Affiliation:
1Department of Biomedical Engineering, Faculty of Medicine and Health Sciences, McGill University, Montreal, QC H3A2B4, Canada
2Shriner’s Hospital for Children – Research Center, Montreal, QC H4A0A9, Canada
ORCID: https://orcid.org/0000-0002-2689-0991
Affiliation:
1Department of Biomedical Engineering, Faculty of Medicine and Health Sciences, McGill University, Montreal, QC H3A2B4, Canada
2Shriner’s Hospital for Children – Research Center, Montreal, QC H4A0A9, Canada
3Faculty of Dental Medicine and Oral Health Sciences, McGill University, Montreal, QC H3A1G1, Canada
ORCID: https://orcid.org/0000-0003-2907-3580
Affiliation:
4Institute of Biomaterials, Department of Material Science and Engineering, University of Erlangen-Nuremberg, 91058 Erlangen, Germany
ORCID: https://orcid.org/0000-0002-7377-2955
Affiliation:
1Department of Biomedical Engineering, Faculty of Medicine and Health Sciences, McGill University, Montreal, QC H3A2B4, Canada
3Faculty of Dental Medicine and Oral Health Sciences, McGill University, Montreal, QC H3A1G1, Canada
Email: maryam.tabrizian@mcgill.ca
ORCID: https://orcid.org/0000-0002-5050-4480
Explor BioMat-X. 2025;2:101327 DOI: https://doi.org/10.37349/ebmx.2025.101327
Received: October 22, 2024 Accepted: January 20, 2025 Published: February 14, 2025
Academic Editor: Rongchang Zeng, Xi’an Jiaotong University, China
The design of effective treatments for critical size bone defects, which result from various conditions such as trauma, infection, injury, or tumor resection, presents a significant challenge in clinical practice. While autologous grafts are commonly regarded as gold standard treatments in these complex healing scenarios, they are often associated with notable limitations, including donor site morbidity and limited graft volume. As a result, recent research trends have shifted towards developing biomaterials that better emulate the inherent complexity of the native bone structure and function through implementation of a “Diamond Concept” polytherapy strategy. Central to this approach is the utilization of biomaterials, increasingly composed of composite materials that integrate bioactive osteoinductive factors and cell sources to enhance healing outcomes. The usage of Wnt signaling specific agonists as osteoinductive mediators has been recently shown to be a promising strategy for promoting healing, as this pathway is well established to have an important role in both osteogenic differentiation and bone formation processes. Implementation of a localized delivery system through scaffold incorporation is necessary in this scenario, however, to minimize any potential off-target effects caused by the Wnt signaling cascade’s non-specificity to bone. Findings in the literature clearly show that this approach holds promise to improve clinical healing outcomes, paving the way for more effective treatment options. In this review, we will generally discuss the design of biomaterials, specifically bulk materials and composites, for the treatment of critical size bone defects. Additionally, we will highlight recent work on the design of chitosan-based scaffolds modified with purine crosslinking, to overcome cytotoxicity issues associated with other chemical crosslinkers. In this context, we focus on optimizing material design for this bone healing application and discuss the benefits of localized Wnt agonist as mediators to improve the scaffold’s osteoinductive behavior.
Bone, as the main part of the body’s skeletal system, is responsible for a variety of crucial functions such as providing locomotion, protecting soft tissues, and acting as a storage site for minerals and bone marrow [1–5]. It’s ability to provide these functions is a testament to its structurally dynamic and complex architecture that is highly adaptive based on mechanical demands [1, 2, 6]. One of the most common traumatic injuries within the musculoskeletal system are bone fractures, which are caused by exertions of significant force that overpower what the bone can withstand, leading to a crack or break [7–10]. The intricate activities and organization of the native tissue, however, allows for a built-in complex healing mechanism for these scenarios, in which fractures can repair themselves following damage without scarring [7, 9, 11–15].
In contrast to this, critical size defects, another source of bone related injuries, are defined by their inability to spontaneously heal despite the introduction of surgical stabilization [16–20]. This category often stems from large bone loss as a result of traumas, infections, diseases, or non-unions, and represents a major clinical challenge in the domain of orthopedics [16, 17, 20–23]. Consensus on the exact definition of this term is not currently well established in the field, as many considerations play a factor including the condition of the soft tissue, anatomical location, presence of adequate blood supply, and overall patient medical history [16, 17, 21, 23]. However, it is generally accepted in the literature that if the defect length is larger than 2 cm, with a concurrent loss of more than 50% of bone circumference, the terminology can be applied, and a surgical intervention is needed for effective bone healing [16, 17, 24].
