Abstract
Improving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification of the biomaterials from which the vascular grafts are constructed has been used to reduce the risk of complications such as thrombosis and infection. Herein with a focus on vascular tissue engineering, we provided an overview of (a) fundamental hemodynamic considerations for blood-contacting biomaterials, (b) surface modification strategies to attenuate nonspecific adhesion of proteins, improve hemocompatibility, and induce the formation of a confluent endothelial lining, and (c) the guidelines for the clinical development of surface modified biomaterials.
Keywords
Blood-contacting implants, thrombogenicity, hemocompatibility, surface coatings, lubricant-infused coatingsIntroduction
Accounting for 23.6 million deaths per year by 2030, cardiovascular disease (CVD) is the leading cause of death worldwide, surpassing cancer and other diseases [1, 2]. In 2020, CVD incurred a financial burden of $22.2 billion in Canada; an amount that is expected to increase alongside sociodemographic characteristics such as aging of the population, urbanisation, and socioeconomic status, as well as environmental risk factors such as air pollution [3]. To address this urgent global health problem, vascular grafts have proven instrumental in enhancing the quality of life in patients with CVD and are widely used to reconstruct, replace, or bypass occluded vessels. Vascular grafts serve as crucial tools in repairing damaged tissues and reinstating blood flow to organs and tissues affected by arterial blockages or damage, particularly when there is a limited availability of autologous tissue. In scenarios where arteries are narrowed or obstructed, these grafts are employed to create alternative pathways for blood circulation, effectively bypassing the occluded segment. This intervention significantly enhances blood flow, easing symptoms associated with diminished blood supply, such as angina or claudication [4, 5]. Furthermore, vascular grafts find extensive application in procedures like coronary artery bypass grafting (CABG) and peripheral artery bypass surgery, where they replace or bolster damaged or diseased blood vessels. Through the surgical implantation of grafts, physicians can successfully reinstate blood flow to critical organs and tissues, mitigating the risk of severe complications such as heart attacks, strokes, or limb loss [5–7]. Nonetheless, adverse events in vascular grafts such as thrombotic occlusion, calcification, and stenosis occur and often lead to device failure [8]. Therefore, there is a pressing need to develop strategies aimed at enhancing the biocompatibility and tissue integration of vascular grafts to address these challenges effectively.
To enhance the biocompatibility of vascular grafts, it is essential to develop strategies rooted in a comprehensive understanding of the molecular mechanisms underlying the host response to foreign bodies, including complex processes like protein adsorption, cellular interactions, inflammatory signaling pathways, immune reactions, and tissue remodeling [5, 9]. Thus, the implantation of synthetic vascular grafts perturbs the homeostatic balance, triggering thrombosis and inflammation through mechanisms such as coagulation cascade activation, platelet adhesion, inflammatory responses, and protein adsorption, ultimately compromising graft function. This disruption, compounded by endothelial layer damage, compromises the blood vessel’s antithrombotic environment, potentially resulting in thrombotic occlusion, fibrous encapsulation, and calcification [10, 1]. Surface modifications to the luminal surface, coupled with the integration of biologically active substances, are aimed towards mitigating these complications by effectively enhancing hemocompatibility, reducing inflammation, promoting endothelial cell (EC) adhesion and proliferation, facilitating controlled drug delivery, and preventing calcification, thereby emulating the multifaceted performance criteria of native blood vessels while preserving the mechanical properties inherent to natural vasculature [1]. Synthetic blood vessels must be sufficiently elastic and compliant to prevent mechanical mismatch and resistance to long-term fatigue. In addition, they must have sufficient suture retention strength to tolerate the hydrodynamic and mechanical forces at the sites of anastomosis [11, 12]. The native arterial blood vessels exhibit an ultimate tensile strength of 2.24 MPa, whereas decellularized tissue has an elastic modulus of 4.34 MPa [13, 14]. Maintaining a load-bearing capacity within this range is crucial when fabricating artificial vascular grafts. The elastic modulus of synthetic blood vessels varies depending on the material used. Common synthetic polymeric vascular grafts, such as Dacron or poly(tetrafluoroethylene), typically possess elastic moduli of 14,000 MPa and 500 MPa, respectively [15]. This modulus is divided into two categories based on the extracellular composition, reflecting the native tissue’s initial modulus values: 0.71 MPa for the elastin network and 12.26 MPa for the collagen network. In contrast, decellularized tissue exhibits modulus values of 1.11 MPa for elastin and 19.81 MPa for collagen, respectively [11]. This elastic value is crucial in preventing resistance to long-term fatigue by ensuring that the vessel can withstand the cyclic stress of blood flow without undergoing plastic deformation or failure [16]. Suture strength is generally affected by the fiber alignment direction, the thickness of the graft wall, and the number of stitches. Typically, the suture strength required at the anastomosis site of vascular scaffolds typically falls within the range of 2 N to 6 N [13, 14, 17], ensuring secure closure and integrity of the connection between the synthetic vessel and the native tissue. These values are comparable to the suture retention strengths observed in human internal mammary arteries (1.4 N) and human saphenous veins (1.8 N) [11, 17]. This strength is crucial for preventing complications such as leaks, hematomas, and other issues that may arise from inadequate closure [13].
The landscape of synthetic polymers utilized in vascular grafts is diverse, with numerous options available for clinicians. Expanded polytetrafluoroethylene (ePTFE), polyethylene terephthalate (PET), and polyurethane (PU) are among the most prevalent choices, showcasing a range of properties suited to different clinical needs [5, 6, 18]. Despite this diversity, ePTFE and PET emerge as the primary biomaterials for prosthetic vascular grafts, owing to their favorable characteristics and established clinical performance [5, 18–23]. PET is a synthetic material typically used in the form of fibers for vascular grafts. These fibers, which may vary in shape, are often woven or knitted into multifilament yarns, creating a structure versatile enough for such applications [18, 24, 25]. In contrast, ePTFE is produced by a process that involves heating, stretching, and extruding to form a porous tube with a distinctive network of nodes and fibrils. Although ePTFE offers a unique structure with promising features, its grafts face challenges due to surface thrombogenicity, which has led to inconsistent patency rates in long-term studies [18, 19]. These rates have ranged widely, from approximately 60% at one year to as low as 14% at approximately three years [22], highlighting the need for ongoing research and refinement in this area. The length of fibers in ePTFE, known as the internodal distance, plays a critical role in governing the material’s properties, including its mechanical strength and biocompatibility [22]. Further investigation into optimizing this parameter could potentially improve the performance of ePTFE grafts and address challenges associated with long-term patency rates as research indicates that at least 50% of ePTFE grafts fail within 10 years mainly because they lack a functional endothelium that resists thrombosis [3, 4].
Surface modifications play a pivotal role in vascular graft engineering by addressing key challenges and enhancing overall graft performance. Surface modifications effectively reduce thrombogenicity, preventing blood clot formation and minimizing the risk of graft occlusion. However, it’s important to note that the effects of such coatings on endothelial layer formation can vary. While some coatings may facilitate EC adhesion, promoting hemocompatibility, others may not directly influence this process. Nevertheless, these modifications are crucial for promoting long-term graft patency. In addition to minimizing thrombotic events, they can also potentially inhibit smooth muscle cell proliferation and intimal hyperplasia by fostering the formation of a functional endothelial layer. This endothelial layer acts as a protective barrier, further enhancing graft stability over time. Additionally, surface modifications stimulate tissue integration and regeneration around the graft by incorporating bioactive molecules or growth factors onto its surface. These molecules facilitate cell migration, proliferation, and extracellular matrix deposition, fostering a microenvironment conducive to tissue ingrowth and remodeling. For instance, binding specific molecules to the surfaces of vascular grafts can significantly enhance cell migration. This is achieved by improving cell adhesion properties, which are crucial for initiating cell movement towards the graft surface. Similarly, these molecules promote cell proliferation by activating cellular pathways that contribute to cell division and growth. Furthermore, they aid in the deposition of the extracellular matrix by stimulating the production of matrix proteins, which are essential for tissue regeneration and integration of the graft with the host tissue [26, 27]. Ultimately, these comprehensive modifications promote the seamless integration of the graft with surrounding native tissue, leading to improved healing outcomes and long-term graft stability [1, 4, 28]. Furthermore, by reducing thrombogenicity and promoting endothelization, surface modifications can eliminate the need for antithrombotic drugs, consequently mitigating the risk of thrombosis. Consequently, this helps mitigate the risk of hemorrhagic complications by reducing the need for medications that increase the likelihood of bleeding [29, 27]. This review focuses on surface modification techniques used in vascular tissue engineering to enhance vascular graft biocompatibility and integration.
Hemodynamics
The study of hemodynamics is concerned with the pressure and flow of blood through arteries and veins in the circulatory system. The flow of blood is driven by the changes in pressure due to the contraction of the heart. Acting as a pump the heart pressurizes the blood, providing it with internal energy that drives its motion through the circulation [30]. In addition to pressure, the flow of blood is regulated by the resistance due to the interaction of blood and the walls of the vasculature. This resistance to blood flow is attributed to many factors such as the diameter of the blood vessels, blood viscosity, and the length of the vessels [30]. Additionally, obstructions to the blood vessel as seen in the case of atherosclerosis or excessive thrombotic events, can result in a decrease in blood flow and result in severe complications such as myocardial infarction and stroke [31]. The relationship of pressure (∆P) and resistance (R) on the flow (Q) is communicated by the basic flow equation (equation 1):
Fundamental of shear stress
When observing the deformation and transmission of materials due to forces, such as blood flowing through an artery, it is difficult to apply Newton’s second law, F = ma, due to the sheer quantity of blood cells [30]. Observing this phenomenon through a continuum approach is required and the study of stress allows for an appropriate model to assess mechanical interactions of materials. Stress is a physical quantity that represents the internal forces distributed within a material, exerted per unit area. It arises when external forces act on a material, causing internal distortions [32]. The use of partial differential equations has been elucidated to relate stress to the motion at each point in the material [30]. A detailed discussion of the mathematics behind continuum mechanics is beyond the scope of this review but was well described by Secomb et al. 2016 [30].
