Affiliation:
Stallergenes Greer, 92160 Antony, France
Email: laurent.mascarell@stallergenesgreer.com
ORCID: https://orcid.org/0000-0001-7199-0345
Explor Asthma Allergy. 2025;3:100968 DOI: https://doi.org/10.37349/eaa.2025.100968
Received: September 16, 2024 Accepted: November 22, 2024 Published: January 17, 2025
Academic Editor: Giovanni Paoletti, Humanitas University, Humanitas Research Hospital, Italy
In a previous study, we conducted an in vitro comparison of two sublingual allergy immunotherapy products, namely, house dust mite (HDM) Staloral 300 IR/mL produced by Stallergenes Greer, and HDM Osiris 300 IR/mL produced by ALK-Abelló. Although both products are labeled with the same unit, that is, the index of reactivity (IR), the definition of this unit is different depending on the product. This resulted in HDM Staloral 300 IR/mL displaying a higher total allergenic activity (TAA) and higher contents in major allergens and proteins. The aim of this study was to extend the comparison to cat sublingual extracts, that is, to cat Staloral 300 IR/mL from Stallergenes Greer and cat Osiris 300 IR/mL from ALK-Abelló. While both cat allergen extracts were qualitatively similar, in that they exhibited similar protein and allergen profiles, cat Staloral 300 IR/mL displayed a 2 times higher TAA than cat Osiris 300 IR/mL (according to an in-house method) and contained 1.6 time more proteins, 1.3 and 1.9 time more major allergens Fel d 1 and Fel d 4, respectively. No conclusions on clinical efficacy or safety can be drawn from these data.
Following house dust mites (HDM), cats are the most important indoor sources of airborne allergens [1]. Currently, eight cat allergens, labeled Fel d 1 through 8, are officially recognized (see www.allergen.org), among which Fel d 1 and Fel d 4 are major allergens [2].
Allergen immunotherapy (AIT), the only known treatment capable of altering the course of allergic conditions, involves the administration of either increasing or fixed doses of allergens, mainly through the subcutaneous or sublingual route [3]. In Europe, the potency of AIT products is generally expressed using different unit names, depending on the manufacturer. However, different manufacturers may use the same unit name with yet different definitions of the unit. This is the case for the index of reactivity (IR), used by both ALK-Abelló in France and Stallergenes Greer worldwide (a unique case in Europe, to our knowledge). As described elsewhere [4], for Stallergenes Greer, 100 IR/mL corresponds by definition to a concentration that yields a 7-mm wheal diameter on skin prick testing in allergic patients, whereas ALK-Abelló’s 100 IR/mL relates to a 6-mm wheal diameter, the considered diameter being a geometric mean in both cases. In this context, we previously conducted a comparative characterization of two 300 IR/mL sublingual HDM extracts, one from Stallergenes Greer, and the other from ALK-Abelló. The former exhibited a 2.4-fold higher total allergenic activity (TAA) than the latter, along with 1.8 time more protein and 1.5 to 3 times more major allergens (Der p 1, Der f 1, group 2 allergens, and Der p 23) [5].
The objective of this study was to expand this comparison to cat sublingual extracts.
Three batches of Staloral “Chat” 300 IR/mL and three batches of Osiris “Chat (phanères)” 300 IR/mL were analyzed. All six batches were manufactured from cat dander.
TAA was measured through IgE binding inhibition in ELISA, in comparison to an in-house reference extract, as described elsewhere [5]. Proteins were quantified by the Kjeldahl method. Fel d 1 and Fel d 4 were quantified by ELISA. Protein profiles were obtained using SDS-PAGE, and allergen profiles by western blotting and LC-MS/MS. Supplementary materials and Table S1 contain additional methodological details.
Cat Staloral 300 IR/mL displayed a TAA that was 2 times higher than that of cat Osiris 300 IR/mL (Figure 1A and Table S2). In addition, cat Staloral 300 IR/mL contained 1.3 and 1.9 time more major allergens Fel d 1 and Fel d 4, respectively, as well as 1.6 more protein and a 1.3-fold higher specific activity, the difference being statistically significant for TAA, Fel d 1, and protein contents (Figure 1B–D and Table S2).