A variety of treatment methods for these defects have been explored clinically, both through the incorporation of different grafting materials such as autografts and allografts, as well as surgical techniques including distraction osteogenesis or the induced membrane technique. While the effective management of these defects has been shown to be complex, the current clinical gold standard approach for treatment is the autologous grafting technique [13, 16–18, 22, 25, 26]. This method boasts many advantages including its low cost, high incorporation rate, lack of disease transmission and immunocompatibility, as it is taken directly from a patient’s own body at a distal site from the defect [17, 22, 24, 27, 28]. Despite these beneficial traits, autologous grafts are often associated with numerous disadvantages as well accompanying its usage, with the most important one being its limited graft volume [13, 16, 17, 22, 23]. Other limitations to these grafts include large rates of complications, donor site morbidity, infection, and long hospitalization times [16, 17, 19, 22–24, 29, 30].
The evident limitations associated with clinical treatments have led researchers to use the established principles of engineering and biology to design and fabricate artificial bone specific microenvironments capable of encouraging repair in large defects [31–38]. These substitutes are expected to act similarly to the native tissue in terms of both architectural structure as well as intended function [34, 35, 39]. Thus far, no specific design of these biomaterials has been able to successfully satisfy the complex criteria needed to be considered ideal for bone tissue regeneration [37]. The most important of these requirements is the notion of biocompatibility, meaning that the implanted material should elicit an appropriate host response that is specific for the intended application [33, 35, 37, 40–44]. This new definition of the term considers the material’s functionality as well as its intended target for regeneration, which was not previously included. Triggering of the foreign body response post-implantation can lead to specific fibrin adsorption onto the scaffold’s surface, ultimately leading to faster material resorption and possible harmful effects on the patient [37, 42].
To successfully accomplish the design of these biomaterials, researchers have set forth a polytherapy framework called the “Diamond Concept”, implementing five main components: osteoconductive scaffolds, osteogenic cells and osteoinductive mediators within a suitable mechanical environment incorporating an appropriate vascularization strategy [18, 32–34, 41, 43, 45, 46]. The biomaterial itself in this context is typically composed of either metals, ceramics, polymers or composites, which act as a 3D microenvironment that provides structural support and promotes the functions of encapsulated cells [25, 43, 47, 48]. Among these substitutes, injectable hydrogels, composed of a cross-linked hydrophilic polymer network, have recently gained interest in these clinical applications due to their advantageous properties, including the potential for less invasive surgeries and decreased hospitalization times [19, 49–51]. In our laboratory, we have previously developed one of these injectable hydrogel scaffolds, which forms through electrostatic attractions between anionic phosphate groups of a guanosine diphosphate (GDP) purine crosslinker and cationic amine groups of the chitosan polymer [19, 49, 52–57]. Results found that crosslinking occurs rapidly (<1.6 seconds) in this scaffold without the need for external stimuli, such as pH or temperature changes, thus limiting unwanted solution diffusion into surrounding tissues, ensuring localization at the defect site, and allowing for an easy and efficient encapsulation of cells and factors [58–60].
The notion of this polytherapy approach follows the more recent progression from the implementation of bioinert to bioactive materials within bone applications, with research showing that osteoconductivity and osteoinductivity are equally important design parameters, and that the scaffold should have the ability to interact with cells in the local microenvironment [31, 33, 38, 39, 42, 61–64]. The combination of these five aspects is expected to more carefully mimic the natural healing environment of bone, thereby encouraging cell and tissue growth as well as bone regeneration. Incorporation of cells within biomaterial designs has been evidenced to significantly improve healing outcomes through the cells’ ability to secrete extracellular matrix proteins as well as different growth factors and cytokines [33, 34, 65]. Different studies in the literature have indeed confirmed this benefit, demonstrating a significant lack in cellular ingrowth from the surrounding host tissue in non-cell-loaded scaffolds [66].
Osteoinductive mediators, such as bone morphogenetic proteins (BMP), platelet derived growth factors (PDGF), and biologically active ions, have also long been explored as encapsulants in biomaterial scaffold design due to their well-established ability to promote cellular growth, differentiation and bone formation [33, 45, 67–74]. While BMPs, particularly BMP2, remain gold standards in osteoinduction, recent research has shifted towards finding alternative approaches that can overcome some of BMPs’ limitations, namely their high cost and possibilities for ectopic bone formation. Usage of glycogen synthase kinase 3 (GSK3) inhibitors, for example, are one of these newer approaches, that have been shown to have a positive effect on both osteogenic differentiation and subsequent bone formation. Some studies in the literature have indeed reported significant increases in the early osteoblastic differentiation marker, alkaline phosphatase (ALP) and osteogenic gene expression levels with this addition in cultures of different cell types compared to respective controls [75–83].
