Abstract
A high percentage of patients with severe asthma also suffer from nasal polyposis, with dupilumab, an anti-IL-4R antibody both diseases can be treated due to its role in type 2 (T2) inflammation. When these conditions are associated with eosinophilic vasculitis, they may be classified as eosinophilic granulomatosis with polyangiitis (EGPA), which can be treated with mepolizumab. This case report presents an atypical form of EGPA, following an unusual course that began with the final signs and symptoms and then proceeded backward to the prodromal stages. Our patient, indeed, a 46-year-old woman, was asymptomatic throughout her youth, with the exception of nasal polyps. Later, at the age of 34, she developed late onset asthma, which became increasingly difficult to treat, until old and previously unacknowledged evidence of vasculitis was discovered, giving a twist to our patient’s medical history and, subsequently, to the therapeutic strategy adopted to treat her. This case report aims to highlight the importance of conducting a thorough anamnesis in patients suffering from both severe asthma and nasal polyposis, taking into account the high prevalence of EGPA in this category of patients, as well as its wide range of clinical manifestations, not to mention the various available therapeutic strategies, including mepolizumab.
Keywords
EGPA, mepolizumab, severe asthma, vasculitis, dupilumabIntroduction
Once upon a time pathologies used to have a typical course, with prodromic signs and symptoms, followed by an acute phase of the disease itself. This clinical case completely overturns this paradigm, beginning with the final signs and symptoms and then moving on to the prodromal stages, over the years.
Just as in “The Curious Case of Benjamin Button”, by beginning with the end of the story, we can happily affirm that our patient is alive and in excellent health, which might lead you to believe that no further reading is necessary. However, we suggest doing that, to learn an interesting twist story that began as a typical and common case of severe asthma, then evolved into an unusual and challenging case for physicians, but let’s proceed in order.
It is well known that asthma and nasal polyps (NP) are commonly associated with each other in a high percentage of patients [1]. In recent years, biological drugs that can act individually or simultaneously on both diseases have been approved. The principal mechanisms underlying the action of these biological drugs target type 2 (T2) inflammation, whose effectors include T2 innate lymphoid cells (ILC2), cytokines—particularly interleukin (IL)—or their receptors. Among these drugs, one of the most prescribed and used, especially in patients with asthma and NP, due to its excellent efficacy in treating the aforementioned T2 diseases, is dupilumab, an anti-IL-4R antibody that can act on either disease, both individually and in combination [2]. It is also well known that most patients suffering from severe asthma have concomitant NP; furthermore, if the combination of these conditions is accompanied by eosinophilic vasculitis, it can be classified as a more complex systemic disease, known as eosinophilic granulomatosis with polyangiitis (EGPA), for which, to date, mepolizumab remains the only available anti-eosinophilic treatment.
Case report
As in a movie, we have a leading actor—our patient—and the supporting cast, represented by the physicians who have treated her; on the other hand, there are the antagonists of the story, represented by the disease with its signs and symptoms, not to forget, in the end, the strategies and weapons, including drugs and surgery, used to fight the pathology at stake.
Let’s now proceed step by step, beginning by introducing patient, G.L., a 46-year-old woman, with a late onset asthma, which became evident at the age of 34, and who has never been symptomatic—either with bronchial or nasal evidence—in her youth. Throughout her life, her most obstinate disease problem was chronic rhinosinusitis with NP (CRSwNP), more than asthma, although it progressively became more and more difficult to control with inhaler therapies.
The first supporting character to appear in this story was the otolaryngologist. In particular, he intervened four times in the life of the patient, surgically addressing NP on each occasion: first in 1997, then in 1998, again in 2003, and finally in 2013, the first year in which the histological analysis of the surgical specimen revealed an eosinophilic inflammation. In addition to this, the patient’s asthma has progressively worsened.
It was just that year that she started referring to a general practitioner (GP) to try to improve respiratory symptoms. Here, the first pharmacological approach prescribed to treat the patient’s respiratory symptoms was fluticasone propionate/formoterol 25/250 mcg 1 inhalation bid.
