Affiliation:
1Department of Allied Health Sciences, De Montfort University, LE1 9BH Leicester, UK
2Enderby Medical Centre, LE19 4LY Leicester, UK
Email: Samantha.K.Harrison@dmu.ac.uk
ORCID: https://orcid.org/0009-0009-2354-7854
Affiliation:
1Department of Allied Health Sciences, De Montfort University, LE1 9BH Leicester, UK
2Enderby Medical Centre, LE19 4LY Leicester, UK
ORCID: https://orcid.org/0000-0002-7218-3278
Explor Asthma Allergy. 2025;3:100969 DOI: https://doi.org/10.37349/eaa.2025.100969
Received: November 06, 2024 Accepted: December 20, 2024 Published: January 20, 2025
Academic Editor: Carlo Lombardi, Fondazione Poliambulanza Istituto Ospedaliero, Italy
Aim: Short-acting β2-agonist (SABA) overuse adversely affects asthma-related outcomes and the environment. The latest Global Initiative for Asthma (GINA) report no longer recommends SABA-only therapy. Since 2020, we have implemented an inhaled corticosteroid (ICS)-containing reliever strategy for our moderately severe asthmatics within our practice population. We only administered budesonide/formoterol (BUD/FORM) via a single device maintenance and reliever therapy (MART) across this cohort and eliminated the use of SABA therapy.
Methods: Our asthma registry revealed 195 patients in the cohort. All patients were invited for assessment and 101 patients agreed to this new strategy [MART + simultaneous anti-inflammatory reliever (AIR)]. The remaining 94 patients continued with MART and SABA inhalers. Both groups were followed up for 24 months.
Results: There were no deaths in either group. Asthma-related hospitalizations fell by 91.5% in the SABA-free group (p = 0.003). Exacerbations requiring oral corticosteroids (OCS) fell by 82.1% in the SABA-free group over the 24-month period (p = 0.041). At the end of 24 months, 97.5% of patients in the SABA-free group remained SABA-free, with an average of 4.9 cannisters of BUD/FORM prescribed per year, compared with 7.68 cannisters in the MART/SABA groups (p = 0.00347).
Conclusions: This data provides real world evidence that the use of MART/AIR with BUD/FORM simultaneously with the elimination of SABA is safe and effective for moderate asthmatics within primary care.
Asthma is a chronic respiratory condition associated with airway inflammation and bronchial hyper-reactivity [1]. Airway inflammation occurs as a result of mucus plugging and bronchoconstriction due to mast cell degranulation and histamine release in response to allergen exposure [2].
Asthma is the most common lung disease, with over 12% of the United Kingdom (UK) population receiving an asthma diagnosis [3]. In 2019, asthma affected an estimated 262 million people and caused 455,000 deaths [4]. These deaths are largely due to exacerbations. When they are not life-threatening, they cause significant morbidity, increase healthcare costs, and in some patients cause a progressive loss in lung function [5]. Key outcomes such as hospital admissions and mortality have not really improved in the past 10–15 years despite escalating pharmacological costs both financially and environmentally [6].
Historically, asthma has been managed with a stepwise approach which involves varying doses of inhaled corticosteroids (ICS) depending on the severity of symptoms with a short-acting β2-agonist (SABA) as required across all stages [7]. However, studies have shown that overuse of SABA is associated with an increase in the frequency of asthma exacerbations, airway inflammation, and mortality [1, 8, 9]. Furthermore, SABA has been shown to have a high carbon footprint contributing to global warming, with a 100-dose Ventolin inhaler amounting to 28 kg of carbon dioxide [10]. Hence, the latest Global Initiative for Asthma (GINA) report no longer recommends SABA-only therapy [1].
The use of a single inhaler containing ICS and formoterol (FORM) for both maintenance and quick relief therapy maintenance and reliever therapy (MART) is recommended by GINA in steps 3–5 [1]. ICS-FORM provides quick relief for asthma symptoms similar to that of SABA while reducing the risk of severe asthma exacerbations, at an overall lower ICS exposure. Given well known adverse effects of SABA overuse on asthma outcomes and the environment, and the update in GINA guidelines, it is important to determine whether MART therapy with the elimination of SABA is effective for controlling asthmatics.