A key type of stress relevant to the study of biomaterials that contact with blood is shear stress, which impacts material behavior. This is the component of the stress vector that acts parallel to the surface. Factors that affect sheer stress (τ) are the force applied (F) and cross-sectional area (A) of the surface as seen in equation 2:
The resulting sheer stress will deform the structural element of interest and result in a displacement parallel to the force applied resulting in shear strain. When applied to laminar flow where the profile of blood velocity is parabolic the sheer stress is related to the viscosity of the blood, flow rate, and vessel radius. Vascular shear stress of large conduit arteries varies between 5 and 20 dynes/cm2; while instantaneous values can range from negative measures to nearly 40 dynes/cm2 during states of increased cardiac output [33].
Shear stress considerations for biomaterials
The development of prosthetic vascular grafts requires a clear understanding of hemodynamic principles to ensure effective patency and long-term mechanical integrity. Typically, early graft failures (< 30 days) are attributable to technical failures while late-stage failure is a result of the development of atherosclerosis and an ill-defined pathologic lesion known as anastomotic neointimal hyperplasia [34, 35]. As grafts are blood-contacting devices they are subjected to the stressors by the flow of blood, such as shear stress. Based on the Hagen-Poiseuille formula shown in equation 3, the radius of the vessel plays a significant role in the magnitude of shear stress experienced by the graft.
where τ represents the shear stress, μ denotes the dynamic viscosity, Q signifies the flow rate, and r stands for the radius of the conduit.
Therefore, optimizing the diameter of the graft does allow for the modification of shear stress to ensure, the diameter is large enough not to limit flow but small enough to promote a thin, luminal surface long term.
A study examining 40 polytetrafluoroethylene (PTFE) grafts across varying diameters (3, 6, and 8 mm) and anatomical positions (carotid and femoral arteries) in 10 mongrel dogs revealed the impact of flow rate and shear stress on thrombosis and intimal hyperplasia formation [34]. Results revealed that 3 mm grafts exhibited heightened shear stress, reduced flow rate, and thrombosis occurrence at 15 weeks compared to 6 mm and 8 mm grafts; furthermore, implant location influenced shear stress, with carotid artery implants experiencing higher shear stress due to increased flow rates, ultimately suggesting that while low to normal shear stress leads to thinner intimal hyperplasia, elevated shear stress levels (> 70 dyne/cm2) result in exponential thrombus formation and platelet activation [34].
Hemocompatibility of artificial vascular grafts
Sustained hemocompatibility of implants is critical for their long-term success and poses a current limitation for current blood-contacting implants. Since implants are recognized as foreign objects by the body, adverse interactions between the implant and human blood need to be analyzed to ensure that there is little or no activation of the blood coagulation and inflammatory response [3, 36]. Such adverse reactions include coagulation cascade activation, platelet adhesion and activation, inflammatory response, protein adsorption, and foreign body reaction [9, 37]. Upon exposure to blood, biomaterials can instigate the coagulation cascade, leading to clot formation, while a diverse range of plasma proteins swiftly adsorb to the surface. This activation may result in thrombosis, impeding blood flow, and causing ischemic complications. This initial event sets off a dynamically changing, progressively thromboinflammatory microenvironment mediated by coagulation and innate immunity activation, with subsequent recruitment of platelets and inflammatory leukocytes [2, 37]. As implants are perceived as foreign bodies, they can incite a foreign-body reaction characterized by the formation of a fibrous capsule around the implant, ultimately leading to implant failure over time. Failure to control these adverse interactions can instigate interconnected pathological processes, leading to thrombosis, hemodynamic instability, bleeding complications, and organ damage, stemming from platelet, erythrocyte, and leukocyte alterations, generation of activation products in plasma, and deposition of proteins and cells on the material surface [5, 6, 38]. Furthermore, in addition to the complement system, which is a crucial component of the body’s immune system comprising a group of proteins that work together to recognize and clear pathogens, activating both the intrinsic and extrinsic coagulation pathways may occur (Figure 1A). The intrinsic pathway, also known as the contact activation pathway, is initiated when blood comes into contact with negatively charged surfaces such as collagen exposed upon vessel injury. This contact activates factor XII, which subsequently activates factors XI and IX, ultimately leading to the conversion of factor X to its active form, factor Xa, in the presence of factor VIII and calcium ions. This activated factor X then combines with factor Va, phospholipids, and calcium ions to form prothrombinase, which converts prothrombin to thrombin. Thrombin, in turn, converts fibrinogen into fibrin strands, forming a fibrin network (Figure 1B) that stabilizes the platelets and reinforces clot formation. Conversely, the extrinsic pathway is initiated by tissue factor (TF), which is present outside the bloodstream and is exposed upon vascular injury. TF forms a complex with factor VIIa, leading to the activation of factor X directly. This extrinsic pathway is the primary initiator of coagulation in vivo, especially in response to tissue injury. Additionally, platelets possess the capability to adhere to and aggregate on the surface. Meanwhile, the adhesion and activation of leukocytes can prompt the release of polymorphonuclear (PMN) elastase and TF, thereby initiating the activation of the extrinsic pathway [5, 8, 38]. Such responses primarily depend on the surface properties of the artificial vascular materials such as pore size (Figure 1C and D), implantation site, and those of interacting proteins, cells, and neighboring blood flow [28, 24]. Increased absorption is likely due to a higher surface area to volume ratio, a characteristic often associated with greater porosity, although the chemistry of the surface can also play a significant role in this phenomenon. Consequently, heightened porosity can lead to increased protein adhesion, with specific proteins binding to the surface. While surfaces with increased porosity may exhibit greater surface roughness, which could potentially be considered more thrombogenic, research findings vary. Some studies suggest reduced platelet activation on materials with heightened porosity, particularly those featuring pore sizes around 30 µm [39–41]. However, it is essential to note that the relationship between porosity and protein absorption, as well as thrombogenicity, is complex and influenced by various factors beyond just surface characteristics. For instance, while smooth surfaces are generally preferred for enhanced hemocompatibility, porous materials remain essential for blood-contacting materials to allow for suturing or facilitate local tissue integration in certain applications [42–44]. In the literature, it is widely acknowledged that materials with higher porosity and larger pore sizes may lead to increased calcification [23, 38, 45–48]. Nonetheless, observations indicate that vascular graft implants with reduced porosity can also instigate degenerative effects, resulting in elevated calcification. Research has shown a complex relationship between material porosity and calcium deposition. While studies indicate that materials with water porosities exceeding 5,000 mL of water per minute per square centimeter at a pressure of 120 mmHg can help prevent calcification and other adverse responses [41, 49], it is generally observed that higher porosity can also correlate with increased calcium deposition. This paradox suggests that, in addition to porosity, inflammatory responses play a significant role in the mechanisms of mineralization. It is essential to balance these factors when designing materials to mitigate adverse biological responses effectively [41, 50]. Therefore, it is imperative to account for local tissue responses and design vascular grafts that minimize inflammatory reactions.
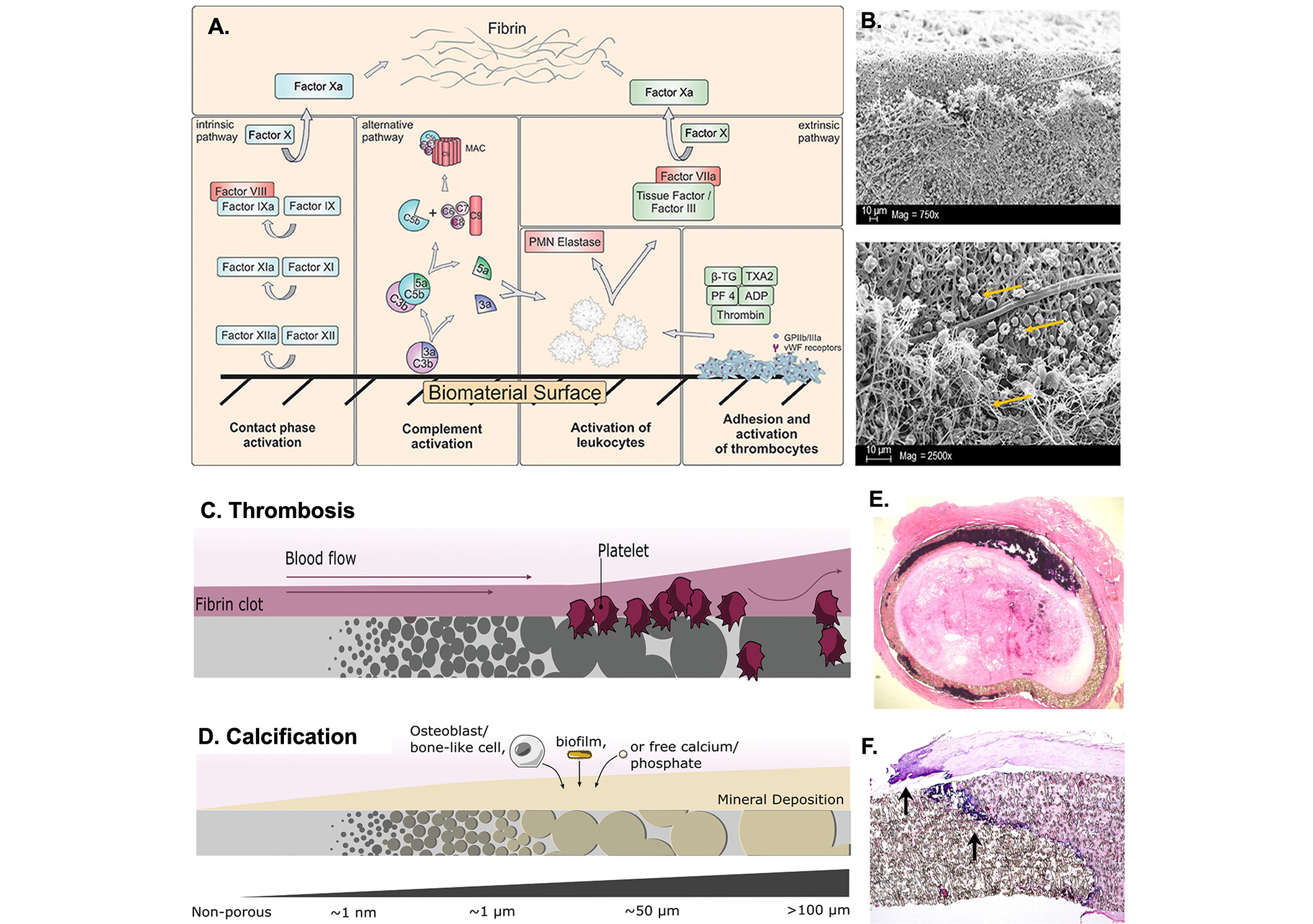
Adverse reactions induced by blood-contacting implants. (A) Schematic illustration of the activated intrinsic and extrinsic coagulation pathways that result in a fibrin network [38]; (B) scanning electron microscopic (SEM) analysis of artificial vascular grafts after activation of the blood coagulation system illustrating the adhered platelets and the 3D-fibrin meshes shown by the yellow arrows [38]; (C) biocompatibility trends in respect to biomaterial pore size. Thrombogenicity development and shear-mediated platelet activation with large porosity and surface roughness [41]; (D) calcification deposition in larger pored materials sourced from osteoblast-like cells, a biofilm, or free minerals [41]; (E) thrombus formation on PTFE vascular grafts [5]; (F) transmural calcification within PTFE vascular grafts at the edge of fibrin deposits as shown by the arrows [5]. PTFE: polytetrafluoroethylene; MAC: membrane attack complex; PMN: polymorphonuclear; β-TG: β-thromboglobulin; TXA2: thromboxane A2; PF 4: platelet factor 4
Note. Figure 1A and B was adapted from “Blood-contacting biomaterials: in vitro evaluation of the hemocompatibility” by Weber M, Steinle H, Golombek S, Hann L, Schlensak C, Wendel HP, et al. Front Bioeng Biotechnol. 2018;6:99 (https://doi.org/10.3389/fbioe.2018.00099). CC BY; Figure 1C and D was adapted from “Medical applications of porous biomaterials: features of porosity and tissue-specific implications for biocompatibility” by Hernandez JL, Woodrow KA. Adv Healthc Mater. 2022;11:e2102087 (https://doi.org/10.1002/adhm.202102087). © John Wiley & Sons; Figure 1E and F was adapted from “Pathology of explanted polytetrafluoroethylene vascular grafts” by Mehta RI, Mukherjee AK, Patterson TD, Fishbein MC. Cardiovasc Pathol. 2011;20:213–21 (https://doi.org/10.1016/j.carpath.2010.06.005). © Elsevier Inc.