The patient was then referred to a lung disease specialist who, in order to better endophenotype the patient, conducted skin allergy tests, revealing sensitization to cats, dogs, and rabbits, as well as a blood test showing an eosinophil count of 450 cells/µl.
The honeymoon period between the patient and her respiratory disease did not last long. After 4 years, inhaled therapy was no longer effective and was switched to beclometasone/formoterol 100/6 µg 2 inhalations bid, then in 2019 tiotropium Respimat was added. Lastly, she was switched to beclometasone dipropionate, formoterol fumarate dihydrate, glycopyrronium bromide 172/5/9 µg 2 inhalations bid in 2023. Her eosinophils level, in all these years, never exceeded the level of 550 cells/µl, ranging between 400 cells/µl to 550 cells/µl. During the last two years, the patient has needed to be treated with frequent courses of oral corticosteroids (OCS), arriving at a mean of 3 cycles/years.
Here, another key figure enters the scene: the pulmonologist specializing in asthma who was working at a severe asthma center. During the visit, a CT scan was requested, which later revealed a small emphysematous bubble, some bronchiectasis, thickening of the bronchial walls, and some bronchi filled with mucus. Baseline data are summarized in Table 1.
Main parameters of the patient, before the introduction of biologics, at the switch to mepolizumab, and after 3 months of therapy
| Main parameters | Baseline | After 5 weeks | At switch | After 3 months of mepolizumab |
|---|---|---|---|---|
| Blood eosinophils | 500 cells/µl | 6,600 cells/µl | 1,900 cells/µl | 100 cells/ µl |
| FeNO | 57 ppb | 22 ppb | 38 ppb | 29 ppb |
| FEV1 (%) | 45% | 56% | 51% | 62% |
| FEV1 (L) | 1.15 L | 1.43 L | 1.30 L | 1.55 L |
| ACT | 18 | 23 | 25 | 24 |
| SNOT-22 | 58 | 28 | 20 | 24 |
Parameters of principal markers, found during single observations, concerning lung function tests and control of the disease before the first biologic used, dupilumab (baseline), after 5 weeks of treatment with dupilumab, at the switch to mepolizumab and after 3 months from the first administration of 300 mg of mepolizumab. FEV1: forced expiratory volume in 1 second; ACT: asthma control test; SNOT-22: sinonasal outcome test-22
At this point, the asthmologist chose a treatment to address both the patient’s NP and the airways. The chosen “weapon” was dupilumab, a drug that can act synergistically on both the NP and the airways, acting on IL-4R and IL-13, and useful furthermore on allergic patients [3].
After starting treatment, disease control was quickly achieved in both the upper and lower airways, with subjective and objective improvements in lung function tests and in questionnaire scores.
Just when the narrated story seemed to have reached its longed-for happy ending, a new and insidious sign appeared, one that could jeopardize the certainty of the diagnosis as well as the effectiveness of the treatment: hypereosinophilia. A blood count of 6,600 cells/µl was recorded. Despite its known presence in literature that dupilumab can increase eosinophils, without any signs or symptoms, it is prudent to better address the patient’s anamnesis. The patient was asymptomatic, with no signs of any diseases related to elevated eosinophil levels. At this point, the drug was prudently discontinued, and prednisone was administered to the patient for three weeks, starting with a dose of 25 mg, which was halved each week thereafter. No sign of vasculitis could be observed, and a complete panel of blood samples was requested, particularly to assess renal function, and returned normal. Furthermore, an echocardiogram and a chest X-ray were also performed, all of which were reported as normal. Anti-neutrophil cytoplasmic antibodies (ANCA) antibodies were searched, before prednisone administration, but without finding them.
At this point, the discerning reader may wonder why we mentioned an abnormal form of EGPA in the introduction. Despite using an old biopsy, we reach the criteria about “2022 American College of Rheumatology/European Alliance of Associations for Rheumatology”, for the diagnosis of EGPA, with the presence of EGPA of small medium vessels, two of the major criteria (obstructive airway diseases, NP) and the laboratory criteria of more than eosinophil 1.0 × 109/L.
