are pivotal activators of 12/15-LOX, their impact in the context of asthma might be limited by the actual concentration levels achievable in the lung environment. The therapeutic modulation of IL-4 and IL-13 levels or direct inhibition of 12/15-LOX by agents such as VIT-E could still offer benefits, but the effectiveness of these interventions must consider the realistic concentrations of these cytokines and the specific cellular contexts within the lung. By reducing these remodeling features, VIT-E helps maintain normal airway structure and function. VIT-E reduces levels of OVA-specific IgE, which are associated with allergic responses in asthma [92, 118]. This reduction helps to mitigate allergic airway inflammation and hyperresponsiveness [92, 118]. VIT-E restores mitochondrial function by reducing oxidative stress, enhancing antioxidant defenses, and normalizing COX activity and mitochondrial protein regulation. These actions lead to the attenuation of asthma features, including reduced airway inflammation, hyperresponsiveness, and remodeling, as well as lower levels of OVA-specific IgE [92, 118].
Esculetin, a potent antioxidant derived from coumarin, demonstrates significant capabilities in reducing oxidative stress induced by linoleic acid [104]. It operates through multiple mechanisms, including the reduction of oxidative free radicals, scavenging of free radicals, and diminishing lipid peroxidation [104]. Notably, esculetin has shown promise in mitigating mitochondrial dysfunction [104]. Additionally, esculetin acts as a potent nonspecific inhibitor of 12/15-LOX, suggesting its potential in addressing asthma-related features [104]. To explore this hypothesis, researchers administered esculetin to asthmatic mice, evaluating its effects on key mitochondrial structural changes and functions [104].
Derived from various plants, esculetin exhibits broncho-dilating properties in vitro, particularly evident in carbachol-induced airway spasms. However, its clinical efficacy in treating asthma remains unexplored [104]. Furthermore, esculetin demonstrates immunomodulatory properties, potentially contributing to its diverse pharmacological effects [119, 120].
Esculetin directly inhibits 12/15-LOX, which is involved in the production of pro-inflammatory metabolites such as HODE and HETE [121]. By blocking 12/15-LOX activity, esculetin reduces the formation of these inflammatory mediators, thereby decreasing inflammation and oxidative stress associated with asthma. The inhibition of 12/15-LOX by esculetin leads to diminished levels of HODE and HETE, metabolites that play a critical role in promoting inflammation and further damage to structural cells of the lung [104].
Esculetin possesses antioxidant properties that mitigate oxidative stress [122], which is crucial for protecting mitochondrial function. By decreasing oxidative damage, esculetin helps maintain the integrity and efficiency of mitochondrial respiratory complexes. Esculetin also promotes mitochondrial biogenesis, the process by which new mitochondria are formed. This enhancement helps replace damaged mitochondria and improves overall mitochondrial function, leading to better cellular energy production and reduced apoptosis [123, 124].
Esculetin’s inhibition of 12/15-LOX activity leads to a reduction in inflammation and oxidative stress [104]. By inhibiting ALOX, a source of oxidative stress, esculetin not only reduces inflammation but also restores mitochondrial function [104]. These combined actions make esculetin a promising agent for mitigating conditions such as asthma, where both inflammation and mitochondrial dysfunction play critical roles [102]. In this context, the inhibition of 12/15-LOX is particularly significant as it addresses both inflammation and oxidative stress, which are central to asthma pathogenesis [102, 104]. These therapeutic benefits, coupled with its potential for use in combination therapies, make esculetin a promising candidate for improving asthma management and patient outcomes [102].
Baicalein, a compound found in the dry roots of “Scutellaria baicalensis” Georgi, is recognized for its diverse biological activities, including antiviral, antioxidant, and anticancer properties [125, 126]. Notably, its inhibition of 12/15-LOX makes it a promising candidate for asthma management. However, it is important to note that baicalein is not a specific ALOX15 inhibitor; it also inhibits other ALOX isoforms, including ALOX5 [127]. Since ALOX5 is responsible for the biosynthesis of leukotrienes, the beneficial effects of baicalein in asthma models might be attributed to its inhibition of ALOX5 also [127]. By targeting multiple ALOX isoforms, baicalein effectively reduces the production of pro-inflammatory metabolites such as HODEs and HETEs, thereby alleviating airway inflammation [91, 128]. Furthermore, baicalein modulates the immune response by decreasing Th2 cytokine levels, which are pivotal in asthma pathogenesis [91, 128].
Baicalein’s antioxidant properties are particularly noteworthy for scavenging ROS, which significantly contributes to mitochondrial damage in asthma [129, 130]. By protecting mitochondria from oxidative stress, baicalein preserves mitochondrial function and stimulates mitochondrial biogenesis via the peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) pathway [131]. This leads to increased ATP production, essential for maintaining cellular energy levels and supporting airway epithelial cell function [131]. Additionally, baicalein safeguards mitochondrial DNA, regulates mitochondrial dynamics, and maintains a healthy mitochondrial network, all of which are crucial for preventing apoptosis and preserving cellular integrity [131].
In conclusion, while baicalein demonstrates promise as a therapeutic agent for asthma through its multifaceted mechanisms targeting inflammation, oxidative stress, and mitochondrial dysfunction, its potential is not without controversy. The non-specific nature of its inhibitory activity and the broad range of its antioxidant effects could lead to adverse side effects if used frequently in clinical settings. Therefore, further clinical investigations are necessary to validate its efficacy, establish optimal treatment protocols, and thoroughly assess its safety profile in asthma management [104].