Thrombosis
Nonspecific protein adsorption is the initial event at the interface between the surface of the implant and human blood, wherein the dynamics of protein adsorptions are governed by kinetic and thermodynamic principles. Although the blood contains over 300 proteins of varying shape, size, and concentration, the main contributors to the formation of the non-specific protein adsorbed layer are fibrinogen, albumin, and immunoglobulins [36]. Typically, the first plasma protein to be adsorbed to the surface is fibrinogen following other contact factors, and adsorption of even small amounts of fibrinogen can initiate a cascade of coagulation events including platelet adhesion and activation of coagulation (Figure 1D). Locally generated thrombin converts fibrinogen to fibrin and the fibrin monomers then polymerize to form fibrin. Thrombin also serves as a potent platelet agonist, which activates ambient platelets that adhere and aggregate on the graft surface [51]. Formation of platelet-rich thrombi on the graft surface fouls the device and can provide a nidus for infection, or can reduce graft patency and lead to embolic complications (Figure 1E) [52]. Complement activation by synthetic vascular grafts could also lead to the prevention of EC seeding, resulting in the aggregation of platelets and the in situ production of tissue thromboplastin by PMN leukocytes. Aside from thrombosis, fibrosis and calcification are other long term complications of synthetic vascular grafts [6].
Calcification
The calcification of prosthetic vascular grafts, characterized by the deposition of crystalline calcium phosphate, primarily hydroxyapatite, occurs gradually due to multiple factors. These factors include the graft material’s composition, inflammatory responses, and the presence of risk factors like hypercalcemia or chronic kidney disease. Calcification poses a significant risk to graft function, resulting in vessel stiffening, narrowing, or complete blockage, ultimately leading to graft failure or other complications. It is a common complication associated with insufficient biocompatibility of conduits (Figure 1F) [6, 53, 54]. A study reported that of 40 failed PTFE bypass grafts, 68% revealed calcifications within 1 month after implantation [5]. Characterized by ectopic mineral formation in the form of calcium phosphate or other calcium salts, calcification can increase the risk of heart attack, stroke, and limb amputation by contributing to vascular occlusion and through embolization of calcific deposits [6]. Additionally, vascular mineralization results in mechanical dysfunction of implants. While studies indicate that calcification is a cell-mediated process, the exact mechanisms underlying its pathogenesis remain unclear [6, 53]. Notably, recent research has identified a notable and statistically significant association between protein infiltration into the leaflet during the early stages and eventual calcification of the leaflet upon long term implantation. This finding suggests that protein infiltration could be a key factor initiating the progression of leaflet calcification [55, 56]. Factors related to this phenomenon include matrix remodeling, endoplasmic reticulum stress, apoptosis, inflammation, and the generation of reactive oxygen species (ROS) [6, 51]. Given the widespread occurrence of interstitial calcification [6, 42, 43, 53, 54], there is a pressing need for vascular graft materials resistant to this phenomenon. Functional modification strategies play a pivotal role in countering calcification. This is crucial as these modifications inhibit the material’s interaction with serum proteins, which is facilitated by the microporous structure inherent to ePTFE and the emergence of minute interstices on the leaflet surface post-implantation, serving as a scaffold for protein adhesion. Therefore, the modification of ePTFE to reduce its microporous structure and fortify against interstice development holds promise in mitigating leaflet calcification by preventing protein infiltration [42, 49, 55].
Functional modification approaches for vascular grafts
Surface characteristics including surface wettability, potential, energy, topography, and reactivity govern the hemo and cell compatibility of artificial grafts (Figure 2). Superhydrophobic surfaces, characterized by low water sliding angles (< 10°) and high water contact angles (> 150°), exhibit exceptional water repellency and self-cleaning properties. These surfaces can reduce protein adsorption and minimize contact between blood and the material interface, due to the composite solid-air surface formed beneath water droplets [57–59]. In addition, surface chemistry, energy, and topography, including wrinkles, wells, pillars, and other structural formations, play crucial roles in attenuating thrombosis by modulating cell and protein adhesion. These factors collectively contribute to the interfacial behavior adjacent to the biomaterial, continuously influencing the protein/electrolyte (ions dissolved in blood at the blood-biomaterial interface)/water layer and eliciting responses from host cells and tissues. Surfaces with zero interfacial energy and reduced enthalpic and entropic effects demonstrate weaker support for cell/thrombin adhesion [47, 25]. Similarly, surface charge and chemical reactivity may alter hemocompatibility by modulating the interaction of surface groups and blood species at the blood-material interface. Chemical structures such as sulfonic groups can reduce protein adsorption and contact activation with blood, whereas carboxyl groups tend to heighten platelet activation and thrombin generation. The surface energy, surface charge, and chemical composition of materials act in concert to determine the likelihood of thrombosis and calcification [3, 4, 29]. Below is a review of current functional modification strategies that have been applied to blood-contacting materials, as summarized in Table 1.
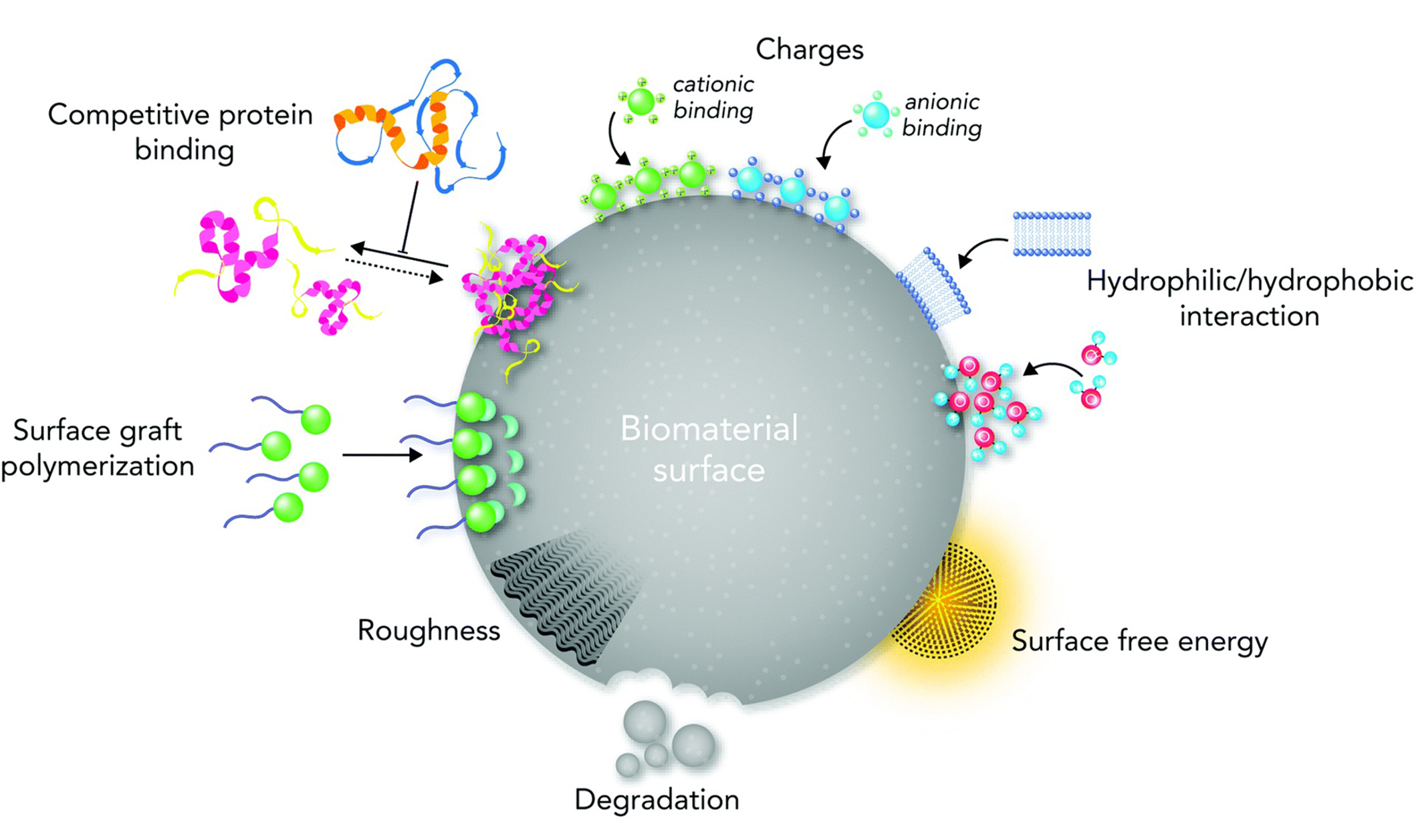
Biological responses of a biomaterial surface based on the key surface physiochemical properties. Manipulation of the molecular and cellular signaling pathways based on topography, stiffness, functional groups, biological moieties, ions, charges, and surface free energy [60]
Note. Reprinted from “Biological responses to physicochemical properties of biomaterial surface” by Rahmati M, Silva EA, Reseland JE, Heyward CA, Haugen HJ. Chem Soc Rev. 2020;49:5178–224 (https://doi.org/10.1039/d0cs00103a). CC-BY.
