Affiliation:
1AlergiaMx, Allergy & Clinical Immunology, Benito Juarez, CDMX 03810, Mexico
ORCID: https://orcid.org/0000-0002-5310-7958
Affiliation:
1AlergiaMx, Allergy & Clinical Immunology, Benito Juarez, CDMX 03810, Mexico
2Facultad Mexicana de Medicina Universidad La Salle, Tlalpan, CDMX 14000, Mexico
3Hospital Infantil de Mexico Federico Gómez, Cuauhtémoc, CDMX 06720, Mexico
ORCID: https://orcid.org/0009-0002-3800-3066
Affiliation:
1AlergiaMx, Allergy & Clinical Immunology, Benito Juarez, CDMX 03810, Mexico
ORCID: https://orcid.org/0009-0008-7446-5923
Affiliation:
2Facultad Mexicana de Medicina Universidad La Salle, Tlalpan, CDMX 14000, Mexico
ORCID: https://orcid.org/0009-0004-9996-1537
Affiliation:
1AlergiaMx, Allergy & Clinical Immunology, Benito Juarez, CDMX 03810, Mexico
2Facultad Mexicana de Medicina Universidad La Salle, Tlalpan, CDMX 14000, Mexico
Email: dr.victorgonzalezu@gmail.com
ORCID: https://orcid.org/0000-0001-9053-7108
Explor Asthma Allergy. 2025;3:100966 DOI: https://doi.org/10.37349/eaa.2025.100966
Received: September 14, 2024 Accepted: November 29, 2024 Published: January 14, 2025
Academic Editor: Somasundaram Arumugam, National Institute of Pharmaceutical Education and Research, India
The article belongs to the special issue Atopic Dermatitis – Pathology and Treatment modalities
Atopic dermatitis (AD) is a prevalent chronic inflammatory skin disorder affecting millions worldwide, with significant variations in clinical presentation influenced by socioeconomic, racial, and environmental factors. This review explores the current understanding of AD pathophysiology, emphasizing immune dysregulation, epithelial barrier dysfunction, and the role of cytokines, particularly interleukin (IL)-4 and IL-13, in disease progression. Safety and efficacy concerns limit traditional corticosteroids, phototherapy, and systemic immunosuppressants, prompting interest in innovative therapies. New biologic agents, including monoclonal antibodies (mAbs) and Janus kinase (JAK) inhibitors (JAKis), target specific immune pathways, promising outcomes in moderate-to-severe AD cases. Biologics like dupilumab and emerging JAKis have shown substantial efficacy and safety in clinical trials, with notable reductions in inflammation and pruritus. However, these advancements present challenges, including hypersensitivity risks and the high costs of biologics, underscoring the need for further research on long-term safety and accessibility. The shift toward precision medicine in AD management marks a significant evolution, with future approaches likely to integrate targeted therapies alongside multidisciplinary care to enhance patient outcomes and quality of life (QoL).
Eczema, also known as atopic eczema or atopic dermatitis (AD), affects a considerable world population, with 101.27 million adults and 102.78 million children experiencing this condition. The overall prevalence rates were 2.0% [95% confidence interval (CI) 1.4–2.6] and 4.0% (95% CI 2.8–5.3) in adults and children, respectively.
Furthermore, this chronic inflammatory condition significantly burdens health services globally [1, 2]. Socioeconomic status (SES) influences the severity of AD and access to medical care, which are critical to managing the disease effectively. For families in low socioeconomic strata, the direct and indirect costs associated with AD, from health care expenses to days lost by caregivers, can exacerbate the socioeconomic challenges they face. Furthermore, disparities in access to health care, particularly in urban and disadvantaged areas, suggest a complex interaction between the severity of AD and available healthcare resources, underscoring the need for more inclusive healthcare strategies that can mitigate these disparities. Therefore, understanding the socioeconomic impact of AD is crucial for developing comprehensive management plans that extend beyond medical treatment to address the social determinants of health that affect patient outcomes [3].
Notably, the presentation of this disease is highly heterogeneous and sometimes unpredictable [4]. This condition manifests itself differently between racial and ethnic groups, which requires an individualized diagnostic and therapeutic approach. Typically, AD presents as erythematous and pruritic plaques. Still, the appearance of AD varies: Asian patients may present with more defined lesions and greater lichenification, whereas those of African descent often present with more extensor surfaces than flexor surfaces, with notable papules and periorbicular accentuation. In dark-skinned individuals, erythema may appear purplish or go unnoticed, complicating evaluations with standard severity scoring systems. In addition, postinflammatory pigment changes are more pronounced in dark skin. Recognizing these variations is crucial for accurate diagnosis and effective management of AD in diverse populations [5].
For a long time, it has been widely reported that, depending on the severity of this pathology, alterations are associated with different aspects of the quality of life (QoL), mental health, and labor productivity of patients, both in adult and pediatric populations [6–10]. In addition, the presence of AD is associated with other atopic comorbidities, such as asthma, allergic rhinitis, food allergies, and eosinophilic esophagitis. In addition, many other nonatopic comorbidities, such as psychiatric disorders (generalized anxiety disorders, depressive disorders, and suicidal ideations), infections, and cardiovascular diseases, are associated with AD (Figure 1) [11]. Although these comorbidities may be specific to pathology, lifestyle changes, particularly smoking, can affect the frequency of these comorbidities [12].
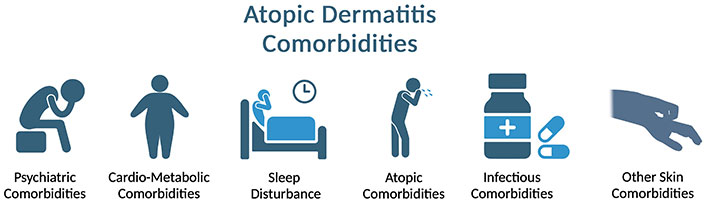
Comorbidities of atopic dermatitis. Created in BioRender. Gonzalez, V. (2024) https://BioRender.com/b47m963
The causes of AD are complex and multifactorial. For a long time, it was considered the first atopic manifestation, marking the beginning of the so-called “atopic march”, which eventually led to asthma and allergic rhinitis [13]. However, the current understanding highlights the mutation in filaggrin (FLG) as the genetic factor associated with the highest risk of AD. Despite this, the importance of variants that influence the skin and the immune system is recognized [14].