The overall success of these GSK3 mediators can be heavily attributed to their well-established role as agonists to the Wnt/β-catenin signaling pathway, which plays a critical role in osteogenic processes, including both differentiation and bone formation [84–92]. Canonical Wnt signaling is initiated when Wnt proteins bind to lipoprotein receptor-related protein 5 and 6 (LRP5/6) and frizzled co-receptors, triggering an intracellular cascade that activates bone formation [87, 93]. As the loss of function of the LRP5 co-receptor has been shown to be linked to a decrease in bone mass, similar to cases of osteoporosis, this further highlights the importance of this pathway in skeletal development [87, 94, 95]. The binding of Wnt proteins to these receptors results in a disbanding of the destruction complex, which in turn prevents the phosphorylation and subsequent degradation of cytoplasmic β-catenin [84, 87, 93]. Due to this action, the stabilized β-catenin that has accumulated in the cytoplasm can then translocate into the nucleus, where it binds to transcription factors such as lymphoid-enhancer binding factor (LEF) and T-cell specific transcription factor (TCF) to successfully upregulate osteogenic target genes [84, 87, 93]. Interestingly, activation of Wnt signaling has also been demonstrated to regulate osteoclastogenesis, by increasing osteoprotegerin expression, which can aid further in healing [94–97]. The dual function of Wnt signaling renders it an attractive target for enhancing the osteoinductive properties of biomaterials, offering a promising potential as a mediator for improving bone repair and regenerative outcomes.
Therefore, in this review, the landscape of materials for scaffold design is discussed with respect to bone regenerative applications in defect healing specifically, emphasizing the recent shift from single material scaffolds to those made of composites, or modified using coatings and nanoparticles (Figure 1A). Additionally, we provide a summary of previous work on the usage of purine crosslinkers, for the first time, within our laboratory’s chitosan based crosslinked scaffold with an aim of improving bone repair and regeneration. The current advancements on the incorporation of Wnt signaling mediators in biomaterial scaffold design are also presented, reflecting the growing interest in these strategies for promoting osteoinduction in these biomaterials. Although only 42 articles have been published on this topic over the past decade, the increasing number of publications each year, along with promising findings, suggest a clear trajectory for future research (Figure 1B, Figure 1C).
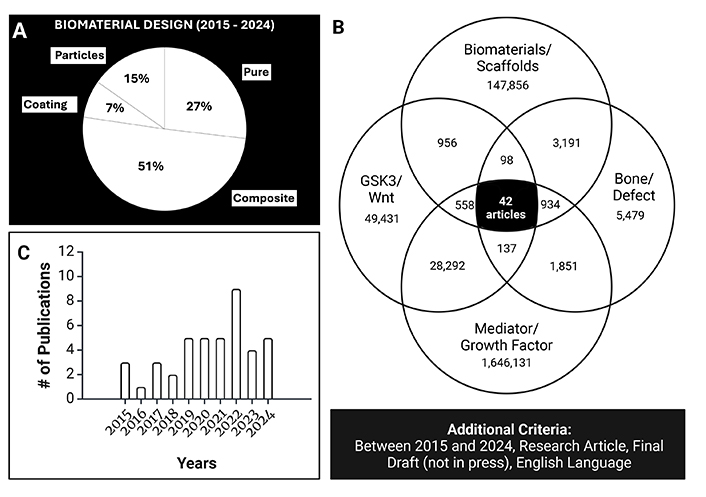
Scopus review of the recent literature (2015 to 2024). (A) Percentage of articles using either single material scaffolds, composites, coatings, or particle additions (n = 908 articles). (B) Number of articles amidst the different keywords in Scopus search. (C) Graph of the increase in publications over time examining and considering Wnt signaling or targeting Wnt signaling. B and C were created in BioRender. Agnes, C. (2025). https://BioRender.com/b68w447
The consideration of the specific clinical application is crucial within the design process as singular situations will necessitate different priorities with respect to scaffold properties [37, 39, 42]. Since bones often serve load-bearing purposes, such as the continued compressive and torsional loading of long bones, the mechanical properties should be one of the key considerations for choosing a material [33, 35]. The intended substitute needs to be able to withstand the expected loading conditions so as not to allow for tissue collapse, and modulus values need to match those of the native tissue surrounding the defect site [33, 37, 38]. In cases where the mechanical properties exceed that of the natural bone tissue, a phenomenon called stress shielding occurs, which correlates with excessive resorption [37].
The scaffold’s architecture is another important consideration with respect to material selection, including the geometrical shape and porous structure [18, 42]. Geometrically, scaffolds have been shown to exist in a variety of forms for bone related applications such as porous or fibrous scaffolds, microspheres and hydrogels, each of which has its own associated advantages (Figure 2A) [37, 46, 98]. Porous and fibrous scaffolds consist of a 3D solid matrix that contains a large number of pores, thereby providing a supportive environment for cellular adhesion, growth and differentiation [31, 32, 34, 35, 46, 98]. Indeed, a high porosity, interconnectivity and surface to volume area have shown to be beneficial not only in terms of cellular functionality, but also in their ability to allow for the ingrowth of bone and invasion of blood vessels thereby providing nutrients and oxygen [32, 42, 67–69, 98]. It has been established that designed scaffolds for bone should have pore sizes between 100 to 750 μm as values outside of this threshold lead to impairment of processes needed for successful healing [32].