During the period Jan 2020–Dec 2021, patients registered and coded with moderately severe asthma on long-acting beta-agonist (LABA)/ICS therapy at a single UK general practice site were invited to an initial asthma clinic appointment (n = 195). Inclusion and exclusion criteria are outlined in Table 1. In this observational, non-intervention study; the SABA strategy was discussed in detail, and consent was sought. A total of 101 patients agreed to a new strategy of MART + anti-inflammatory reliever (AIR), and the remaining 94 patients continued with MART + SABA inhalers.
Inclusion and exclusion criteria used to identify participants in the study
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
ICS: inhaled corticosteroid; LABA: long-acting beta-agonist; SABA: short-acting β2-agonist; LAMA: long-acting muscarinic antagonist; BDP: beclomethasone dipropionate; COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease
Participants were reviewed at 3, 9, 12, and 24 months to assess their inhaler use, number of exacerbations, and hospital admissions. All hospital admissions were analysed from hospital records and confirmed with clinical coding. As this was an observational study there were no changes in routine asthma management. ICS doses were adjusted in response to the presence or absence of exacerbations in accordance with routine asthma care. Participants continued to have annual asthma reviews where inhaler technique and adherence were assessed.
The sample size had a power of 85 and an alpha two-sided of 0.05 to detect a difference between the two groups. For non-normal distribution data, the Mann-Whitney test was used and Fisher’s exact test for the comparison of categorical variables. The R programme was employed as statistical software.
This study complies with the Declaration of Helsinki [11]. The study was approved by the De Montfort University Institutional Review Board Faculty Research Ethics Committee (FREC/019-68). All participants consented to participate in the study with all data being anonymised to comply with data protection legislations.
A total of 195 asthmatic patients participated in the study. At the beginning of the study period, there was no significant difference between the group’s general characteristics (Table 2) including history of asthma, predicted forced expiratory volume in 1 second (FEV1), FEV1 change post-bronchodilator, poorly controlled asthmatics, and the use of rescue medication. However, there were significantly fewer participants in the SABA-free group that used regular ICS at the beginning of the trial period (p = 0.0497).
General characteristics and main differences between group of patients that did not use SABA as rescue medication and the control group that did use SABA at the start of study period
| Variables | SABA-free (n = 101) | SABA (n = 94) | p value |
|---|---|---|---|
| Age (yrs mean ± SD) | 31.5 ± 18.4 | 34.8 ± 17.7 | 0.78 |
| Male/Female | 52/49 | 41/53 | 0.574 |
| History of asthma (yrs mean ± SD) | 18.2 ± 11.4 | 17.6 ± 10.4 | 0.81 |
| ACT (mean ± SD) | 18.2 ± 2.7 | 15.7 ± 6.4 | 0.067 |
| FEV1% predicted | 80.2 ± 10.4 | 79.2 ± 6.1 | 0.3547 |
| FEV1 change post bronchodilator (%) | 10.8 ± 11.2 | 14.2 ± 11.19 | 0.088 |
| Hospital/ED visit 12 months prior (n) | 19 | 18 | 0.347 |
| Subjects with regular ICS (n) | 57 | 61 | 0.0497* |
| ICS dose (mcg) mean (95% CI) | 162.52 (58–347) | 118.57 (63–214) | 0.0512 |
| Poorly controlled asthma (ACT < 16%) | 18 | 16 | 0.543 |
| Daily maximum of rescue medication mean (95% CI) | 0.96 (0.45–1.21) | 1.05 (0.1–1.9) | 0.81 |
SABA: short-acting β2-agonist; yrs: years; SD: standard deviation; ACT: asthma control test; FEV1: forced expiratory volume in 1 second; ED: emergency department; ICS: inhaled corticosteroid; CI: confidence intervals. * p value of < 0.05 is considered statistically significant
A total of 101 asthma patients aged 14–69 receiving budesonide (BUD)/FORM MART therapy without SABA over a 24-month period had a statistically significant reduction in asthma-related hospitalisations (p = 0.003) (Figure 1) and asthma exacerbations requiring oral corticosteroids (OCS) (p = 0.041) compared to 94 individuals taking MART/SABA (Figure 2). There were 26 asthma exacerbation episodes in the SABA group requiring OCS compared to only 5 exacerbations in the SABA-free group over the 24-month study period (Figure 2). There were a total of 12 asthma-related hospitalizations in the patients across both groups (11 in the SABA group vs. 1 in the SABA-free group) with no mortality recorded. A similar pattern was seen when visits to emergency/same-day emergency departments (Figure 1) with a total of 23 visits in the total cohort (19 in the SABA group vs. 4 in the SABA-free group).