The pathogenesis of this disease is dependent on three main interrelated factors: immune dysregulation, dysfunction of the epithelial barrier, and pruritus.
Immune dysregulation in the acute phase is mediated by innate immune cells, such as T-helper cell type 2 (Th2) lymphocytes, type 2 innate lymphoid cells, dendritic cells, and basophils, which generate Th2-type cytokines [interleukins (ILs) 4, 5, 13, 31]. In addition, epidermal keratinocytes can amplify the Th2-type response, producing alarmines such as thymic stromal lymphopoietin (TSLP), IL-23, and IL-33.
In the chronic phase of the disease, in addition to the Th2-type response, Th1, Th22, and Th17 lymphocytes are involved in the release of IL-4, IL-13, and IL-22, which inhibit the expression of FLG, increasing the deterioration of the epithelial barrier and perpetuating the pathophysiology of the disease [15].
As mentioned above, dysfunction of the epithelial barrier has an immunological implication. Still, in the same way, it has been widely associated with genetic components, mainly mutations in the FLG gene. FLG is a protein produced by the epidermal keratinocytes of the upper layer and promotes the production of moisturizing factors and a lipid matrix. This protein acts as an adhesive, holding the keratinocytes of the horny layer together. The mutation in this gene has been described mostly in the white population, and it can have a highly variable presentation in 10–50% of patients with AD. A decrease in the lipid matrix leads to a decrease in the production of antimicrobial peptides, which changes the skin microbiome. This phenomenon is called dysbiosis and predisposes patients to complications associated with AD, such as impetigo and epidermal abscesses. Alterations in epidermal barrier function allow allergens to penetrate the skin, generate allergic sensitization, and perpetuate the clinical picture of patients [16].
Pruritus is one of the fundamental components that results in mechanical disruption of the epithelial membrane. This process is generated by the production of pruritogens released in the acute phase of the disease by the inflammatory cells involved. The main molecules involved are IL-4, IL-13, TSLP, and histamine. These bind to receptors on C-type sensory nerve fibers and Aδ nerve fibers in the dermis and epidermis, which perceive itching and pain [17]. Importantly, these effects are generated not only by the aforementioned inflammatory process but also by the scratching process. This results in hypersensitization of the nerve fibers secondary to the itch-scratch cycle. They act on IL-4Rα, expressed in peripheral nerves, transmitting chronic itching through the Janus kinase (JAK) 1 signaling pathway [16].
Recent epidemiological studies indicate substantial variation in the global prevalence of AD, influenced by factors such as age, sex, geographic location, and race. The global prevalence is approximately 2.0% in adults and 4.0% in children. Women are generally more affected than men, with prevalence rates of 2.8% in women (impacting an estimated 108.29 million) and 2.4% in men (95 million) [2, 18]. Notably, the incidence of AD in industrialized countries has doubled or tripled in recent decades, likely due to increased exposure to environmental pollutants [19].
A 12-month, international cross-sectional web-based survey by Barbarot et al. [20] conducted across regions in the Americas, Europe, and Asia reported that the prevalence of AD in these areas ranges from 2.1% to 4.9%. Similarly, Silverberg et al. [21], focusing on children aged 6 months to 18 years in regions including the Americas, Europe, the Middle East, and Eurasia, observed a prevalence range of 2.7% to 20.1%, reflecting significant regional variability.
SES plays a significant role in the prevalence and severity of AD. Individuals with lower SES often experience higher rates and more severe manifestations of AD. This increased burden is largely due to limited access to healthcare resources, exacerbating the condition by hindering timely diagnosis and effective management. In the United States, studies have shown that Black and Latinx populations have a greater prevalence and severity of AD compared to white populations. These disparities are influenced more by socioeconomic, environmental, and healthcare access factors rather than genetic predispositions [22].
Environmental exposures associated with lower SES contribute to the worsening of AD symptoms. Underserved communities are more likely to face increased exposure to air pollution, overcrowded living conditions, and inadequate housing—all factors that can exacerbate skin inflammation and barrier dysfunction characteristic of AD. Furthermore, barriers to healthcare access, including cost, lack of insurance, and limited availability of specialized services, are more pronounced in low SES groups. These barriers lead to underdiagnosis, delayed treatment initiation, and undertreatment of AD, perpetuating the cycle of disease severity and poor outcomes [23].
Social determinants of health, such as economic stability, education level, and neighborhood conditions, significantly impact the health outcomes of individuals with AD. For instance, lower educational attainment may limit understanding of disease management strategies, while economically disadvantaged neighborhoods may lack access to quality healthcare facilities. Addressing these social determinants is crucial for reducing health disparities and improving overall disease management in AD [24].
Efforts to mitigate these disparities should include developing inclusive healthcare strategies that provide equitable access to medical care, increasing community awareness about AD management, and implementing policies to reduce environmental exposures in underserved communities. By tackling the socioeconomic and environmental factors contributing to AD, it is possible to improve health outcomes and QoL for affected individuals across diverse populations.
In Europe, the financial impact of moderate-to-severe AD is substantial. A systematic review found that the total costs, including direct medical expenses and productivity losses, can reach up to €20,695 per person annually for adults with uncontrolled moderate-to-severe AD symptoms. Key contributors to these expenses include:
Direct medical costs: These expenses cover prescription medications, specialist visits, and other healthcare services.
Impact of biologics: Although effective in managing symptoms and improving outcomes, biologics are a major cost driver in AD treatment [25].
Biologic treatments for AD involve both direct and indirect costs, which can be considerable:
Direct costs: Expenses for prescription medications and specialist consultations range from €307 to €6,993 per year per patient.
Indirect costs: Productivity losses, especially for patients with uncontrolled disease, can represent up to 60.8% of the total economic burden [26].