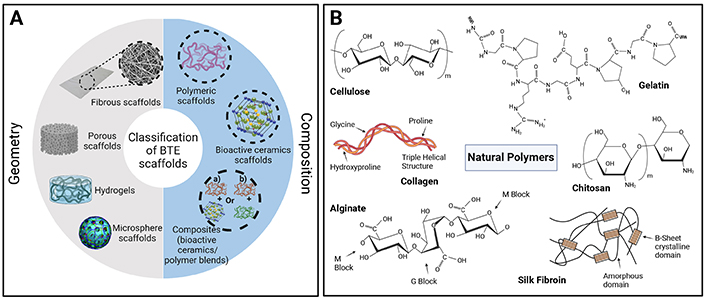
Tissue engineered scaffold structure and composition. (A) Methods for defining scaffolds in terms of both their geometry and composition. Reproduced from Ref. [3] under CC-BY conditions from MDPI. (B) Examples of natural polymers used in bone tissue engineering applications. Schematic B was created in BioRender. Agnes, C. (2025). https://BioRender.com/z45x380
Hydrogel based scaffolds, on the other hand, consist of a 3D hydrophilic network, and are known to be beneficial in terms of their easy ability for encapsulation and protection of cells and factors [46, 98, 99]. However, due to their inherent properties and fabrication through means of physical or chemical crosslinking, these scaffolds are often associated with low mechanical strength, which is not significantly beneficial for load bearing applications [46]. The third geometrical class of scaffolds belongs to micro- and nanospheres, which are sphere shaped scaffolds containing a dense core layer [46]. These scaffolds are mostly used as delivery mechanisms for cells and factors and can be fabricated through phase separation or solvent extraction [46, 98].
As the scaffold is expected to generally promote bone ingrowth over time, the base material selected likewise needs to be able to undergo gradual biodegradation through chemical or enzymatic breakdown within the body [35, 39, 40]. The rate of degradation, however, needs to correlate with the rate of bone deposition, so that it can gradually transfer the mechanical load to the newly formed tissue [37, 43, 44, 100]. A consideration for the byproducts of scaffold degradation is also warranted as these need to be biocompatible and able to be removed by the body without significant alteration to mineralization or healing processes [37, 38].
Material options for substitute design in bone tissue engineering are derived from a variety of sources both natural and synthetic and are typically characterized as metals, ceramics or polymer based (Figure 2A, Table 1) [18, 32, 34, 37, 38, 61, 99]. Metals such as tantalum [101–104], stainless steel [105, 106], cobalt chromium [107, 108], nickel [109], titanium [102, 104], magnesium [110], and their alloys have long been used in bone repair scenarios as implants, due to their exhibited high mechanical properties, including both stiffness and strength, which satisfy load bearing conditions of bone replacement [18, 32, 34, 40, 42, 61–63, 111, 112]. However, this category of materials is usually associated with limitations due to the stress shielding phenomenon caused by an imbalance in mechanical properties to native tissue, thus resulting in material corrosion, and implant loosening [35, 113].
To overcome this challenge, researchers have begun to implement porous structures to the material, thereby allowing for a reduction in the elastic modulus and an increase in cellular functionality and bone ingrowth [33, 114–116]. Specific types of metals such as titanium and its alloys have also been more recently explored as base materials, since the alloys can maintain a passive film on their surface protecting them from corrosion [32, 117]. Titanium and its alloys are heavily present as biomaterials along with cobalt alloys within the orthopedic field, with uses as long-term implants such as hip and knee replacements as well as bone plates [32]. Yet, as a temporary scaffold for bone defect regeneration, this category presents a challenge as their characteristic non-degradable tendency necessitates a second surgery for scaffold removal [40].
Summary of common scaffolding materials for bone tissue engineering. The main advantages and disadvantages are presented with examples for each material. Adapted from Ref. [37] with permission from Springer Nature, © 2025 Springer Nature
| Material Type | Main Advantages | Main Disadvantages | Examples | Refs |
|---|---|---|---|---|
| Natural polymers | Biomimetic; some contain cell-adhesion sites; low cost | Low mechanical properties (for example, stiffness); potential immunogenicity; batch-to-batch variability | Collagen or gelatinSilkAlginate | [118–120][121–123][124–127] |
| Synthetic polymers | Wide range of compositions and properties; ease of modification | Some produce undesirable or acidic degradation products | Poly(lactic-co-glycolic acid)Poly(propylene fumarate)Poly(ɛ-caprolactone) | [124, 128, 129][130–132][130, 133–135] |
| Bioceramics | High compressive modulus; capable of delivering bioactive ions | Brittleness | Hydroxyapatiteβ-Tricalcium phosphateBioactive glasses (such as 45S5 composition) | [129, 133, 136, 137][128, 138–140][118, 141–143] |
| Biodegradable metals | High compressive strength | High corrosion rate; require high-temperature processing | Magnesium and its alloys | [144, 145] |
| Carbon-based nanomaterials | High tensile strength; ease of functionalization using surface groups | Limited biodegradability; potential cytotoxicity | Carbon nanotubesGraphene or graphene oxide | [146, 147][148–152] |
The next category of materials, which are found to be heavily present in current scaffold designs, are the inorganic bioceramics [42]. Their large presence within this research domain can be explained by their exceptional biocompatibility, osteoconductivity, and bioactivity, yet traditional ceramics exhibit brittleness due to their internal covalent bonding and high porosity, which provide them with a weakness to shear or tension forces. This limitation thus renders their usage challenging for load bearing applications [32, 34, 35, 38, 40, 42, 44, 61, 112, 153].