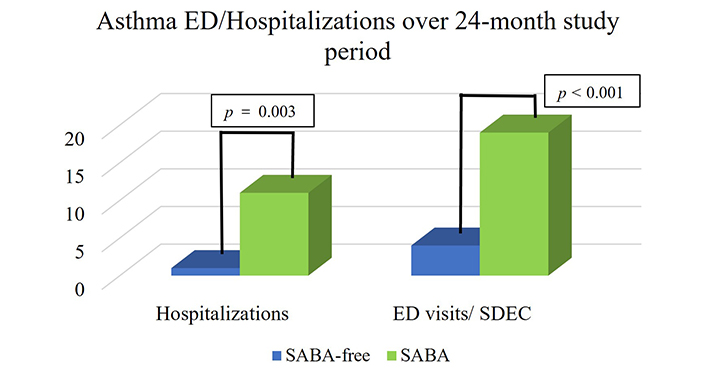
Asthma ED visits and hospitalizations per group within the 24-month study period. There were 11 hospitalisations in the SABA group vs. 1 hospitalisation in the SABA-free group. There were 19 visits to the ED/SDEC in the SABA group vs. 4 visits in the SABA-free group. p value < 0.05 is classed as statistically significant. Thus, there were significantly fewer hospitalisations (p = 0.003) and ED visits/SDEC (p < 0.001) with SABA-free therapy. ED: emergency department; SDEC: same-day emergency care; SABA: short-acting β2-agonist
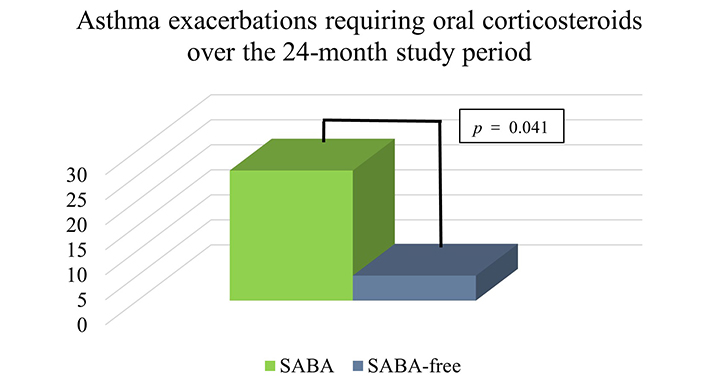
Asthma exacerbations requiring oral corticosteroids per group within the 24-month study period. There were 26 exacerbations in the SABA group compared to only 5 exacerbations in the SABA-free group (p = 0.041). p < 0.05 is statistically significant. SABA: short-acting β2-agonist
Analysis of asthma medication used over the 2-year study period is shown in Table 3. The SABA-free group was prescribed significantly less BUD/FORM over the 24-month period compared to the MART/SABA group (p < 0.001). Interestingly, the SABA-free group on average requested 4.9 cannisters of BUD/FORM per year compared to the SABA group (7.68 cannisters/year; p = 0.00347). This equated to almost 50% less steroid use in total by the SABA-free group when compared to the SABA group. Of note, the SABA group requested an average of 6.48 cannisters of SABA per year.