This review highlights the critical role of 12/15-LOX in asthma pathobiology and the therapeutic potential of targeting this enzyme. However, there are notable limitations to consider. The variability in 12/15-LOX expression and activity among different individuals and asthma phenotypes complicates the generalization of therapeutic strategies. Additionally, while preclinical studies provide valuable insights, the translation of these findings to clinical practice remains challenging due to differences in experimental models, human disease complexity, and pro- and anti-inflammatory properties of 12/15-LOX. The non-specific effects of some inhibitors, such as baicalein, which affects multiple LOX isoforms, further complicate the interpretation of their therapeutic efficacy. Despite these limitations, the strengths of this review lie in its comprehensive analysis of the mechanisms linking 12/15-LOX to mitochondrial dysfunction and oxidative stress in asthma. By integrating recent advancements in understanding the molecular pathways and highlighting promising therapeutic agents, this review provides a solid foundation for future research. The use of multiple antioxidants and their potential to address various aspects of asthma pathology represents a significant strength, offering a multifaceted approach to treatment.
Future research should focus on several key areas to advance the understanding and treatment of asthma. First, further clinical studies are needed to validate the efficacy and safety of 12/15-LOX inhibitors and antioxidants like esculetin, baicalein, and VIT-E in diverse asthma populations. Investigating the long-term effects and optimal dosing regimens will be crucial for translating these findings into effective therapies. Additionally, exploring the mechanisms by which these compounds interact with other signaling pathways and their impact on different asthma phenotypes will help tailor more personalized treatment strategies. Advances in genomic and proteomic technologies could provide deeper insights into individual variations in 12/15-LOX activity and response to treatment. Furthermore, the development of novel delivery systems for these therapeutic agents, including targeted delivery to the lungs, could enhance their effectiveness and minimize side effects. Finally, continued research into mitochondrial dysfunction in asthma could uncover new therapeutic targets and strategies, potentially leading to innovative treatments that address the underlying causes of the disease. By addressing these areas, future studies can build on the current knowledge and significantly improve asthma management and patient outcomes.
This review underscores the pivotal role of 12/15-LOX in the pathogenesis of asthma, emphasizing its significant contribution to mitochondrial dysfunction and oxidative stress. The interplay between 12/15-LOX and mitochondrial dysfunction is central to asthma pathobiology, with 12/15-LOX metabolizing PUFAs like AA into pro-inflammatory mediators such as HODE and HETE. This enzymatic activity exacerbates airway inflammation, epithelial damage, and mitochondrial dysfunction, leading to the exacerbation of asthma symptoms. Recent research highlights the therapeutic potential of targeting 12/15-LOX and its associated pathways. Compounds such as esculetin, baicalein, and VIT-E demonstrate promise in ameliorating the pathophysiological aspects of asthma. Esculetin, a potent antioxidant, not only inhibits 12/15-LOX but also improves mitochondrial function, offering a dual therapeutic approach. Baicalein, derived from “Scutellaria baicalensis”, targets multiple LOX isoforms and reduces inflammatory mediators while promoting mitochondrial biogenesis. VIT-E, known for its antioxidant properties, mitigates 12/15-LOX expression and its metabolites, improving mitochondrial function and reducing asthma symptoms. Despite the insights provided, limitations such as variability in 12/15-LOX activity among individuals, differences between preclinical models and human disease, and the non-specific effects of some inhibitors need to be addressed. Future research should focus on validating the efficacy and safety of these therapeutic agents, exploring their interactions with other signaling pathways, and developing novel delivery systems. Enhanced understanding and targeted therapies could lead to more effective asthma management and improved patient outcomes.
12/15-LOX: 12/15-lipoxygenase
13-(S)-HODE: 13-(S)-hydroxyoctadecadienoic acid
15-HETE: 15-hydroxyeicosatetraenoic acid
AA: arachidonic acid
AHR: airway hyperresponsiveness
ALOX15: arachidonic acid 15-lipoxygenase
ATP: adenosine triphosphate
COX: cytochrome c oxidase
ETC: electron transport chain
H2O2: hydrogen peroxide
IgE: immunoglobulin E
IL-4: interleukin-4
IL-4Rα: interleukin 4 receptor α
O2·–: superoxide anion
OE: overexpressed
OVA: ovalbumin
ROS: reactive oxygen species
SPMs: specialized pro-resolving mediators
STAT-6: signal transducer and activator of transcription-6
Th2: T helper 2
VITs: vitamins
MVG and AB equally contributed to: Writing—original draft, Writing—review & editing. UM: Validation, Conceptualization, Writing—original draft, Writing—review & editing, Supervision. SVM and RKT: Writing—review & editing, Supervision. PAM: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Padukudru Anand Mahesh who is the editorial board member of Exploration of Asthma & Allergy journal had no involvement in the decision-making or the review process of this manuscript. All the other authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This research was supported by CSIR [MLP137-MISSION LUNG] and DST-SERB [GAP-432]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2734
Download: 55
Times Cited: 0
Takahiro Matsuyama ... Hiromasa Inoue