Most commonly in studies, these take the form of calcium phosphates (CaP) such as hydroxyapatite (HA) [154–157], tri-calcium phosphate (TCP) [158–160] or biphasic calcium phosphates (BCP) [161–164], since they contain calcium and phosphate ions that are similar to native bone tissue mineralization structure [18, 34, 35, 38, 40, 42]. The composition of these ceramics encourages additional benefits in that their degradation byproducts can further promote bone formation and mineralization [35]. BCPs have been proven to be a useful alternative to HA and TCP alone, since their combination allows for more ideal scaffold resorption rates with high osteoinductivity [40]. Other common materials that make up this category are bioactive glasses (BGs) in different compositions [34, 40, 165]. BGs are often used in bone regeneration applications as they have been shown to be biocompatible exhibiting high binding affinity to biological tissue [34, 40]. Implantation and subsequent contact of the BGs with bodily fluids, interestingly, allows for the formation of an HA or CaP layer on the scaffold’s surface [38, 40]. In addition, the byproducts of degradation for these materials include various ions such as Na, Ca, Si, and P, which are known to improve osteogenic and angiogenic functionality, leading to enhanced healing outcomes [34, 38, 40]. BGs can also be doped with other ions, notably ions that can induce the osteogenic and angiogenic effects, such as Cu, Co, Sr, B, etc. [73, 166, 167].
Polymeric biomaterials represent the third class of materials and are composed of long repeating monomer chains with covalent bonding in between [37]. Within this category, materials can be further segregated depending on their source, whether that be natural polymers from living organisms or those of synthetic derivation [18, 34, 37, 42]. The natural polymers, including collagen [168–170], gelatin [171], fibrin [172, 173], chitosan [174, 175], and hyaluronic acid [176, 177] amongst others, are well known specifically for their similarity in structure to the native extracellular matrix, rendering them highly biocompatible with minimal adverse reactions (Figure 2B) [32, 34, 38–42, 44, 46]. As an example, collagen, which is one of the most highly used scaffolding materials in this domain, is found within the body as a major organic component of the native extracellular matrix [18, 32, 35, 37, 40, 42, 46]. Indeed, studies in the literature have shown that this structural similarity allows for improved cellular adhesion, in addition to osteoblast function and vascularization [31, 40]. However, due to the acquisition of these materials from natural sources, this renders the possibility for inherent batch-to-batch variations, as well as a characteristically lower mechanical strength and a difficulty in effectively controlling the degradation rate [31, 32, 35, 40, 44, 48].
In contrast to these, synthetic polymers, such as polylactic acid (PLA) [178, 179], poly(lactic-co-glycolic) acid (PLGA) [180, 181], poly(glycolic) acid (PGA) [182], and polycaprolactone (PCL) [183], provide researchers with more control and tunability to a scaffold’s mechanical and degradative properties, allowing for the high strength and stiffness moduli needed for bone specific applications [32, 35, 38, 40, 41, 184]. While this can be seen as beneficial, the synthetic derivation of these polymers has been shown to result in limited biocompatibility with poor cellular attachment [31, 32, 34, 39, 41].
To overcome the evident limitations of the one-dimensional base material options, researchers have more recently shifted their attention towards the use of composites to better tune and match the material properties and functionality of the target host tissue [35, 39, 44, 54, 185, 186]. This is often accomplished in bone tissue engineering through either the blending of multiple polymers [187] or by a combination of polymers with bioceramics such as CaPs [18, 35, 44, 185, 188–190] or BGs [191, 192].
Incorporation of ceramics in polymeric scaffolds allows for biomaterials to more closely resemble bone structure, since the native tissue is also considered as a composite with HA mineral deposits dispersed in a collagen extracellular matrix [44]. This evident similarity, in turn, improves the bioactivity of the composite polymeric/ceramic scaffolds, with results in the literature showing a marked improvement in osteogenic differentiation and new bone formation in animal defect models [39, 185, 188–190]. A study by Nguyen et al. [188] also demonstrated that the incorporation of BCP with a polymeric hyaluronic acid and gelatin scaffold yielded a significant increase in compressive strength as well as porosity. This combination not only enhances the mechanical properties (compressive strength) of polymeric scaffolds, but also reduces the inherent brittleness found in bulk ceramic biomaterials [39, 44, 185, 186].