In Germany, the contrast in costs between patients using biologics (€20,983 per year) and those who do not (€2,470 per year) underscores the financial weight of these therapies. Despite the high expense, biologics typically lead to better clinical outcomes and higher patient satisfaction [27].
In the United States, AD also carries a significant economic burden:
Out-of-pocket expenses: Patients report a median annual out-of-pocket cost of approximately $600, with 42% facing expenses that exceed $1,000, influenced by disease severity and flare frequency.
Polypharmacy and coverage gaps: Many patients use multiple prescription therapies, with nearly half reporting out-of-pocket expenses for medications not covered by insurance.
Indirect costs: Work absenteeism and reduced productivity add to the financial strain of AD management [28].
Dupilumab, a key biologic therapy for AD, contributes significantly to both direct and indirect costs:
Polypharmacy impact: Patients using multiple therapies often experience higher out-of-pocket expenses, with polypharmacy linked to more severe disease, poorer control, and frequent flares, all of which drive up healthcare costs [29].
Direct costs: Dupilumab incurs substantial expenses, with a projected lifetime cost of $509,600 per patient, including $267,800 specifically for the drug. However, it provides an additional 1.91 quality-adjusted life years (QALYs), leading to an incremental cost-effectiveness ratio (ICER) of $124,500, which is considered cost-effective, particularly for severe AD cases [30].
Dupilumab has demonstrated significant benefits in reducing productivity losses:
Work productivity: According to the Dutch BioDay Registry, work impairment due to AD decreased from 35.5% at baseline to 11.5% after 52 weeks of treatment, significantly lowering productivity losses.
Absenteeism and presenteeism: This reduction in work impairment is essential for mitigating the indirect costs of AD, such as missed workdays and decreased productivity during work hours [31].
In Europe, dupilumab has significant implications for AD management costs:
Direct cost justification: Despite its high price, dupilumab is cost-effective, particularly for severe AD cases, as it considerably improves QALYs [26].
Overall clinical benefits: Economic evaluations reveal that dupilumab not only incurs high costs but also delivers marked improvements in disease severity, pruritus reduction, and QoL, supporting its value as a therapeutic option for AD [32].
The clinical manifestations of AD vary depending on age, sex, race, and geographic location. The characteristic dermatological findings of AD include pruritus (itching), xerosis (dry skin), and eczema. Eczema in AD progresses through different stages—acute, subacute, or chronic—each with distinct clinical features and severity levels classified as mild, moderate, or severe [16].
Acute stage: Typically involves intense itching and red, inflamed skin.
Subacute stage: Presents with less intense inflammation and scaling of the skin.
Chronic stage: Features thickened skin due to prolonged scratching and rubbing.
For a better understanding of the morphological presentation of AD, it was classified into three phases according to the age of presentation and the topography of the lesions (Table 1) [33].
Classical classification of atopic dermatitis according to the age range and topography of the lesions
| Age range | Distribution or affected area |
|---|---|
| Infant phase (birth to 2 years old) | Eczematous lesions typically occur on the cheeks, forehead, scalp, and extensor surfaces |
| School phase (2 to 18 years old) | They occur on the flexor surfaces (antecubital fossae, popliteal fossae, neck, wrists, and ankles) |
| Adult phase (from 18 years old) | Lesions on flexor surfaces may persist, but they are classically associated with lesions in the head segment (the upper portion of the eyelids and lips) |
The diagnosis of AD is made primarily through clinical evaluation and specific diagnostic criteria, such as those developed by the American Academy of Dermatology (AAD) and other specialized organizations. An accurate assessment of disease activity and burden is essential, which is best measured via composite scores that assess both objective and subjective symptoms, such as SCORing Atopic Dermatitis (SCORAD) [34].
The diagnosis of AD remains primarily clinical, but with the increasing availability of new therapies, there is a need to update and consolidate current diagnostic criteria to ensure that they reflect recent developments in treatment. Recent sources highlight the importance of correct identification and management of AD, which includes not only attention to skin symptoms but also to comorbidities and complicating factors that can influence the course of the disease [35].
One stands out in the different clinical criteria we can currently use since it had been used previously; however, the following article highlights the sensitivity and specificity of the Hanifin and Rajka (HR) criteria compared with the others. This article discusses the variability and challenges associated with the diagnostic criteria for AD used in clinical trials. Notably, the HR criteria are the most commonly used criteria, appearing in 69.2% of the tests that specify diagnostic criteria. Other criteria, such as the UK’s refinement of the HR criteria, the Japanese Dermatological Association (JDA) criteria, and the AAD criteria, are also mentioned but are used less frequently. Variability in the diagnostic criteria, which may affect the comparability of the results of the trials and introduce biases in the inclusion of patients, the need to standardize the diagnostic criteria in the clinical trials of AD is emphasized to improve the interpretability and inclusiveness of the results, especially for racial and ethnic minorities. Vakharia et al. [36] suggested that initiatives such as the Harmonizing Outcome Measures for Eczema (HOME) group should work toward establishing an international consensus on the diagnostic criteria for AD.
In general, the HR criteria are currently the most important and widely used in clinical trials, but there is a clear need for a more standardized approach to improve the consistency and reliability of research results in AD [36].
Effective AD management must have a comprehensive and individualized approach that addresses the disease’s symptoms and underlying causes. Central to this approach is educating patients about factors that can trigger or exacerbate AD symptoms, such as environmental irritants, allergens, stress, and weather changes. By identifying and avoiding these triggers, patients can reduce the frequency and severity of flare-ups. Maintaining the integrity of the skin barrier is also crucial; regular use of emollients supports skin hydration and barrier function. Topical treatments, including corticosteroids and calcineurin inhibitors, are primary therapies for reducing inflammation and alleviating symptoms during acute episodes. In more severe or persistent cases, systemic immunosuppressants and emerging biologic therapies, such as monoclonal antibodies (mAbs) and JAK inhibitors (JAKis), offer promising alternatives. Although evidence varies, complementary and alternative medicine (CAM) therapies have also gained interest for their potential benefits. Patient education and self-care programs, like “eczema schools”, empower individuals and their families with knowledge and skills to manage AD effectively, improving QoL. This section explores the multifaceted strategies involved in treating AD, emphasizing the importance of a holistic approach integrating medical therapies with patient education and proactive skin care.