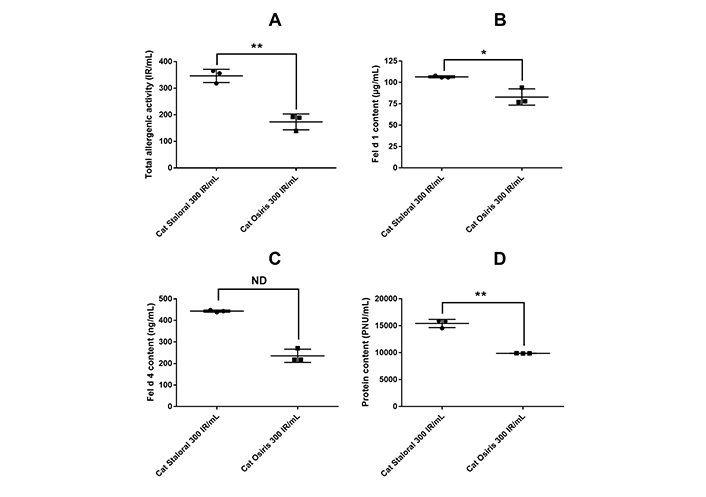
Comparison of cat Staloral and Osiris 300 IR/mL according to different quantitative parameters. Total allergenic activity (TAA) (A), and contents in Fel d 1 (B), Fel d 4 (C), and protein (D) in three batches of cat Staloral 300 IR/mL and three batches of cat Osiris 300 IR/mL. Bars represent the means ± SD; *: p < 0.05; **: p < 0.01. ND: not determined (see Table S2)
According to the protein and allergen profiles (Figure 2A–C and Table 1), the two products are qualitatively similar. Notably, Fel d 1 and Fel d 4 derived peptides, as well as peptides derived from Fel d 2, Fel d 3, and Fel d 7, were found with extremely high global allergen identification scores in all batches tested, whereas Fel d 8 was detected in none of them (Table 1).
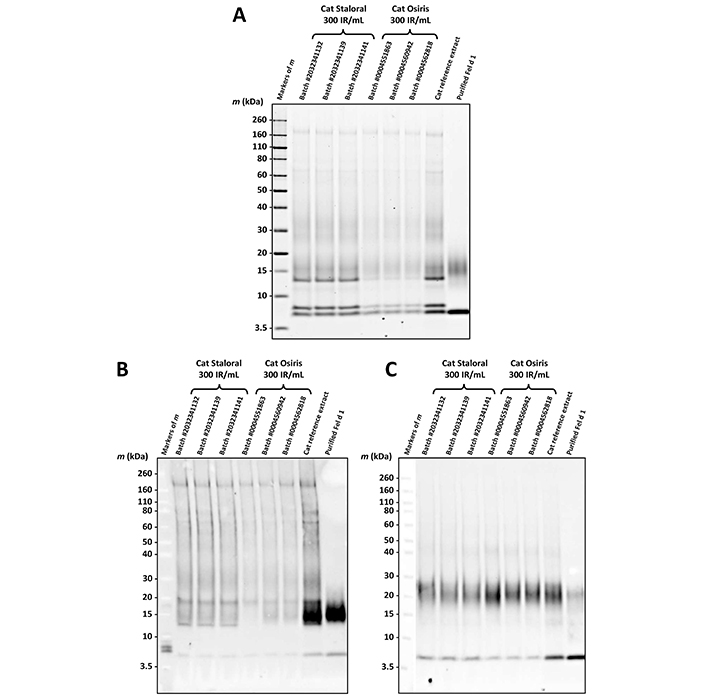
Comparison of cat Staloral and Osiris 300 IR/mL according to different qualitative parameters. Protein profiles (A) and allergen profiles after detection using a pool of sera from cat-sensitized patients (B) or mAb of clone number 6F9 to Fel d 1 (C), of three batches of cat Staloral 300 IR/mL and three batches of cat Osiris 300 IR/mL
LC-MS/MS allergen profiles of three batches of cat Staloral 300 IR/mL and three batches of cat Osiris 300 IR/mL
| Allergen | Identification score [–10 × log10(p-value)]* | |||||
|---|---|---|---|---|---|---|
| Cat Staloral 300 IR/mL | Cat Osiris 300 IR/mL | |||||
| Batch #2032341132 | Batch #2032341139 | Batch #2032341141 | Batch #0004551863 | Batch #0004560942 | Batch #0004562818 | |
| Fel d 1 |  |  |  |  |  |  |
| Fel d 2 |  |  |  |  |  |  |
| Fel d 3 |  | 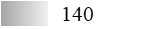 | 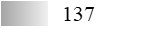 | 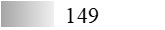 | 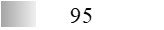 | 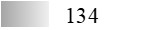 |
| Fel d 4 | 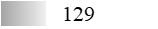 | 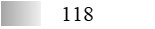 | 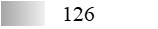 | 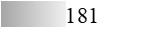 | 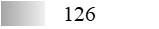 | 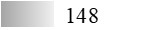 |
| Fel d 7 | 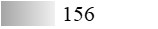 | 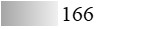 | 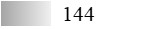 | 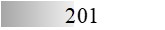 | 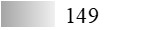 |  |
| Fel d 8 | Not applicable** | |||||
* This score does not reflect the allergen amount; ** See main text
The two main outcomes of this study are the same as those of our previous study comparing Staloral and Osiris sublingual AIT products [5].