Upon reviewing the patient’s clinical history, given the unexpected increase in eosinophils, the patient was invited to provide one more time all her clinical documentation. This included a skin biopsy performed in 2003 on a small and unique supraciliary centimetric, not symptomatic, papular lesion surgically removed for aesthetic reasons: its histological examination revealed a peri-vasal eosinophilic granulomatous infiltrate. The lesion had no other signs of vasculitis and similar cutaneous manifestations were found in the subsequent 21 years.
Although you already know how the story ends—with a happy ending—we confirm that the patient is asymptomatic to date, she has been switched to mepolizumab 300 mg/28 days, as indicated for EGPA, and she achieved a good asthma control, along with a satisfactory control of the nasal component.
Discussion
The case we wanted to recount in a somewhat fictional manner is in our opinion a very special case that is worth analyzing carefully and seriously. It is known that hypereosinophilia could be caused by other diseases [i.e. hypereosinophilic syndrome (HES), parasitic infestations……] before judging the patient as an EGPA, we performed analysis to exclude other main differential diagnoses. Despite it being a single case, and therefore not appliable to a more ample population, it is interesting to observe and discuss the strange presentation of a complicated disease as EGPA is, with the aim to underly several points, as an accurate anamnesis and the necessity to think about a disease also if the most common symptoms are not clearly present. Generally speaking, EGPA presents in a fairly typical way, a prodromal phase with allergic or non-allergic asthma, and rhinosinusitis, followed by a phase marked by eosinophilic infiltration and significant blood eosinophilia, and finally the vasculitis phase [4]. Steps are represented in Figure 1, where we would like to easily and briefly synthesize the most usual presentation of EGPA.
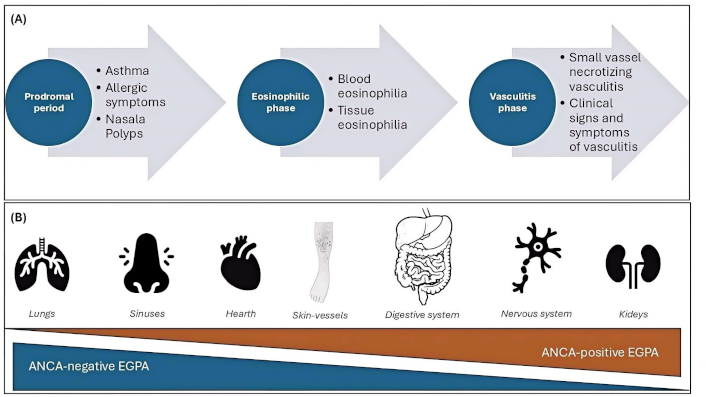
Usual trend of EGPA. In contrast to the clinical case we want to present, the common steps related to the onset of eosinophilic granulomatosis with polyangiitis (EGPA) have been summarized, with the main organs involved and the probability of Anti-neutrophil cytoplasmic antibodies (ANCA) positivity. A. Usual progression of EGPA disease with beginning of prodromal phases, subsequently an eosinophilic and finally a vasculitis phase; B. principal organ involved in the disease, according to the frequency of ANCA presence
In this case, there was a small vasculitic infiltrate, which was treated surgically so that it no longer presented for 21 years, followed by a prodromal phase with asthma and polyposis, and finally a slating of the eosinophilic phase, an event that is known as to have occurred [5]. In this particular case, predicting the patient’s response to dupilumab therapy would have been quite challenging, which was fully indicated given the data available to clinicians. Had we known about the vasculitis evidence in histology from 21 years earlier, we might have approached the situation differently. Although the patient was asymptomatic at the time and the clinical investigations performed were normal, we decided to treat her preventively with steroids to rapidly reduce the proportion of circulating eosinophils.