Patients must understand the factors that can trigger or aggravate AD symptoms. These include environmental irritants, allergens, stress, and weather changes. Educating patients in identifying and avoiding these factors can decrease the frequency and severity of outbreaks. In addition, maintaining the integrity of the skin barrier through the regular use of emollients is a central strategy in managing AD. Patients should be instructed in the daily application of emollients and the proper use of topical corticosteroids (TCS) during acute episodes to reduce inflammation [37]. The therapeutic approach for AD should be individualized based on the severity of the disease and the response before treatment. Options include topical treatments, such as corticosteroids and calcineurin inhibitors, and, in more severe cases. The emergence of new biological therapies offers significant promise for patients with moderate to severe AD who do not respond adequately to conventional treatments [38].
Patient education through structured programs such as “eczema schools” has proven effective in improving patients’ AD management and QoL. These programs provide patients and their families with the knowledge necessary to manage the disease more effectively and reduce the negative impact on their daily lives [34].
The updated guidelines for managing AD underscore the critical role of emollients as a foundational therapy for supporting skin hydration and maintaining the integrity of the skin barrier.
According to the European EuroGuiDerm guidelines, emollients are recommended as a primary component of AD management, with regular application at least twice daily to prevent flare-ups and mitigate symptoms. Emollients containing humectants, such as urea or glycerol, are advised for their ability to increase stratum corneum hydration. At the same time, occlusive agents like lipids or petrolatum are recommended to prevent moisture loss through evaporation [39].
The Japanese guidelines emphasize emollients as key agents for addressing barrier dysfunction, which is fundamental to AD management. These guidelines recommend emollients be applied regularly to maintain skin hydration, reduce irritation, and prevent flare-ups. Notably, they caution against the use of pure oil products like coconut or olive oil, as these can increase transepidermal water loss and potentially worsen the skin barrier. Instead, formulations with a balanced combination of humectants and occlusives are preferred to optimize skin hydration and barrier function. Overall, both the European and Japanese guidelines highlight the ongoing, supportive role of emollients in managing AD, emphasizing their continuous use to enhance barrier function and prevent disease progression [40].
The guidelines also introduce the concept of “emollients plus”, non-medicated products that include active ingredients such as flavonoids or bacterial lysates. These ingredients aim to enhance the therapeutic effects of standard emollients by offering additional anti-inflammatory or immune-modulating properties, further supporting the prevention of AD exacerbations [41].
CAM options for AD have garnered interest due to the chronic and often refractory nature of the disease. While the evidence supporting these therapies varies in quality and consistency, several options have shown some promise based on randomized controlled trials (RCTs) and systematic reviews.
Some of the examples of alternative medicine as a treatment for AD are:
Acupuncture and acupressure: Some level I evidence suggests that acupuncture and acupressure can reduce the signs and symptoms of AD. However, the studies are generally small and of limited quality, making it difficult to draw definitive conclusions [42].
Stress-reducing techniques: Techniques such as hypnosis, massage, and biofeedback have been explored. Massage therapy, in particular, may improve symptoms and reduce anxiety levels in patients and their parents. However, the evidence is limited and of low quality [42].
Balneotherapy: This involves bathing in mineral-rich waters and has shown some benefit in AD management. The therapeutic effects are thought to be due to the anti-inflammatory and moisturizing properties of the minerals [42].
Herbal preparations and botanical oils: Various herbal preparations, including Chinese herbal therapy, have been studied. While some studies suggest potential benefits, the results are conflicting, and there are significant safety concerns, such as hepatotoxicity. Botanical oils like coconut oil, sunflower oil, and evening primrose oil have also been explored, with some evidence supporting their use [43].
Vitamin supplementation: Vitamin D and topical vitamin B have shown some promise in small studies. However, the evidence is not robust enough to make strong clinical recommendations [44].
Probiotics and prebiotics: The use of probiotics and prebiotics for AD treatment has shown inconsistent results. Current guidelines do not recommend their routine use due to the lack of consistent evidence [44].
Dietary interventions: Food elimination diets based solely on allergy test results are not recommended. However, children with moderate to severe AD may benefit from food allergy evaluations if they have persistent AD despite optimized treatment or a reliable history of immediate reactions to specific foods [44].
Other therapies: Wet wraps and bleach baths are non-prescription treatments that have shown promise when used in conjunction with conventional treatments. These methods help maintain skin hydration and reduce bacterial colonization, respectively [45].
Although several CAM therapies show potential, the evidence is generally limited and of variable quality. Further research with larger, well-designed RCTs is needed to establish the efficacy and safety of these treatments in the management of AD.
In cases involving inflammation, TCS are advocated for their effectiveness in rapidly reducing inflammation and alleviating symptoms, working by suppressing the release of proinflammatory cytokines and modulating the immune response. Currently, 4–7 classes of topical steroids are considered, ranging from mild to super potent, and are the first anti-inflammatory options for AD.
The difference in absorption depending on the region of the body to be treated must be taken into account, since in areas such as the face, eyebrows, flexor regions, and scrotum, they present much more significant absorption than other parts of the body do. Thus, the potency of the same drug must be directed and calibrated depending on the area; therefore, powerful TCS can be used in chronic lesions located in the palmoplantar area and those of medium strength in acute treatments in areas such as the face and intertriginous areas. Importantly, there is a careful selection of topical steroids to optimize both acute and long-term use.
Although there is no consensus in the literature regarding the duration of this treatment owing to potential adverse effects (AEs) [46], systemic AEs, such as adrenal insufficiency, are associated with the use of high-power TCS, as well as local vasodilation and skin atrophy [47–50].