On the one hand, cat Staloral and Osiris 300 IR/mL display similar protein and allergen profiles (Figure 2 and Table 1). Especially, they both contain the allergens Fel d 1 through Fel d 4, as well as Fel d 7 (Table 1). The fact that Fel d 8 was not detected in the tested batches suggests the absence of cat salivary components, insofar as this allergen has been identified from cat submandibular salivary gland [6].
On the other hand, the two products differ quantitatively, especially in terms of TAA (Figure 1A and Table S2). As previously, this difference is consistent with their labelings in IR/mL being based on two different definitions of IR. Since anti-Fel d 1 IgE levels correlate strongly with IgE levels to a whole cat extract [7], the observation that cat Staloral and Osiris 300 IR/mL differ quantitatively in terms of Fel d 1 concentrations was also expected (Figure 1B and Table S2). In addition, the two products differ quantitatively in terms of concentrations of Fel d 4, the other major cat allergen (Figure 1C and Table S2).
The presence in cat AIT products not only of Fel d 1 but also of other cat allergens, especially Fel d 4, is expected to play an important role in the observed efficacy of AIT [8]. This might explain why therapeutic approaches focusing only on Fel d 1, whether relying on peptides or mAbs, ultimately proved unsuccessful [9, 10].
In summary, while cat Staloral and Osiris 300 IR/mL appear to be qualitatively similar products, they differ quantitatively in terms of allergenic activity and major allergen concentrations. That said, no conclusions on clinical efficacy or safety can be drawn from these data.
AIT: allergen immunotherapy
HDM: house dust mite
IR: index of reactivity
TAA: total allergenic activity
The supplementary materials and tables for this article are available at: https://www.explorationpub.com/uploads/Article/file/100968_sup_1.pdf.
TB and LM: Conceptualization, Writing—original draft. SD, KJ, and DB: Formal analysis. CP and SV: Supervision. HC and CD: Supervision, Writing—review & editing. MB: Formal analysis, Writing—review & editing.
All authors are employees of Stallergenes Greer, Antony, France.
Individual sera constituting the pool, used as a reagent, come from blood donation at the French Blood Establishment (EFS) holding an Ethics and Professional Conduct Committee as per Decision No. 2019-05.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The data of this manuscript could be available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