We still don’t fully understand the mechanisms behind dupilumab-induced blood hypereosinophilia. The most reasonable reason for the increase in circulating eosinophils, seen in certain individuals, appears to be the suppression of eosinophil migration from blood into tissues by IL-4/IL-13. Indeed, eotaxin-1 [motif C-C chemokine 11 (CCL11)], eotaxin-3 (CCL26), RANTES (CCL5), and MCP-4 (CCL13) are among the chemotactic factors for eosinophils whose expression is regulated by IL-4 and IL-13. Additionally, vascular cell adhesion molecule 1 (VCAM-1) is expressed on endothelial cells by IL-4 and IL-13, which facilitates eosinophil transmigration into tissues [6].
We talked about the ability to interact with ICAM and VCAM by keeping the eosinophils in circulation [7] and preventing them from entering tissues, where they can become pathological. After discontinuing the drug, our concern was that a fraction of these endothelial adhesion molecules might allow the eosinophils to infiltrate the tissue and consequently lead to pathology. Therefore, we preferred to treat the patient with a short course of systemic steroids. Several studies have begun to explore the possibility of combined therapy with dupilumab and mepolizumab in EGPA patients, to target two different mechanisms, with interesting results [8], as well as the use of mepolizumab alone in treating both EGPA and HES [9].
The decision then to switch our patient to mepolizumab was based on the scientific evidence supporting the drug’s efficacy in treating both nasal polyposis and asthma [10]. Additionally, it also took into account that, in our country, it is the only biological drug approved for treating EGPA, on the strength of the significant findings from clinical trials [11] and in real life [12]. In the future, benralizumab may also represent a therapeutic strategy for EGPA, as the MANDARA trial confirmed [13]. However, looking at what can be seen from the attached table, we can observe the effect dupilumab had in reducing inflammation in terms of FeNO reduction, and the effect on respiratory function, in both cases greater than what was obtained with prednisone.
This case report aims to highlight the intrinsic complexity of EGPA, a condition that can present very differently depending on factors such as the presence or absence of p-ANCA antibodies. In this case, however, the disease followed an atypical progression, opposite to its usual course: it started with vasculitis, followed by the polyposis and asthma phase, and eventually progressed to the eosinophilic phase. With suspect that patient could be affected by EGPA, due to the abovementioned mechanisms of improving number of eosinophils, the therapeutic choice would have been different in the first instance, falling on different drugs such as those acting directly on IL-5 or a combination of both antibodies (i.e. dupilumab + mepolizumab) despite it is not till now been approved.
As this case highlights, a very careful anamnesis is crucial before treat a patient, also considering things that may seem less relevant or far behind in time, in order to minimize the risk of misinterpreting a diagnosis—that, in this case, was actually quite complex—and to allow the patients to live happily ever after.
Abbreviations
| ANCA: | anti-neutrophil cytoplasmic antibodies |
| CCL11: | motif C-C chemokine 11 |
| EGPA: | eosinophilic granulomatosis with polyangiitis |
| HES: | hypereosinophilic syndrome |
| IL: | interleukin |
| NP: | nasal polyps |
| T2: | type 2 |
| VCAM-1: | vascular cell adhesion molecule 1 |
Declarations
Author contributions
DB, BB: Conceptualization, Investigation, Writing—original draft. VC, FRMC, CDT, SF, VM, MM, MR, MB: Supervision, Validation. FB: Writing—review & editing, Validation, Supervision. All authors read and approved the submitted version.
Conflicts of interest
Diego Bagnasco who is the Editorial Board Member and Fulvio Braido who is the Associate Editor of Exploration of Asthma & Allergy had no involvement in the decision-making or the review process of this manuscript. The other authors declare that there are no conflicts of interest.
Ethical approval
The study was approved by the Genoa Ethics Committee (EC 2017, ID 3663) and complies with the Declaration of Helsinki.
Consent to participate
Informed consent to participate in the study was obtained from the patient.
Consent to publication
Informed consent to publication was obtained from relevant participants.
Availability of data and materials
The data of this manuscript could be available from the corresponding authors upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.