In addition, topical calcineurin inhibitors (TCIs), such as tacrolimus and pimecrolimus, act by inhibiting calcineurin to reduce inflammation and the immune response in the skin. For more persistent or severe cases, newer agents, such as phosphodiesterase-4 (PDE-4) inhibitors and JAKis, such as ruxolitinib, which block specific inflammatory pathways by interfering with signaling, have been introduced. JAKs block the PDE-4 enzyme, thus reducing the degradation of cyclic adenosine monophosphate (cAMP) and the release of inflammatory mediators [51].
Since 1890, phototherapy has been used as a medical treatment. It is recommended to use it when the previously mentioned or traditional treatment does not work adequately. It is recommended for patients with acute or chronic presentations of AD [52].
Solar or artificial radiation has a profound immunosuppressive effect in part through the apoptosis of activated T lymphocytes (Figure 2). This radiation can be classified into ultraviolet B (UVB) radiation, which emits wavelengths between 280 nm and 320 nm, and UVA radiation, which emits wavelengths between 320 nm and 400 nm; among these, the immunosuppressive effect plus depth is determined by the UVB [53]. However, in the observational study carried out by Steyn et al. [54], which determined that narrowband UVB phototherapy (NB-UVB) is the most commonly used method for adult and pediatric patients in much of Europe, such as the United Kingdom, there is currently a lack of various prescribing consensus for phototherapy in many aspects, so there is a great opportunity to develop standard and more systematic prescriptions in the future with high-quality RCTs.
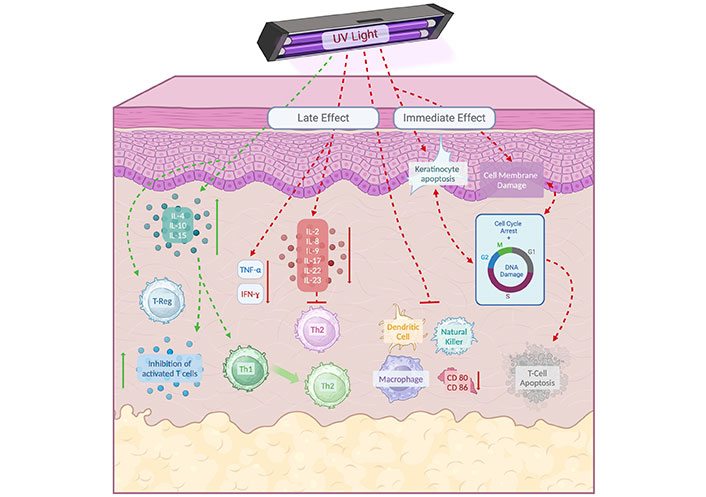
Immunosuppressive effect on T-CD4+ lymphocytes by ultraviolet (UV) radiation. The effects of UV radiation can be divided into immediate and late effects. The immediate effects include membrane and DNA damage, leading to cell growth arrest and apoptosis. UVB phototherapy can induce apoptosis in T-cells and keratinocytes, which is key to immune suppression and the amelioration of diseases such as psoriasis. UV radiation reduces the number of antigen-presenting cells, such as Langerhans cells, and alters the secretion of cytokines, decreasing inflammatory cytokines and promoting immunosuppressive cytokines, which contributes to reducing inflammation in the skin. IL-4: interleukin 4; Th2: T-helper cell type 2. Created in BioRender. Gonzalez, V. (2024) https://BioRender.com/p70v197
Oral immunosuppressants have been an affordable option for patients who do not respond adequately to topical therapies. Medications such as methotrexate, azathioprine, and mycophenolate act by modulating the immune response to reduce inflammation and itching, improving the QoL of patients [55].
Until recently, the only systemic treatments that have been used for the treatment of AD were broad-acting immunosuppressants, which are mentioned below [56].
Systemic corticosteroids: Steroid hormones that bind to the glucocorticoid receptor to increase the expression of anti-inflammatory proteins and suppress the expression of proinflammatory proteins.
Methotrexate: Folic acid antagonists that prevent cell division, DNA/RNA synthesis, and protein synthesis to suppress the activity of the immune system.
Azathioprine: A drug that converts to a 6-mercaptopurine antimetabolite. Its thioguanine nucleotides are believed to be incorporated into DNA and inhibit its synthesis.
Mycophenolate mofetil: A prodrug of mycophenolic acid that is responsible for depleting the guanosine nucleotides of T and B lymphocytes and thus inhibiting their proliferation; in addition, it inhibits the expression of adhesion molecules and the recruitment of lymphocytes and monocytes during inflammation.
Cyclosporine A (CsA) is an immunosuppressive agent effective in managing moderate-to-severe, refractory AD by inhibiting T-cell activation and cytokine production, essential processes in AD pathophysiology [57]. Clinical studies show that CsA significantly reduces AD severity and symptoms like pruritus and inflammation, with efficacy demonstrated across both high and low doses [58]. However, potential AEs, such as nephrotoxicity and hypertension, limit its use to short-term interventions with careful monitoring [59]. Additionally, CsA’s antipruritic effects may involve reducing epidermal nerve density and modulating immune responses, contributing to symptom relief [60]. CsA remains a valuable option for short-term AD management, particularly when rapid symptom control is required.
Other indication for systemic therapy occurs in patients who, in addition to being refractory to treatment with TCS, are dependent on high doses of this anti-inflammatory measure for a long time and with extensive skin extension; therefore, systemic therapy is indicated as a measure that reduces the amount of corticosteroids [56]. However, long-term use of these drugs is limited by their possible side effects and the need for constant monitoring. This is strongly associated with the risk of AEs, which require continuous medical monitoring [61].
Recent advances in the understanding and management of AD have identified numerous drug targets, marking a shift toward precision medicine in treating this complex disease. With over 70 new drugs currently in development, there is promising potential to address the significant gap in safe and effective options for moderate-to-severe AD, a challenge that persists despite decades of reliance on TCS, phototherapy, systemic immunosuppressants, and calcineurin inhibitors [62]. Current treatments are often limited by issues such as inconvenient application over large skin areas or concerns about long-term safety and efficacy, especially with topical immunosuppressants [63].