Further research involving the addition of layered silicate ceramic, montmorillonite (MMT) into a methacrylated glycol chitosan hydrogel demonstrated an enhancement of the scaffold’s material properties, allowing the scaffold to more closely meet the set criteria for bone tissue engineering scaffolds (Figure 3A) [193]. While Young’s modulus values were increased proportionally with concentration of MMT, the addition of the highest content of MMT (0.07 MPa) still failed to produce a value that could meet that of native bone, which often ranges between 5 to 21 GPa depending on anatomical location. This discrepancy can, however, be overcome compared to some other material properties through the addition of an external fixator to bear some of the mechanical load or through usage in non-load bearing conditions.
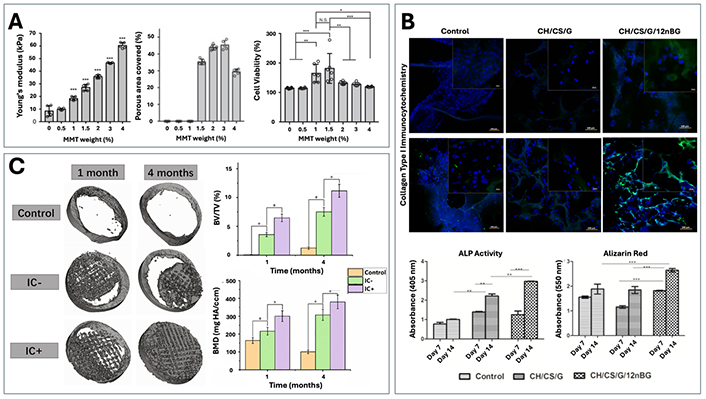
Examples of composite and modified scaffolds for optimizing material properties and encouraging bone regeneration. (A) Changes in Young’s modulus, porosity and BMSC cellular viability with the addition of layered silicate, montmorillonite (MMT), in various concentrations. Reproduced from Ref. [193] under CC-BY conditions from Springer Nature. (B) Evaluation of in vitro osteogenic differentiation (ALP, Alizarin Red, COLI) for a chitosan/chondroitin sulfate (control) scaffold with the addition of gelatin (CH/CS/G) and nano-BG (CH/CS/G/12nBG). Reproduced from Ref. [194] with permission from Elsevier. (C) Assessment of bone regeneration in a beagle calvarial defect model for calcium phosphate and sodium alginate scaffolds unloaded (IC–) or loaded with icariin (IC+). Reproduced from Ref. [195] with permission from Elsevier
Additionally, work by Singh et al. [194], found that the addition of nano-BG led to significant increases in collagen type I (COLI) expression, ALP activity and mineralization compared to control scaffolds made of chitosan, chondroitin sulfate, and gelatin (Figure 3B). These findings indicate that incorporating ceramics into composites can enhance biomineralization and matrix deposition in these designed scaffolds, while also improving their mechanical and material properties.
Another of the modifications to bulk materials that has been widely explored is the application of coatings often onto metallic or ceramic based implants to improve bone ingrowth and material bioactivity with the host tissue [32, 196–202]. A recent study by Su et al. [199] successfully demonstrated that by coating the surface of a titanium alloy scaffold with strontium and CaP, they were able to observe increased calcium ion deposition, as well as osteogenic gene expression [runt related transcription factor 2 (RUNX2), ALP, COLI] and new bone formation in a rabbit femoral defect model compared to control groups.
In addition to coatings, some studies have also investigated the usage of scaffolds as carrier mechanisms for bioactive molecules, as means to similarly promote bioactivity and osteoinductivity [193, 195]. Often, growth factors such as transforming growth factor (TGFβ), vascular endothelial growth factor (VEGF), BMP and PDGF are used for these scenarios as they are native to bone tissue and have been shown to have important roles within the fracture healing cascade [185]. Interestingly, others have also shown that more non-traditional mediators such as the flavonoid compound icariin, are able to effectively support further osteogenic activity when delivered in a controlled manner from biomaterials [185, 193, 195, 203]. For example, Sun et al. [195] examined the influence of icariin in CaP/sodium alginate composite scaffolds, with results showing an increase in new bone ingrowth, the bone volume fraction (BV/TV) and bone mineral density (BMD) (Figure 3C). The incorporation of these drugs introduces a new important characteristic of ideal scaffolds, which is the ability to maintain a controlled and gradual release of mediators from the biomaterials, without an initial bulk release as is often seen in the literature [185].