Asthma medication data for the 24-month study period
| Variable | SABA-free (MART only) | SABA/MART | p value |
|---|---|---|---|
| BUD/FORM cannisters (n = mean/year ± SD) | 4.9 ± 1.8 | 7.68 ± 2.9 | 0.00347 |
| Steroid dose (mcg) mean (95% CI) (BDP equivalent) | 156.78 (60–274) | 317.21 (98–759) | < 0.001 |
It shows that the SABA-free group used significantly fewer BUD/FORM inhalers with an overall significantly lower steroid dose than the SABA/MART group. p < 0.05 is classed as statistically significant. SABA: short-acting β2-agonist; MART: maintenance and reliever therapy; BUD/FORM: budesonide/formoterol; BDP: beclomethasone dipropionate
A total of 101 asthma patients receiving BUD/FORM MART therapy without SABA as recuse medication over a 24-month period had a statistically significant reduction in asthma-related hospitalisations (p = 0.003) and asthma exacerbations requiring OCS (p = 0.041) compared to 94 individuals taking MART/SABA.
The SABA-free group was prescribed significantly less BUD/FORM over the 24-month period compared to the MART/SABA group (p < 0.001).
These data are supported by previous studies [12–14], which have shown that MART therapy significantly reduces asthma exacerbations compared with fixed dose ICS/LABA plus SABA. Nannini et al. [15] showed that SABA-free MART therapy reduced asthma-related hospitalisations by 92%. Our observations are very much in line with this which leads us to believe that over-reliance on SABA and poor adherence to preventer therapy are more than likely both faces of the same coin. The SABINA programme more recently investigated the global burden of SABA use and associations with asthma-related outcomes under three pillars. Data from the SABINA studies have been published in several peer-reviewed articles [16–18], and some of these have been cited in articles challenging the role of SABA in asthma management as well as in recent revisions to the GINA strategy document [1]. The challenges faced with the SABINA programme were not too dissimilar to the ones the authors faced in this study. We also demonstrated better outcomes in the SABA-free group along with less ICS use but we accept that although our study shows the benefit of SABA-free MART therapy up to 24 months, there is hardly any data about the long-term benefits of SABA-free MART therapy beyond this. Furthermore, only 195 participants were included in this study. This is a very small sample size considering over 300 million people are estimated to have asthma globally [4]. This highlights the need for further randomized controlled trials within the community cohort to elicit the best rescue medication strategy in asthmatics requiring LABA/ICS therapy. Our study is also complicated by the design of it being observational but enhanced by it being prospective in nature. We accept SABA prescriptions were used as a proxy for SABA use and inherently there is a flaw in that argument. This data along with others discussed would suggest that there is a clear link between worse outcomes and SABA use. This may be due to the increased anti-inflammatory treatment related to the MART-AIR approach compared to the MART-SABA approach which may explain the fewer exacerbations and hospitalisations in the former group. However, there is no definitive proof of a causal link to explain this. The argument that lack of asthma control would alone explain the results has somewhat been questioned by our results as the asthma control test (ACT) scores in this study were not statistically significant between the two groups and only < 20% of the patients had poor control. The authors accept that to demonstrate that SABA use is causally related to poor outcomes, the adjustments made would require completely removing the confounding factor of poor asthma control and the factors which lead to it; something that a well-designed randomized study would need to address in the future.
In conclusion, the data provides real world evidence that the use of MART with BUD/FORM with the elimination of SABA is safe and effective for moderate asthmatics within primary care, significantly reducing asthma-related hospitalisations and the need for OCS. SABA-free MART therapy provides effective asthma control without the risks associated with SABA overuse at an overall lower ICS dose.
AIR: anti-inflammatory reliever
BUD/FORM: budesonide/formoterol
GINA: Global Initiative for Asthma
ICS: inhaled corticosteroids
LABA: long-acting beta-agonist
MART: maintenance and reliever therapy
OCS: oral corticosteroid
SABA: short-acting β2-agonist
SH: Conceptualization, Project administration, Resources, Validation, Visualisation, Writing—original draft, Writing—review & editing. EG and JR: Investigation, Resources. SG: Conceptualization, Data curation, Formal analysis, Resources, Methodology, Validation, Visualisation, Writing—review & editing.
The authors declare that they have no conflicts of interest.
The study was approved by the De Montfort University Institutional Review Board Faculty Research Ethics Committee (FREC/019-68) and complies with the Declaration of Helsinki.
Informed consent to participate in this study was obtained from all participants.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher. However, data sets will be anonymised to maintain patient confidentiality.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 6208
Download: 54
Times Cited: 0