A pivotal discovery has been the role of cytokines, particularly IL-4 and IL-13, in AD pathogenesis. IL-4 is critical in activating the Th2 immune pathway, central to the disease’s inflammatory processes [64]. Targeting these pathways has led to the rise of biologics, therapeutic agents derived from living organisms using recombinant DNA technology, which are now transformative in managing immune-mediated and inflammatory disorders, including AD [65]. mAbs, a subset of biologics, have shown particular promise by selectively inhibiting specific molecular pathways involved in type 2 inflammation, a hallmark of AD, as well as other allergic diseases like asthma and chronic rhinosinusitis [66, 67]. Biologics not only provide a safer, steroid-sparing alternative but also show substantial efficacy in symptom control and disease management in AD [68]. Despite their advantages, challenges remain, as biologics can elicit hypersensitivity reactions or lose effectiveness over time due to immunogenic responses, including the development of antidrug antibodies [69, 70]. Nonetheless, the advent of biologics in AD treatment is a significant advancement, promising more precise, targeted, and enduring approaches for patients with moderate-to-severe disease.
Dupilumab is a recombinant immunoglobulin G subclass 4 (IgG4) mAb that targets the IL-4Rα to inhibit its signaling pathway in type 2 inflammation [71].
Clinical trials and efficacy. Paller et al. [72] conducted a randomized, double-blind, placebo-controlled (PC) phase III trial from November 2017 to September 2019 with 367 patients from 6 different countries in Europe and America between 6 and 11 years of age called LIBERTY AD PEDS to determine the efficacy and safety of dupilumab in children with severe AD. At sixteen weeks, patients treated with dupilumab + TCS had a significant improvement in the Investigator Global Assessment (IGA) score. With respect to AEs, no severe AEs were observed in patients treated with dupilumab, but the most common AE was conjunctivitis. A total of 20.6% of patients treated with 100 mg of dupilumab every 2 weeks developed conjunctivitis.
Simpson et al. [73] conducted a phase 3, randomized, double-blind trial in 251 adolescent patients between 12–18 years of age with uncontrolled moderate-severe AD who were treated with TCS, called LIBERTY AD ADOL, where the objective of the study was to determine the efficacy and safety of dupilumab. Compared with patients in the placebo group, those in the dupilumab group had a marked improvement in the Eczema Area and Severity Index (EASI) score (75%), IGA score (0–1 point), score ≥ 3- and ≥ 4-points on the numerical rating scale (NRS) of maximum pruritus, QoL, and improvement in sleep determined by the patient-oriented eczema measure (POEM). The dose of dupilumab administered every 2 weeks was greater than the dose of dupilumab administered every 4 weeks, and the 200 mg dose achieved better results than the 300 mg dose.
Safety profile. Regarding the AEs observed in the trial, no serious AEs associated with the use of the mAb were reported; however, the most common AE reported in the study was conjunctivitis.
Mechanism of action. It is a high-affinity monoclonal IgG4 antibody directed at IL-13. This mAb prevents the formation of the IL-4Rα-IL-13Rα1 signaling complex, thus blocking IL-13Rα1. In this way, IL-13 signaling is inhibited without interfering with IL-4 signaling [74].
Clinical trials. Silverberg et al. [75] conducted 2 identically designed, randomized, double-blind, PC phase III trials with 52-week durations, ADvocate1 and ADvocate2, to evaluate the efficacy and safety of lebrikizumab in adult and adolescent patients with moderate-severe AD. Patients aged 12–18 years were included in the group of adolescents, and patients older than 18 years were included in the group of adults. The patients had to have a diagnosis plus inadequate treatment for AD, with an EASI of at least 16–72% and an IGA of at least 3–4 points.
In both trials, the dose of 250 mg lebrikizumab every 2 weeks was superior to the placebo in improving the IGA score, achieving an EASI of 75%, and improving the score on the NRS of maximal pruritus. A 90% EASI was achieved in 38.3% of the lebrikizumab patients versus 9% of the placebo patients in the ADvocate1 trial. In the ADvocate2 trial, 30.7% of the lebrikizumab patients and 9.5% of the placebo patients managed to reach it.
Safety profile. The most frequently reported adverse reaction was conjunctivitis, which was observed in 129 patients out of 283 with lebrikizumab compared with 73 patients out of 141 with placebo in ADvocate1 patients and 150 patients out of 281 with lebrikizumab compared with 96 patients out of 145 with placebo in ADvocate2 patients. A comparison of different studies revealed that this AE is less common in mAbs that inhibit only IL-13 than in those that jointly inhibit both IL-4 and IL-13.
It is a high-affinity mAb that is responsible for blocking the IL-13 signaling pathway by binding to its respective receptor chains, IL-13Rα1 and IL-13Rα2 [76].
Clinical trials. Wollenberg et al. [76] conducted 2 randomized, double-blind, PC phase III trials called ECZTRA 1 and ECZTRA 2 in adults with moderate-severe AD for 52 weeks to determine their efficacy and safety in treating moderate-severe AD. Patients older than 18 years with a previous diagnosis of AD of one year or more of evolution with a poor response to systemic and topical treatment, an EASI ≥ 12%, an IGA score ≥ 3, and a score ≥ 4 on the rating scale were selected for numerical peak pruritus. Sixteen weeks after the initiation of tralokinumab, symptoms were reversed in both groups of patients treated at a dose of 300 mg every 2 weeks compared with those in the placebo group. An evaluation of the NRS of maximal pruritus revealed that 20% of the patients treated with tralokinumab versus 10.3% of the patients treated with placebo achieved a score of ≥ 4 points.
In the NRS evaluation of maximal pruritus, 25% of the tralokinumab patients versus 9.5% of the placebo patients had scores that decreased by ≥ 4 points. Regarding the safety of the drug, the most common AE in patients administered tralokinumab was mild-moderate-intensity conjunctivitis, which occurred in ≤ 10% of patients in the initial stage of treatment and in < 9% of patients in the maintenance stage.