Surface modifications of vascular grafts
| Material | Fabrication | Surface modification | Time of effect | Target effect | Additional notes | Reference |
|---|---|---|---|---|---|---|
| Segments of the vasculature of porcine and rabbit | Glutaraldehyde-cross-linked | Heparin | 5 months | Calcification | Effective in porcine thoracic aorta (AO), pulmonary artery (PA), and ineffective in jugular vein (JV) and rabbit aorta (RA) | [25] |
| Porous polyurethane (PU) vascular graft | Dip-coated | Sulfonated poly(ethylene oxide) (PEO-SO3)-grafted PU copolymer (PU-PEO-SO3) | 14–39 days | Calcification, platelet adhesion, and thrombosis | PU-PEO-SO3 provided additional structural support.Calcium deposits resurfaced over time. | [4] |
| Polycaprolactone (PCL) vascular graft | Electrospinning with catechol/gallol surface chemistry | Polyethyleneimine, heparin, and epigallocatechin gallate | 28 days | Platelet adhesion/activation and fibrinogen formation | - | [26] |
| PU vascular grafts | Dip-coated with multiple layers of plasticized polyvinyl chloride | Polymer containing a nitric oxide dono (dialkylhexanediamine diazeniumdiolate) | 21 days | Platelet adhesion and thrombosis | - | [28] |
| Poly(ester urethane)urea graft | Amine-carboxyl chemical immobilization | Methacryloyloxyethyl phosphorylcholine | 24 weeks | Platelet adhesion | - | [31] |
| Tissue-engineered decellularized vascular grafts | Covalent attachment of thiol-functionalized hyaluronan onto the thiol-reactive vessel/graft | Hyaluronic acid hydrogel | 5 weeks | Platelet adhesion/activation and fibrinogen and fibrin formation | Decrease macrophage adhesion | [32] |
| PCL fibers | Electrospinning | Cu-metal organic frameworks | 12 weeks | Platelet adhesion/activation | Promote endothelial monolayer | [33] |
| Hydroxyl-terminated poly(ethylene-co-vinyl alcohol) | Covalent attachment through hydroxylgroups | Vascular endothelial growth factor receptor | Not stated | Increase endothelialization of graft | - | [36] |
| Expanded polytetrafluoroethylene (ePTFE) vascular grafts | Covalent attachment of silanized anti-CD34 antibodies (CD34-APTES) to the hydroxyl-terminated ePTFE surface | Perfluoroperhydrophenanthren lubricant and (3-aminopropyl) triethoxysilane (APTES) silanized anti-CD34 | 4 days | Thrombosis | Capture endothelial cells and prevent nonspecific adhesion | [46] |
| PU and polyethylene terephthalate(PET) | Covalent attachment through hydroxylgroups | Slippery-liquid infused porous surface (SLIPS) | 8 hours | Thrombosis; Icing; Scaling; Fouling; Corrosion | - | [49] |
| Titanium alloy | Covalent attachment through hydroxyl groups | SLIPS | 25 hours | Platelet adhesion | - | [6] |
| ePTFE | Dip-coating | SLIPS (Three lubricants tested perfluoropolyether, and perfluorodecalin | 21 days | S. aureus infection | Decrease macrophage inflammatory cytokine | [53] |
| Glass substrates | Chemical-vapor deposition | Tethered-liquid perfluorocarbons | 40 minutes | Platelet adhesion | Measured optimal thickness (between 100 nm and 2 μm) of lubricant to limit loss due to shear stress | [54] |
| Tissue-engineered decellularized vascular grafts | Dynamic culturing | Human endothelial progenitor cells and umbilical cord-derived mesenchymal stem cells | 2 hours | Thrombosis | - | [5] |
| Tissue-engineered decellularized vascular grafts | Dynamic culturing | Human umbilical vein endothelial cells | 14 days (cell viability) | Thrombosis | - | [55] |
-: no data
Prevention of calcification
Early studies in the field of surface modification of vascular grafts mainly focused on the prevention of passive deposition of calcium within the lumen of the grafts and around them. Compared with uncoated grafts, vascular prosthetic grafts coated with heparin exhibited significantly reduced calcium content 5 months after subcutaneous implantation in rats [53]. Likewise, coating a portion of a porous PU graft with sulfonated poly(ethylene oxide) reduced platelet adhesion and dissolved calcium in a canine right ventricle-pulmonary artery shunt model compared with the uncoated portion. These findings are thought to reflect the non-adhesive and mobile characteristics of poly(ethylene oxide), as well as the negative charge of sulfonate acid groups [61]. However, both types of coatings were associated with increasing calcium deposition over time, suggesting that the coatings delayed but did not abolish the calcification process [53, 61]. This is due to the fact like bone formation, calcification is an active process that progressives over time and cannot be hindered by passive measures [6].
Antithrombogenic
Thrombosis is the primary barrier to the long-term patency of vascular grafts. Strategies adapted for anti-thrombogenic applications mainly involve coating the surfaces with anticoagulants such as heparin or vasodilators and inhibitors of platelet activation such as nitric oxide, inhibitors of protein adsorption such as poly(ethylene glycol) (PEG) or zwitterionic polymers, and synthetic drugs [1, 29, 48, 62, 63].
Anticoagulants
Various anticoagulants have been used to improve the hemocompatibility of vascular grafts such as heparin, corn trypsin inhibitor, as well as direct thrombin inhibitors such as hirudin, bivalirudin, and argatroban [3, 29]. These anticoagulants inhibit blood clot formation, thereby preventing thrombosis and enhancing the biocompatibility of vascular grafts. Among these, heparin is the most widely used agent due to both its efficacy in preventing clot formation and its familiarity among physicians [47, 64, 62]. Heparin acts by enhancing the activity of antithrombin III, a natural inhibitor of clotting factors such as thrombin and factor Xa, thereby inhibiting the coagulation cascade. This mechanism makes it particularly effective in preventing thrombotic events in various medical conditions and interventions. Heparin’s effectiveness extends across a spectrum of medical conditions, including acute coronary syndrome, atrial fibrillation, deep venous thrombosis, and pulmonary embolism, where it has demonstrated efficacy in reducing the risk of clot formation and related complications. Furthermore, its use is well-established in clinical procedures such as cardiopulmonary bypass surgery, extracorporeal membrane oxygenation, and hemofiltration, where maintaining blood flow and preventing clot formation are critical [26, 65]. Additionally, heparin’s versatility extends to coating surfaces of diverse medical devices, including venous catheters and those used in vascular surgery, where it improves biocompatibility and reduces the risk of thrombus formation. While its widespread use is partly due to its familiarity among physicians, its demonstrated efficacy and multifaceted applications make it a cornerstone in anticoagulant therapy and medical device coatings [53, 64, 66]. Heparin acts as an anticoagulant by binding to antithrombin and accelerating the rate at which it inhibits thrombin, factor Xa and other coagulation proteases (Figure 3A). Various techniques have been used to immobilize heparin on surfaces including covalent attachment, physical adsorption, electrostatic attachment, and layer-by-layer deposition [3]. Covalent attachment of heparin is the preferred method because each heparin molecule possesses several free carboxyl groups that enable covalent bonds with functional groups on the biomaterial such as amino or hydroxyl groups [29]. However, the efficacy of the immobilized heparin can be compromised because the multiple covalent linkages limit its capacity to bind to antithrombin. End-point immobilization of heparin reduces this problem and materials coated with heparin using this technique exhibit less platelet adhesion and clot formation compared with materials coated with heparin via multivalent techniques [21, 53]. Several heparin-coated grafts are currently available including Gore® and Gentige®, which are fabricated from ePTFE and Dacron, respectively [3]. However, the applicability of these grafts is hindered by the short half-life of the immobilized heparin [1, 67]. Once heparin enters the bloodstream, it binds to plasma and cellular proteins, diminishing its therapeutic effect, and is subsequently cleared with a half-life ranging from 60 to 90 minutes through both rapid and slow mechanisms. For instance, in a 70 kg adult, a 7,000 unit IV heparin dose would exhibit a half-life of 60 minutes, while a 1,800 unit dose would have a half-life of 30 minutes. Conversely, a larger dose of 28,000 units would extend the half-life to 2 hours and 30 minutes [1, 67, 68]. The half-life of heparin may be prolonged by using “sandwiched” layer-by-layer surface coating methods. For example, when an electro-spun polycaprolactone (PCL) vascular graft was modified with polyethyleneimine and heparin using catechol/gallol surface chemistry, there was more heparin on the surface compared with other conventional layer-by-layer coating methods and the graft’s capacity to resist platelet and fibrinogen adhesion were prolonged [62].
Several other anticoagulant agents have been thoroughly investigated for their potential application in anticoagulant coatings, including thrombomodulin, activated protein C, and tissue factor pathway inhibitor (TFPI) [47, 68, 69]. In particular, thrombomodulin has been covalently immobilized onto nitinol surfaces following pre-treatment with amino-terminated organosilanes to create an aminated surface for subsequent reactions. Studies have shown that this immobilization maintains its ability to enhance protein C activation and minimizes platelet adhesion. However, challenges such as in vivo degradation, loss of activity during sterilization, and high costs have been identified as disadvantages [48].
Endothelial cells (ECs)
Vascular grafts coated with ECs have shown great potential in preventing acute thrombosis compared with other agents. As ECs continuously undergo shear stress, they stimulate the release of nitric oxide by endothelial nitric oxide synthase, which triggers vasodilation, inhibits platelet aggregation, reduces restenosis, and enhances human umbilical vein EC adhesion (Figure 3B) [1, 70]. When a PU vascular graft coated with a polymer containing a nitric oxide donor was evaluated in a sheep arteriovenous bridge-graft model, there was no platelet adhesion or thrombus formation after 21 days. In contrast, uncoated grafts exhibited adherent thrombi and red blood cell and inflammatory cell infiltration into the graft wall [63].