Following the selection of appropriate materials and before implementation in clinical settings, designed biomaterials undergo a rigorous optimization process incorporating material property evaluations to ensure that these match optimal scaffold criteria (Figure 4) [37]. Testing mechanisms for this evaluation include scanning electron microscopy (SEM) or micro-computed tomography (μ-CT) for scaffold architecture, rheology and compression/tension testing for viscoelastic and mechanical properties, as well as degradation testing to determine the rate of scaffold resorption [204]. Following this, cell sources such as mesenchymal stem cells (MSC) and pre-osteoblasts are used in vitro to measure the cytocompatibility and differentiation potential of the designed scaffold, most often to determine optimal groups before proceeding to in vivo studies [18, 37, 204]. At this point, a previously established animal model is selected based on the intended application, which could include defects in non-load bearing bones such as cranial/mandible defects, or long bones such as tibial or femoral defects [37, 204].
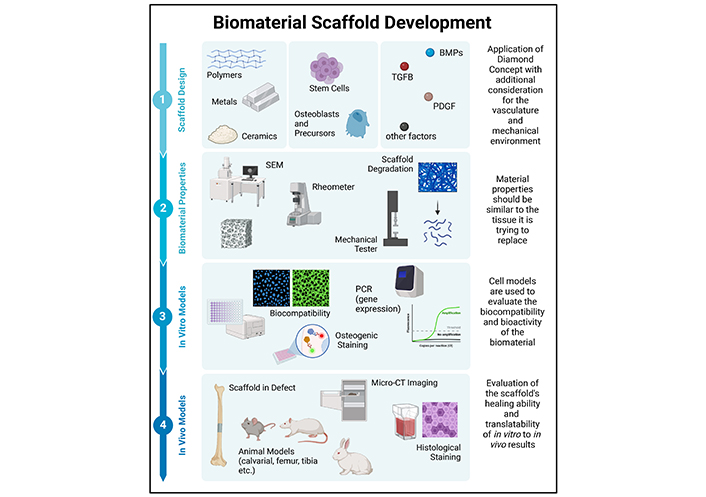
Schematic of the biomaterial developmental process from design up to immediately prior to clinical trials. This image was created in BioRender. Agnes, C. (2025) https://BioRender.com/k40b687
The natural polymer chitosan is one of the bulk materials that has been widely examined with respect to the growing field of injectable hydrogels and scaffolds, encouraging the need for less invasive surgical procedures and thus a decreased hospitalization recovery period [49–51]. This benefit comes from their inherent ability to solidify into 3D structures following in vivo application through the presence of external stimulants such as changes in pH, temperature, and UV irradiation [19, 56]. The gelation mechanism of injectable scaffolds also provides for an easy encapsulation technique for both cells and osteoinductive mediators, providing them with a hydrated internal environment [19, 50, 51, 205–208].
Usage of crosslinkers, such as genipin [209–211], glutaraldehyde [212–214], and tripolyphosphate [215, 216], has been well established in the injectable scaffold domain, meant to stabilize the chitosan scaffold’s internal structure and improve physical properties [54, 56, 217–219]. These evident benefits on material properties, in the scope of chitosan-based biomaterials for bone tissue engineering, have been thoroughly demonstrated in the literature and reviewed in a recent publication by the authors [204].
However, these gold standard crosslinkers tend to present cytotoxicity and compatibility issues in their usage, rendering a need for alternatives [19, 49, 52–57]. One of the more recently developed chitosan-based crosslinked scaffolds in this field, developed by our laboratory, relies on the addition of a purine as an anionic crosslinker for the first time, to overcome these previously demonstrated limitations [19, 49, 52–57]. Purines, such as adenine and guanine, and their derivatives are considered to be naturally biocompatible as they are involved in different biological processes within the body including the formation of nucleic acids, energy transfers and the actions of various signaling pathways [54, 58, 59, 220, 221]. Composed of a pyrimidine and an imidazole, purines tend to have a negative charge in solution, which helps crosslink the cationic chitosan chains together through mixing without the need of external triggers or stimuli [58–60].
Both GDP and adenosine diphosphate (ADP) have been investigated as crosslinkers for chitosan within the scope of this scaffold. Benameur et al. [56] demonstrated that while a sponge will form with either crosslinker through the ionic interaction between phosphate groups on the purines and NH2 groups on chitosan, slight differences are observed when GDP is used compared to ADP. GDP crosslinking allows for additional Hoogsten hydrogen bonding between the guanine groups resulting in the formation of G-tetrad-like structures (Figure 5A) [56]. In comparison, ADP crosslinking is relatively linear with each ADP molecule interacting with two chitosan chains, one through its phosphate group and an additional one through its NH2 group [56].