Advancements in understanding the pathogenesis of AD have led to the development of targeted therapies, including biological agents and JAKis, which show promise, especially in refractory cases [38, 77]. The JAK-signal transducer and activator of transcription (STAT) pathway is pivotal in type 2 inflammation, mediating the effects of cytokines like IL-4, IL-5, IL-13, IL-22, IL-31, and TSLP on immune cells, keratinocytes, and peripheral sensory neurons, contributing to inflammation and pruritus [78, 79].
The JAK family comprises four kinases [JAK1, JAK2, JAK3, tyrosine kinase 2 (TYK2)], and the STAT family includes seven transcription factors (STAT1–6) [78, 79]. Upon cytokine binding, JAKs phosphorylate STATs, which dimerize and translocate to the nucleus to regulate gene transcription (Figure 3) [80]. JAKis prevent this phosphorylation, interrupting cytokine signaling pathways. They are categorized into first-generation (nonselective) inhibitors like tofacitinib and baricitinib, and second-generation (selective) inhibitors such as upadacitinib, abrocitinib, and ritlecitinib [81–83].
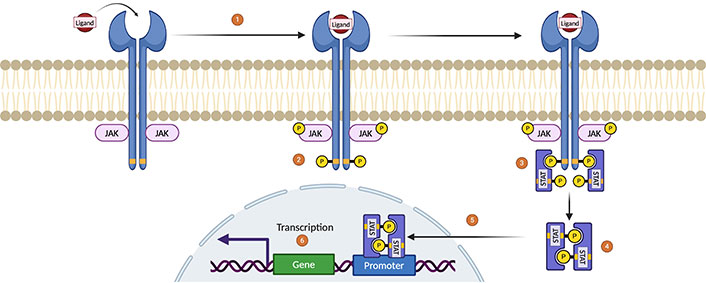
Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway. (1) As a first step, a cytokine binds to the receptor, which induces its dimerization. The activation of the JAK enzymes occurs after this dimerization of the receptor, where once the connection between the ligand and the receptor is made, transphosphorylation is induced by bringing 2 JAK enzymes closer to each other. (2) Activated JAKs induce tyrosine phosphorylation on cytokine receptor residues, thus creating anchor sites for the recruitment of second messengers such as the STAT family of proteins. (3) STAT proteins are phosphorylated by JAKs, (4) so STAT is released from the receptor to form homodimers or heterodimers through the interaction of the Src homology 2 (SH2)-phosphotyrosine domain. (5) Homodimers/heterodimers are mobilized toward the nucleus to (6) induce regulation and hematopoiesis by targeting promoter genes and regulating their transcription. Created in BioRender. Gonzalez, V. (2024) https://BioRender.com/u38q701
Clinical efficacy of JAKis. Wan et al. [84] conducted a network meta-analysis of phase IIb and III studies evaluating newer JAKis versus placebo in AD patients. All JAKis improved the IGA response and EASI. Upadacitinib 30 mg was significantly more effective than abrocitinib or baricitinib. Upadacitinib 15 mg was superior to lower doses of abrocitinib and baricitinib, except for abrocitinib 200 mg, which had similar benefits.
Paller et al. [85] analyzed adolescents in three phase III, randomized, double-blind, PC trials across more than 20 countries. Among 542 adolescents, up to 95% achieved a 75% improvement in EASI at week 16 with upadacitinib 15 mg and 30 mg compared to placebo. A significant proportion attained a Dermatology Life Quality Index (DLQI) of 0 or 1, and most had low scores on the Hospital Anxiety and Depression Scale. The most common AE was acne on the face and trunk; no serious AEs were reported. The study concluded that upadacitinib is effective, well-tolerated, and improves QoL in adolescents with AD.
Blauvelt et al. [86] conducted a phase IIIb study comparing upadacitinib and dupilumab in adults with moderate-to-severe AD over 24 weeks. A total of 348 patients received upadacitinib 30 mg daily, and 344 received dupilumab 300 mg every two weeks. Upadacitinib demonstrated greater efficacy in improving EASI scores, with more patients achieving EASI 75% and EASI 100%. The most common AE with upadacitinib was acne (55 patients versus 9 with dupilumab), whereas conjunctivitis was more frequent with dupilumab (29 patients versus 5 with upadacitinib). No severe adverse reactions were observed.
JAKis, particularly upadacitinib, have shown significant efficacy in improving clinical outcomes for patients with moderate-to-severe AD, offering a promising alternative to existing therapies. Their ability to target specific cytokine signaling pathways presents a valuable approach to managing refractory cases, with an acceptable safety profile demonstrated in clinical trials.
Delgocitinib is a pan-JAKi that targets JAK1, JAK2, JAK3, and TYK2, blocking multiple cytokine signaling pathways involved in the pathogenesis of AD [87].
Nakagawa et al. [88] conducted a multicenter, phase III study in Japan to evaluate the efficacy and safety of topical delgocitinib 0.5% ointment in patients aged 16 years or older with mild to severe AD, diagnosed according to the JDA criteria. Over 52 weeks, 352 patients with 5–30% body surface area (BSA) affected by inflammatory eczema applied approximately 4.8 g of delgocitinib daily. Evaluations every four weeks assessed signs and symptoms using the modified EASI (mEASI), IGA, and NRS for itching.
Safety results. Adverse events occurred in 77% of patients, predominantly mild (61.9%) or moderate (14.8%), with severe events being rare (0.3%). Treatment-related adverse events were reported in 19.6% of patients, leading to discontinuation in 4.5%; serious events occurred in 2%. Common adverse events included nasopharyngitis (28.7%), contact dermatitis (5.7%), and acne (4.8%). Application site reactions, such as folliculitis (3.1%), acne (2.8%), and irritation (2.6%), were infrequent and generally mild.
Compared with TCS, delgocitinib 0.5% ointment rapidly improved clinical signs and symptoms without causing skin atrophy or telangiectasia. Application site irritation was mild and occurred less frequently than with tacrolimus. The safety and efficacy profile of delgocitinib as a topical agent for the treatment of AD is favorable.
IL-31 is a cytokine directly linked to pruritus, the predominant symptom of AD [89]. Nemolizumab is a mAb targeting the IL-31Rα, aiming to alleviate pruritus in AD patients.