Bioinert polymers
Bioinert polymer coatings resist nonspecific adhesion of proteins and cells by binding water molecules to form a hydration layer that attenuates thrombosis. Among different hydrophilic polymers such as dextran and tetraethylene glycol dimethyl ether, the most commonly used synthetic polymer is PEG. The repellency properties of PEG are highly dependent on its chain length and surface density [29]. Some studies have reported enhanced protein and platelet adhesion on high-density PEG-coated surfaces, whereas others found that increasing the surface grafting density creates a more porous and rougher surface coating that promotes cell adhesion [8, 18, 26, 59]. Despite the several promising antithrombogenic results of PEG-coated implants, long-term in vivo tests revealed depletion of the coated PEG layer and degradation of its chain length and surface density due to oxidation by ROS. Therefore, PEG-coated surfaces are only useful for short-term applications [29, 45]. In contrast, vascular grafts coated with zwitterionic polymers have shown superior anti-thrombogenic results, due to their innate antifouling structure composed of a phospholipid-rich cell membrane containing zwitterion head groups of carboxybetaine and phosphorylcholine [5, 8, 26, 27, 59]. Studies of zwitterion structures for blood contacting applications have commonly used 2-methacryloyloxyethyl phosphorylcholine (MPC) due to its simple, high purity, and high-yield fabrication process, in addition to the high free-water fraction levels of its polymer chains that reduce the protein adsorption force at the interface (Figure 3C) [4, 7]. A study using an MPC-coated poly(ester urethane)urea graft implanted as an aortic interposition graft in rats for 24 weeks showed a tenfold decrease in platelet adhesion relative to uncoated grafts. Furthermore, immunohistochemical staining revealed the formation of smooth muscle and EC markers on the coated grafts, as well as oriented collagen and elastin deposition. However, further in vivo experiments are required to substantiate the applicability of these coated grafts for complete remodeling into a native-like artery [7].
Synthetic drugs
Other coatings such as drug-eluting vascular grafts have been designed to slowly release bioactive substances into the bloodstream. Synthetic drugs that release reactive-oxygen-induced antiplatelet ethyl salicylate have been shown to prevent blood clotting [1]. More recently, a decellularized tissue-engineered vascular graft coated with a hyaluronic acid (HA) hydrogel has shown notable progress in providing antithrombotic protection and promoting endothelialization. This protective barrier effectively shields blood platelets from activation triggered by collagen while facilitating endothelial repopulation over time. Using a bifunctional cross-linker between amine groups and maleimide groups, the HA coating was firmly attached to the collagenous graft. This strategic method capitalized on maleimide’s selective reaction with sulfhydryl groups to prevent nonspecific cross-linking reactions within the vascular wall [71]. There were fewer adherent platelets and less fibrinogen and fibrin formation on coated grafts than on uncoated grafts. Furthermore, in vitro studies showed inhibition of macrophage adhesion due to the strongly hydrophilic nature of HA. Studies in vivo in young adult (3-month-old) male Sprague-Dawley rats and 1-year-old Mongrel Hound dog models showed reendothelialization after 5 weeks (Figure 3D) [71]. Application of slow-releasing anti-thrombotic has been achieved using electrospinning technology to develop fiber-based vascular grafts. Copper-metal-organic frameworks (Cu-MOFs) have been shown to induce endogenous S-nitrosothiols (SNO) derived nitric oxide through the enhanced catalytic properties of Cu2+ [72]. A major limitation of this method is Cu-MOFs short half-life. By embedding Cu-MOFs within electrospun PCL fibers, NO production remained at peak levels after implantation for 2, 4, and 12 weeks [72]. Additionally, it is also shown that PCL-Cu-MOFs maintained NO production after multiple administration of endogenous SNO proving that its catalytic behavior could be repeatedly used to provide a stable and controllable generation of NO long-term [72]. These findings pave the way for an “off-the-shelf” tissue-engineered vascular graft for small-diameter applications [71].
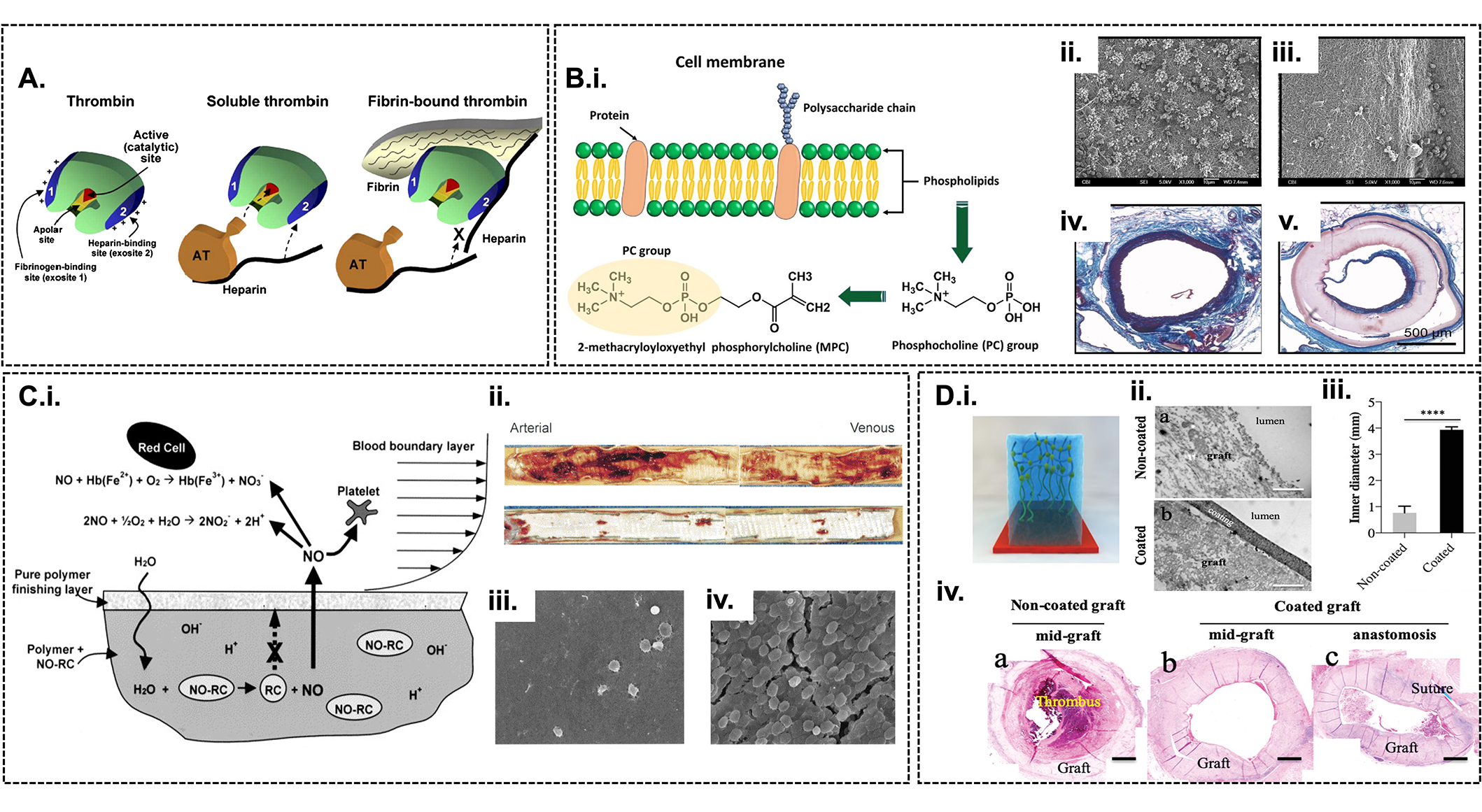
Antithrombogenicity of vascular grafts. (A) Schematic illustration of thrombin and its inhibition [73]; (B) nitric oxide-releasing polymer coating on prosthetic vascular grafts [63]. Schematic illustration of nitric oxide release from polyvinyl chloride biomaterial upon contact with water (b-i). Photographs of explanted and longitudinally opened PU vascular grafts displaying luminal thrombus on uncoated (top) and coated (bottom) grafts (b-ii). SEM images showing platelet adhesion and thrombus accumulation from arterial anastomoses on coated (b-iii) and uncoated grafts (b-iv) (magnification ×1,000); (C) Design and synthesis of the zwitterionic polymer, MPC, depicting its phospholipid rich cell membrane and phosphocholine functional groups (i) [74]. SEM images assessing the thrombogenicity after exposure to ovine blood on uncoated vascular graft (ii) showing platelet aggregates and MPC-coated vascular graft (iii) free of platelet depositions [7]. Histology assessment posts a 24-week period in vivo of the MPC-coated vascular graft, depicting a section of a native rat abdominal aorta prior to the proximal anastomosis of the graft (iv) and section taken in the proximity of the distal anastomosis (v) [7]; (D) hyaluronic acid modified surface coating on decellularized TEVGs vascular graft. (i) Depiction of hyaluronic acid coating on vascular graft. (ii) Transmission electron microscopy (TEM) images of the coating morphology of uncoated and coated hyaluronic acid-coated tissue-engineered vascular graft. (iii) Comparison of the inner diameter of the uncoated and coated allografts in rat aortic implants at 32 days (n = 6, * P < 0.05, ** P < 0.01, by unpaired, two-sided t-test with Welch’s correction). (iv) Images of hematoxylin & eosin stained clotted, uncoated tissue-engineered vascular grafts and coated graft at both the mid-graft (b) and anastomosis regions of canine explants (c). SEM: scanning electron microscope; NO-RC: nitric oxide-releasing compound
Note. Figure 3A was adapted from “Surface modification of cardiovascular materials and implants” by Qi P, Maitz MF, Huang N. Surf Coat Technol. 2013;233:80–90 (https://doi.org/10.1016/j.surfcoat.2013.02.008). © Elsevier; Figure 3B was adapted from “Nitric oxide-releasing biopolymers inhibit thrombus formation in a sheep model of arteriovenous bridge grafts” by Fleser PS, Nuthakki VK, Malinzak LE, Callahan RE, Seymour ML, Reynolds MM. J Vasc Surg. 2004;40:803–11 (https://doi.org/10.1016/j.jvs.2004.07.007). © Elsevier; Figure 3C(i) was adapted from “A small diameter, fibrous vascular conduit generated from a poly(ester urethane)urea and phospholipid polymer blend” by Hong Y, Ye SH, Nieponice A, Soletti L, Vorp DA, Wagner WR. Biomaterials. 2009;30:2457–67 (https://doi.org/10.1016/j.biomaterials.2009.01.013). © Elsevier; Figure 3C(ii–v) was adapted from “In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications” by Soletti L, Nieponice A, Hong Y, Ye SH, Stankus JJ, Wagner WR et al. J Biomed Mater Res A. 2011;96:436–48 (https://doi.org/10.1002/jbm.a.32997). © Wiley Periodicals, Inc.; Figure 3D was adapted from “Glycocalyx-like hydrogel coatings for small diameter vascular grafts. Adv Funct Mater. 2020;30:1908963 (https://doi.org/10.1002/adfm.201908963). © John Wiley & Sons.
Endothelialization of vascular grafts
The formation of a confluent endothelial layer on newly implanted vascular implants is essential for the attenuation of inflammation and coagulation. Recent innovative methods have focused on the in situ engineering of the interface between vascular grafts and the blood to capture circulating ECs with EC-specific ligands or cell adhesion molecules, thereby accelerating endothelialization. Biomimetic nanofibrous scaffolds provide an ideal surface-to-volume ratio and abundant binding ligands to enhance EC adhesion. Alternatively, circulating ECs can be captured with immobilized monoclonal antibodies against CD34. The anti-CD34 antibody stands out as one of the commonly employed EC-capturing antibodies for rapid in situ endothelialization. ECs attached to surfaces functionalized with anti-CD34 antibodies displayed improved viability and metabolic activity compared to those adhered to surfaces lacking antibody functionalization [66, 75]. Studies performed in vitro with implants coated with anti-CD34 antibody in a functionalized multilayer containing heparin-collagen revealed enhanced viability and metabolic activity relative to non-antibody functionalized surfaces. Additionally, in vivo experiments showed less neointimal hyperplasia compared with non-functionalized surfaces [29]. Other studies examined surfaces coated with vascular endothelial growth factor receptor (VEGFR) to capture ECs on hydroxyl-terminated poly(ethylene-co-vinyl alcohol) surfaces. VEGFR surface coating significantly increased the number of cells expressing VEGF after 2 weeks in culture and induced their differentiation (Figure 4A) [76]. Alternatively, coating grafts with active peptides with simple structures and high stability has been shown to promote EC adhesion. The most employed peptide is Arg-Gly-Asp (RGD), which was identified as the minimal essential cell adhesion peptide sequence in fibronectin [77, 78]. RGD can be immobilized on the surface of biomaterials by binding to hydroxyl, amino, or carboxyl groups (Figure 4B). Studies have shown that a high RGD density promotes rapid proliferation of ECs, which can be controlled using bioactive motifs such as Lys and glycine molecules at the N-terminus of peptides that act as spacers (Figure 4C). Although promising, RGD peptide also promotes the adhesion of platelets, thus novel peptides need to be screened with selective binding biofunctions to the special integrins in ECs [27].
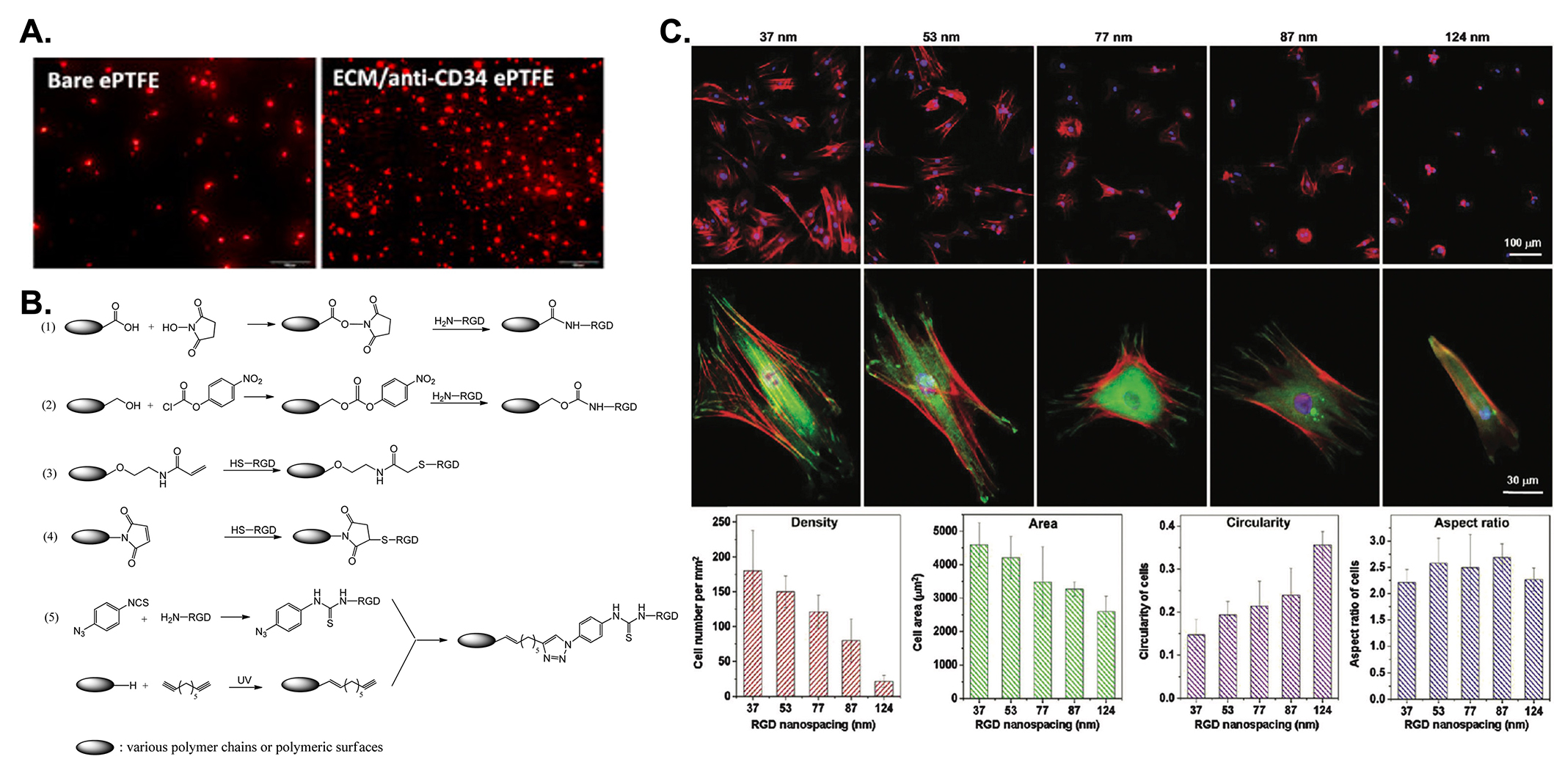
Promotion of endothelization on vascular grafts. (A) Investigation of in vitro cell adhesion on uncoated and anti-CD34 coated ePTFE grafts after 24 hours of blood perfusion [79]; (B) different immobilization methods of RGD peptide on biomaterial surfaces by activation of the carboxyl acid groups on the material’s surface with NHS (1); activation of the hydroxyl groups on the material’s surface with p-nitrophenyl carbonate (2); thiol-RGD peptide reacts with acrylic derivatives via the Michael addition reaction (3); thiol-RGD peptide reacts with maleinimide via the Michael addition reaction (4); immobilization of the RGD peptide via the azido-alkyne click reaction (5); (C) cell adhesion on nanopatterned surfaces with various nano spacings with cells stained red for F-actins, green for vinculins, and blue for nuclei, wherein the top and middle rows reveal low-magnification and high-magnification fluorescent micrographs of smooth muscle cells cultured on nanopatterns for 24 h. The bottom graphs show the statistical results of the cell adhesion [80]. ePTFE: expanded polytetrafluoroethylene; RGD: Arg-Gly-Asp
Note. Figure 4A was adapted from “Surface coating of polytetrafluoroethylene with extracellular matrix and anti-CD34 antibodies facilitates endothelialization and inhibits platelet adhesion under sheer stress” by Chen L, He H, Wang M, Li X, Yin H. Tissue Eng Regen Med. 2017;14:359–70 (https://doi.org/10.1007/s13770-017-0044-3). © The Korean Tissue Engineering and Regenerative Medicine Society and Springer Science+Business Media Dordrecht; Figure 4B was adapted from “Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications” by Ren X, Feng Y, Guo J, Wang H, Li Q, Yang J, et al. Chem Soc Rev. 2015;44:5680–742 (https://doi.org/10.1039/C4CS00483C). © The Royal Society of Chemistry; Figure 4C was adapted from “Effect of RGD nanospacing on differentiation of stem cells” by Wang X, Yan C, Ye K, He Y, Li Z, Ding J. Biomaterials. 2013;34:2865–74 (https://doi.org/10.1016/j.biomaterials.2013.01.021). © Elsevier.
Lubricant-infused surface (LIS) coatings
While the above-mentioned strategies have shown promising results, they rely on a single line of defense that renders thrombosis a problem once the coating is depleted. Although bioinert coatings provide a second line of defense, the lack of endothelialization is problematic resulting in a low patency rate [27, 47, 73]. To overcome the limitations of the coatings reported thus far, LIS provides a highly versatile coating consisting of an underlying flat or rough solid substrate that locks onto the surface through van der Waals and capillary forces and provides a thin, stable, and dynamic per-fluorinated lubricant layer [80–88]. By either exploiting the innate chemical properties of the substrate or chemically modifying the substrate using silanization techniques (Figure 5A), a compatible-lubricant infused platform is created which energetically favors the lubricant layer rather than other contaminating liquids and forms an interface that repels aqueous, organic, and complex biological fluids, as well as prevents cell adhesion and biofilm formation [29].
Since teflon is synthesized from a fluorocarbon-based polymer, it does not require further chemical modification because its porous structure is compatible with per-fluorinated lubricants. In contrast, epoxy-resin-based substrates need to be chemically modified through silanization with a fluorosilane monolayer prior to infiltration with the lubricant layer. Silanization techniques mainly involve liquid phase deposition (LPD) or CVD. However, the results of studies with slippery lubricant-infused coronary catheters modified using LPD or CVD techniques suggest that CVD treatment provides better resistance against thrombosis than LPD treatment. This finding mainly reflects the exposure to acids in the liquid phase during LPD treatment, which produces etching and damage to the inner layers [89–91]. Common lubricants reported in the literature include Krytox-100, Krytox-103, perfluoroperhydrophenanthrene, perfluorodecalin, perfluorohexane, and perfluorooctane [92].