Clinical study. Kabashima et al. [90] conducted a 16-week, phase III randomized, double-blind, PC trial involving 215 patients with moderate-to-severe AD. Patients were randomized in a 2:1 ratio to receive subcutaneous nemolizumab 60 mg (n = 143) or placebo (n = 72) at week 16:
Pruritus reduction: Nemolizumab group showed a 42.8% decrease in the Visual Analog Scale (VAS) for pruritus, compared to a 21.4% reduction in the placebo group.
Eczema severity improvement: EASI scores improved by 45.9% with nemolizumab versus 33.2% with placebo.
Sleep quality: 55% of patients treated with nemolizumab achieved an Insomnia Severity Index (ISI) score of 7 or less (indicating minimal insomnia), compared to 21% in the placebo group.
Safety profile. Adverse events were reported in 71% of patients in both groups, predominantly mild to moderate. Severe drug-related adverse events occurred in three patients receiving nemolizumab, including Meniere’s disease, alopecia, peripheral edema, and worsening of AD. Worsening of AD was noted in 24% of the nemolizumab group and 21% of the placebo group.
Nemolizumab effectively reduced pruritus and improved sleep quality in patients with moderate-to-severe AD. However, the occurrence of AEs, including potential worsening of AD symptoms, suggests that further research is needed to fully assess the drug’s safety and efficacy profile.
Recent developments in understanding the pathogenesis of AD have catalyzed the introduction of biologic treatments and targeted therapies, providing promising options for patients with moderate-to-severe disease. These therapies specifically address type 2 inflammation pathways, which are central to AD pathophysiology, offering targeted efficacy with improved safety profiles.
Biologics like dupilumab, lebrikizumab, and tralokinumab are leading the shift toward precision medicine in AD management:
Dupilumab: This mAb targets the IL-4Rα chain and has shown substantial efficacy in both pediatric and adolescent populations, significantly improving IGA and EASI scores. Dupilumab’s safety profile is favorable, with conjunctivitis as the most common AE, though no severe complications have been reported.
Lebrikizumab and tralokinumab: These IL-13-targeted mAbs have also demonstrated efficacy in enhancing IGA and EASI scores among adults and adolescents. By selectively inhibiting IL-13 while sparing IL-4 pathways, these agents offer a tailored therapeutic approach, potentially reducing the risk of conjunctivitis seen with dual IL-4/IL-13 inhibitors.
JAKis, such as upadacitinib, represent a significant advancement for managing refractory cases of AD:
Upadacitinib: This JAKi disrupts key cytokine signaling pathways involved in inflammation and itching, leading to marked improvements in EASI scores and patient QoL. Clinical trials report an acceptable safety profile for upadacitinib, making it a potent option for challenging AD cases.
Topical JAKis (e.g., delgocitinib): Used as a topical agent, delgocitinib offers rapid symptom relief with fewer AEs compared to traditional TCS, presenting a valuable alternative for milder cases or adjunct therapy.
An antibody targeting the IL-31Rα, is specifically designed to address pruritus, a predominant symptom in AD:
Efficacy in pruritus and sleep quality: Clinical studies indicate that nemolizumab effectively reduces itching and improves sleep quality, enhancing the QoL for AD patients. However, some AEs, such as exacerbations of AD symptoms, require further research to fully validate its safety and therapeutic potential.
These targeted therapies represent a paradigm shift in AD treatment, moving towards a precision medicine approach that leverages specific immunological pathways to achieve better disease control and improve QoL. While biologics and JAKis offer significant therapeutic promise, ongoing research is essential to clarify their long-term safety profiles, refine dosing strategies, and identify patient populations that will benefit most.
The integration of these advanced therapies into clinical practice should be guided by a personalized approach, vigilant monitoring, and continuous assessment to maximize therapeutic outcomes while mitigating potential risks.
AD is a chronic inflammatory disease with a complex pathophysiology that involves epithelial barrier dysfunction, immune dysregulation, and pruritus, which contributes to its high degree of clinical heterogeneity. The global prevalence of AD continues to increase, particularly in industrialized countries, underscoring the need for a multidimensional approach to its management.
The development of new therapies, such as those mentioned above, especially in moderate to severe cases, not only offers significant improvements in clinical symptoms but also addresses associated comorbidities, improving the QoL of patients. However, challenges persist, such as the need to standardize diagnostic criteria and long-term safety assessment, as well as the accessibility of these emerging treatments.
The treatment of AD is in a transition phase toward precision medicine, where the identification of specific markers and the development of targeted therapies promise to personalize and optimize the management of this condition. The integration of new therapeutic strategies with a multidisciplinary approach, which also considers psychosocial factors and patient education, will be key to improving the results of each patient.
AD: atopic dermatitis
AEs: adverse effects
CAM: complementary and alternative medicine
CsA: cyclosporine A
EASI: Eczema Area and Severity Index
FLG: filaggrin
HR: Hanifin and Rajka
IGA: Investigator Global Assessment
IL: interleukin
JAK: Janus kinase
JAKis: Janus kinase inhibitors
mAbs: monoclonal antibodies
NRS: numerical rating scale
PC: placebo-controlled
QoL: quality of life
RCTs: randomized controlled trials
SES: socioeconomic status
STAT: signal transducer and activator of transcription
TCS: topical corticosteroids
Th2: T-helper cell type 2
TSLP: thymic stromal lymphopoietin
UVB: ultraviolet B
The authors would like to express their gratitude to AlergiaMx for providing all the necessary materials and the extensive knowledge that contributed to the development of this article.
VGU: Conceptualization, Methodology, Writing—original draft, Investigation. LAHZ: Data curation, Formal analysis, Visualization, Writing—review & editing. CAGN: Investigation, Resources, Data curation, Writing—review & editing. SNL and GMV: Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Gael Tchokomeni Siwe ... Stefan Barth
Serap Maden
Perpetua U. Ibekwe ... Bob A. Ukonu
Antara Baidya, Ulaganathan Mabalirajan