LIS coatings have been extensively used in healthcare applications and have shown compatibility with medical-grade materials such as poly(methyl methacrylate), PET, ePTFE, polyether amide, polycarbonate, and PU [3, 93]. LIS-coated implants have been shown to outperform conventional surface coatings by efficiently reducing clot formation and device-associated infection. For example, PTFE-based LIS coating infiltrated with Krytox-103 revealed a 35-fold reduction in biofilm formation compared with PEG-coating. Additionally, compared with untreated grafts implanted in male Wistar rats, lubricant-infused ePTFE grafts exhibited a 99% reduction in Staphylococcus aureus (S. aureus) adhesion and a reduced local inflammatory response [25].
Slippery-liquid infused porous surface (SLIPS), a specific type of LIS that involves infusing a lubricating liquid into a micro- or nano-structured porous substrate, has also been explored to reduce thrombosis [27, 40, 94–97]. The two primary features for the design of SLIPS that are required to create an immobilized liquid surface include the capacity for physical entrapment of a liquid within a porous or nanostructured solid substrate and high chemical affinity between the liquid and solid as defined by surface energy parameters [98]. ePTFE infused with various perfluorocarbon liquids generated SLIPS surfaces that exhibited a 99% reduction in S. aureus adhesion with preservation of macrophage viability, phagocytosis, and bactericidal function. Notably, SLIPS modification of ePTFE prevents device infection with S. aureus in vivo, while eliciting a significantly attenuated innate immune response [94]. Studies also showed that SLIPS-modified implants decrease macrophage inflammatory cytokine expression in vitro, which likely contributed to the presence of a thinner fibrous capsule in the absence of bacterial challenge [94, 96]. Furthermore, research comparing liquid infusion on flat versus porous hierarchical surfaces has demonstrated superior anticoagulant properties on rough porous surfaces, showing significantly reduced thrombus formation when tested in whole blood circulation in vitro. This effect is attributed to the enhanced stability of the infused liquid on rough surfaces [95].
Although LIS coatings have shown exceptional anti-thrombogenic results, they lack bio-functionality and inhibit all bio-interactions with the surface. To address this issue, bio-functional lubricant-infused (BLIS) surfaces were developed to allow targeted binding of ECs and tissue integration on the interface without compromising the repellency of the surface (Figure 5B). BLIPS were created on oxygen plasma-treated ePTFE vascular grafts using (3-aminopropyl) triethoxysilane (APTES) silanized anti-CD34 bio-links, infiltrated with perfluoroperhydrophenanthren lubricant. Relative to uncoated ePTFE grafts, BLIPS attenuated thrombin generation and resisted blood clot formation and non-specific protein adhesion. In addition, a confluent endothelial layer was observed on the coated surfaces after incubation with a blood/EC mixture for four days reflecting the targeted binding of ECs from the complex mixture (Figure 5C) [29]. The simple fabrication process of BLIPS and their outstanding anti-thrombogenic performance render these coatings a promising candidate for the next generation of synthetic vascular grafts. Further studies are necessary for the translation of these coatings to real-life applications, such as optimization of the bioactivity of immobilized agents, as well as the stability and durability of the coatings for long-term applications [99, 100].
A limitation of LIS is its vulnerability to the hemodynamic nature of blood in vivo. Many studies illustrate that LIS exhibits shear-driven failure because of the flow of blood [101–104]. Vega-Sánchez and Neto [104] illustrated that LIS perpendicular to the flow direction experienced significant lubricant depletion. Furthermore, 20% loss of lubricant was associated with a loss of function as it was observed that the lubricant became superfluous regardless of viscosity relative to the flowing liquid [104]. A study examined the surface modification of glass tubes coated with lubricant to assess the potential of liquid perfluorocarbon to prolong to lifespan of LIS in physiological conditions [101]. Exposing the LIS-treated tubes to a shear rate similar to that experienced by arteries (2,900 s–1), untreated samples lost their coating after 8 seconds whereas those treated with liquid perfluorocarbon, maintained their integrity and remained functional for many tens of minutes [101].
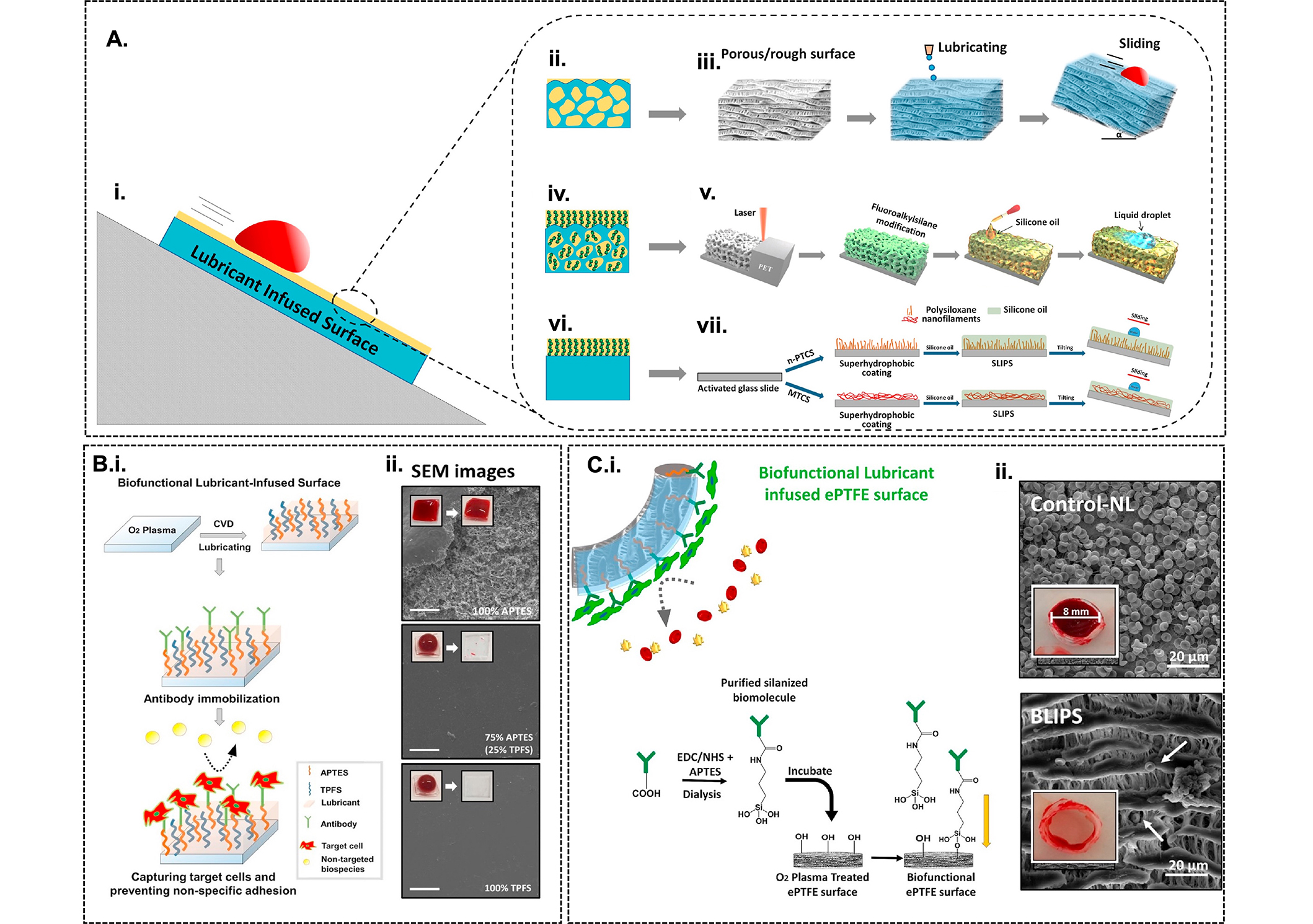
LIS-coated vascular grafts. (A) Illustration of the wetting behaviour of LIS coatings [29]. LIS PTFE/ePTFE nanofibrous membranes are created using innate chemical properties of the rough surface (ii and iii). Chemical modification of porous PET surfaces by femtosecond laser direct writing and functionalization with fluoroalkyl-silane followed by inflation with silicone oil (iv and v). Chemical modification using oxygen plasma treatment on flat glass substrates and functionalization using n-propyl trichlorosilane (n-PTCS) or methyl trichlorosilane (MTCS) and infiltrated with silicone oil to create polysiloxane nanofilament coatings (vi and vii); (B) fabrication of BLIS coated with anti-CD34 antibody using CVD of mixes silanes (i) and SEM images depicting the plasma clotting assay experiments (ii) [105]; (C) Development of BLIS on ePTFE grafts using silanized bioinks and ii) SEM images from blood clotting assay depicting the significant reduction of blood clot adhesion on BLIPS relative to non-lubricated control ePTFE grafts. LIS: lubricant-infused surface; ePTFE: expanded polytetrafluoroethylene; PET: polyethylene terephthalate; BLIS: bio-functional lubricant-infused; CVD: cardiovascular disease; SEM: scanning electron microscopic
Note. Figure 5A and C was adapted from “Single and multi-functional coating strategies for enhancing the biocompatibility and tissue integration of blood-contacting medical implants” by Badv M, Bayat F, Weitz JI, Didar TF. Biomaterials. 2020;258:120291 (https://doi.org/10.1016/j.biomaterials.2020.120291). © Elsevier; Figure 5B was adapted from “Lubricant-infused surfaces with built-in functional biomolecules exhibit simultaneous repellency and tunable cell adhesion” by Badv M, Imani SM, Weitz JI, Didar TF. ACS Nano. 2018;12:10890902 (https://doi.org/10.1021/acsnano.8b03938). © American Chemical Society.
Surface modification of decellularized grafts
Novel approaches of developing vascular grafts have resulted in tissue-engineered decellularized models. These grafts are created by removing vasculature from animal models such as canines or cows and exposing them to multiple rounds of decellularization solution. The remaining vessel is disinfected and ready for in vivo implantation. The previously mentioned article by Dimitrievska et al. [71], functionalized their decellularized vascular grafts using HA which improved its long-term biocompatibility. Another study showed surface modification of bovine carotid arteries with human endothelial progenitor cells and umbilical cord-derived mesenchymal stem cells. This resulted in additional thromboresistance as there was a reduction in CD41 positive platelets compared to unmodified grafts [106]. The proposed mechanism of reduced thrombosis formation by promoting endothelization of vascular grafts [106]. Another approach used high hydrostatic pressure (HHP) to decellularize slices of porcine aortic endothelium and reintroduce human umbilical vein ECs [107]. This study concluded similar findings with recellularized tissue-engineered graphs showing low clot formation similar to that in the negative control [107].
Translation of anti-thrombogenic coatings to clinical applications
The implementation of surface modification techniques for vascular grafts presents several challenges. These include achieving uniform coating thickness and coverage, ensuring long-term stability and durability of the coating under physiological conditions, avoiding cytotoxicity or immunogenicity of the coating materials, and maintaining desired surface properties despite mechanical wear or environmental factors [20, 47, 69, 108]. Additionally, scalability and cost-effectiveness are crucial considerations, especially for clinical translation. Addressing these challenges is vital for the successful application of surface modification techniques to enhance the performance of blood-contacting biomaterials [4, 18, 109].
Prior to implantation, the hemocompatibility of implants needs to be analyzed and must fulfil the guidelines developed by the International Organization for Standardization (ISO 10993-4). Several in vitro testing models are available to assess the hemocompatibility of implants by incubating them in human blood under static, agitated, or shear flow conditions (Figure 6A) [24, 38].
The first step is the evaluation of the thrombogenicity of blood-contacting devices under static conditions. Subsequently, assessment of the hemodynamic behaviour under dynamic flow is crucial, namely the shear-induced activation of blood cells and proteins, as well as the flow-dependent transport of procoagulant cells and proteins. Recognizing the importance of the interaction of blood flow and the biomaterial surface in a dynamic context, studies have focused on developing in vitro testing platforms such as tubular and cone-and-plate viscometers, parallel plate flow chambers, and microfluidic flow chambers [23, 110, 111]. Among these techniques, the use of microfluidic devices provides the most efficient approach because of the short analysis times, versatility of fabrication protocols, minimal consumption of reagents, high resolution and sensitivity of fluid manipulation, and portability (Figure 6B). The advantages of microfluidic devices have driven the development of organ-on-a-chip (OOC) technology to model conditions present in the human bloodstream, specifically vascular-like flow conditions and vascular-like microenvironments. Recently, a microfluidic platform for in vitro testing was fabricated to compare the thrombogenicity of LIS and anti-CD34 coated ePTFE vascular grafts under arterial wall shear stress and with and without ECs under flow conditions. Using this “vascular graft-on-a-chip” device, the thromboresistance of the coated vascular grafts was established under flow conditions and the attenuated thrombin generation and fibrin deposition was monitored in real-time, thus facilitating the translation of synthetic grafts into the clinical space (Figure 6C). Once results from benchtop testing have met the guidelines developed by ISO 10993-4 and the biomaterial is safe for use in vivo, computational in silico testing and studies in animals are the next steps [3, 38]. Common animal species used for such testing include mice, rabbits, goats, and pigs. Computational fluid dynamics and mathematical algorithms are mainly used to model thrombus growth, platelet activation, and platelet aggregation for in silico models [3].
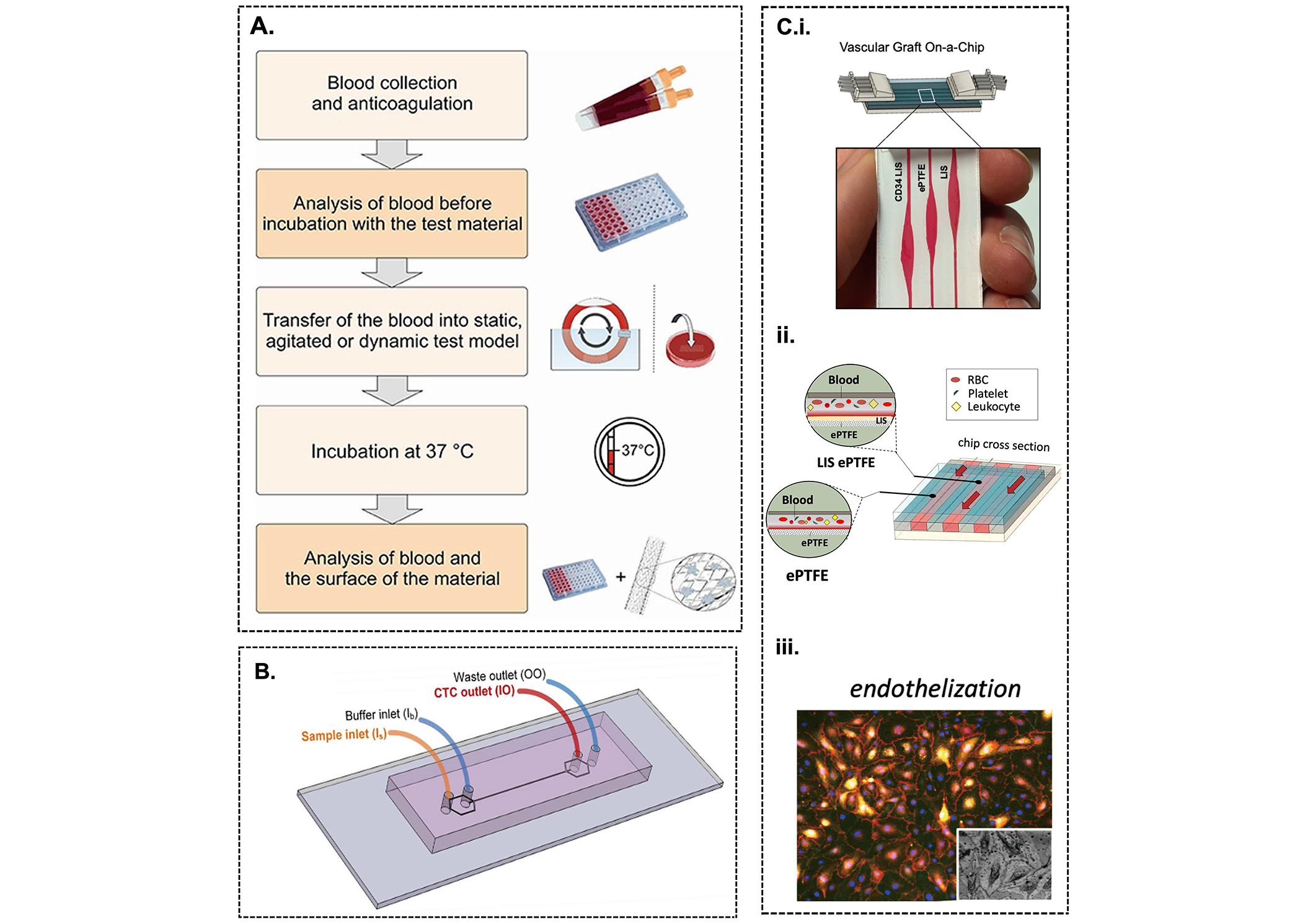
Evaluation of the hemocompatibility of synthetic grafts. (A) Representation of the procedure to analyze the interaction of blood cells and proteins with a biomaterial surface [38]; (B) testing the hemocompatibility of a vascular graft using a microfluidic chip; (C) schematic of a vascular graft-on-a-chip to test the thrombogenic performance of LIS and anti-CD34 coated ePTFE vascular grafts in the presence of arterial wall shear stress with and without endothelial cells (i-iii) [112]. LIS: lubricant-infused surface; ePTFE: expanded polytetrafluoroethylene; RBC: red blood cell
Note. Figure 6A was adapted from “Blood-contacting biomaterials: in vitro evaluation of the hemocompatibility” by Weber M, Steinle H, Golombek S, Hann L, Schlensak C, Wendel HP. Front Bioeng Biotechnol. 2018;6:99 (https://doi.org/10.3389/fbioe.2018.00099). CC BY; Figure 6B was adapted from “Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel” by Zhou J, Kulasinghe A, Bogseth A, O’Byrne K, Punyadeera C, Papautsky L. Microsystems & Nanoengineering. 2019;5:8 (https://doi.org/10.1038/s41378-019-0045-6). CC BY; Figure 6C was adapted from “A vascular graft on-a-chip platform for assessing the thrombogenicity of vascular prosthesis and coatings with tuneable flow and surface conditions” by Bot VA, Shakeri A, Weitz JI, Didar TF. Adv Funct Mater. 2022;32:2205078 (https://doi.org/10.1002/adfm.202205078). CC BY.
Conclusions
Despite the progress in the fabrication and modification of synthetic vascular grafts over the years, many of these coatings have only been tested in vitro and if evaluated in animal models, have only shown promise for short-term applications. Novel strategies that exploit the regenerative biological pathways may lead to new synthetic vascular materials or/and coatings that permit vascularized, non-fibrotic tissue reconstruction. LIS coatings hold great promise as a surface modification strategy for vascular grafts, although further research on the stability and durability of the lubricant layer is necessary.
The development of stable, biocompatible, and efficient surface coatings is essential for long-term applications. The fabrication of biocompatible synthetic vascular grafts that promote endothelization will pave the way for more advanced treatment options and customizable products for various clinical applications and pathological environments.
Abbreviations
| Cu-MOFs: | copper-metal-organic frameworks |
| CVD: | cardiovascular disease |
| ePTFE: | expanded polytetrafluoroethylene |
| HA: | hyaluronic acid |
| LIS: | lubricant-infused surface |
| LPD: | liquid phase deposition |
| MPC: | 2-methacryloyloxyethyl phosphorylcholine |
| PEG: | poly(ethylene glycol) |
| PET: | polyethylene terephthalate |
| PU: | polyurethane |
| RGD: | Arg-Gly-Asp |
| SLIPS: | slippery-liquid infused porous surface |
| TF: | tissue factor |
| VEGFR: | vascular endothelial growth factor receptor |
Declarations
Author contributions
NAJ: Conceptualization, Writing—original draft, Writing—review & editing. AC: Writing—original draft, Writing—review & editing. JIW: Validation, Writing—review & editing. TFD: Validation, Proof-reading, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Conflicts of interest
Tohid F. Didar, who is the Editorial Board Member of Exploration of BioMat-X, had no involvement in the journal review process of this manuscript. The other authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was supported by an NSERC Discovery grant and an Ontario Early Researcher Award to TFD as Tier II Canada Research Chair. JIW holds the Canada Research Chair (Tier I) in Thrombosis and the Heart and Stroke Foundation J. F. Mustard Chair in Cardiovascular Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The author(s) 2024.