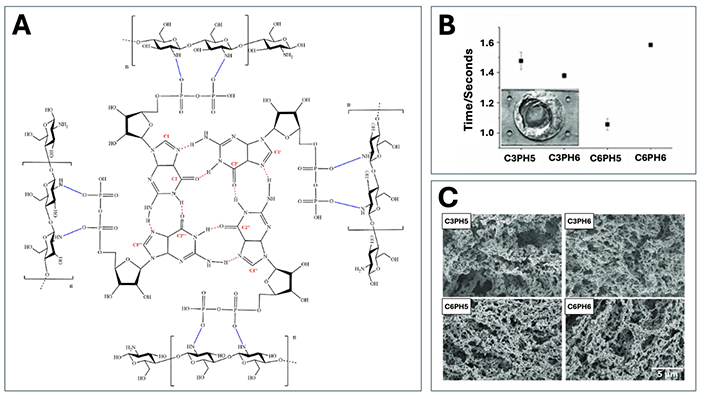
Summary of previous scaffold design and material property work conducted on the purine-cross-linked chitosan scaffold by the Tabrizian Laboratory. (A) Cross-linking structure of the scaffold with guanosine diphosphate showing the formation of a G-tetrad like structure. Reproduced from Ref. [56] with permission from the Royal Society of Chemistry. (B) Structural architecture using SEM for scaffolds with 3 mg/mL of chitosan at pHs of 5 and 6 (C3PH5, C3PH6), and 6 mg/mL of chitosan at the same pHs (C6PH5, C6PH6). (C) Gelation time measurements for the same groups using impedance spectroscopy. (B, C) Reproduced from Ref. [49] with permission from John Wiley and Sons
Further optimization studies on these designed scaffolds have examined the effect of different chitosan concentrations and solution pH values on the gelation and structure of the scaffolds (Table 2) [49, 52, 56]. Since pH greatly affects chitosan’s solubility and the availability of protonated sites for interaction with anionic purines, maintenance of the pH value below that of the pKa was necessary [56]. In this scenario, pH values of 5 or 6 were selected for scaffold fabrication as they allowed for the most rapid interactions between the chitosan chains and the purine crosslinkers [49]. Similarly to pH optimization, the concentration of chitosan in solution before crosslinking (3 mg/mL and 6 mg/mL) was also important to optimize preliminarily as it was shown to affect the integrity of the formed scaffold [49]. Mekhail et al. [49] observed that when too low of a concentration was used, viable sponges were not able to be produced, and in too high of a concentration, a very viscous chitosan solution was present thereby undermining the injectability of the sponge. Combinations of these two concentrations and pH values were used in early scaffold studies to examine their influence on the scaffold’s material properties [49, 52, 56]. However, results suggested that these factors did not significantly affect the scaffold formation as Fourier transform infrared spectroscopy (FTIR) showed no changes in peak intensities for any of the participating groups and cellular metabolic activity was similar amongst scaffolds with the same crosslinker [56].
Summary of modifications, as well as osteogenic factors and cell sources incorporated within the GDP crosslinked chitosan scaffold
| Scaffold Modifications | Factors Encapsulated | Cell Sources |
|---|---|---|
Crosslinker Concentration of chitosan pH of chitosan solution Scaffold structure | Osteogenic factors Non-osteogenic factors
| Osteogenic cells 3T3 Fibroblasts [49] Vascularization cells
Non-osteogenic cells
|
Initial material property studies demonstrated the scaffold’s rapid gelation potential with time measurements ranging from 1.06 seconds for the pH 5 scaffold (concentration 6 mg/mL) to 1.58 seconds in scaffolds with the same concentration but at pH 6 (Figure 5B) [49]. This speed of gelation is still considered to be one of the fastest reported times in the literature, providing significant benefits in terms of limiting unwanted solution diffusion into surrounding tissues as well as easy encapsulation of factors and cells within the scaffold [19, 49, 52, 53]. Addition of different osteoinductive mediators such as pyrophosphatase and various ceramics (HA and β-TCP), as well as changes in the purine crosslinker from GDP to ADP, were not found to significantly alter the rapid gelation property with all studies showing full scaffold formation in under 4 seconds [53, 54].
Regarding structural architecture, SEM and μ-CT imaging demonstrated that formed scaffolds were highly porous with interconnected pores throughout, regardless of chitosan concentration or solution pH (Figure 5C) [49, 56]. Evident changes were observed amidst different purine crosslinkers, with GDP crosslinked scaffolds having a higher pore density and interconnectivity compared to those with ADP crosslinking [56]. Generally, the pore sizes within the scaffolds were heterogeneous, thereby closely resembling the native in vivo tissue, and averaged in size between 100 nm and 500 μm depending on the sponge formulation in study [53, 54, 56, 57, 222]. Work by Jahan et al. [54] examining the incorporation of ceramic apatites in ADP crosslinked sponges showed that their addition directly influenced the microarchitecture of the scaffold, not with respect to total porosity but in the resulting pore sizes and interconnectivity. Since β-TCP particles are larger in size, they were observed to occlude the pores, thereby yielding smaller pore sizes [54]. Yet, the particles were also polydispersed within the sponge, which helped to increase the interconnectivity of the pores [54]. The opposite was observed with the nanosized HA particles, where pore sizes were larger, but more homogenous dispersion of the particles yielded less overall interconnectivity [54].
Mechanical assessments of the scaffold were also conducted in multiple studies as means to get a more complete profile of the scaffold’s material properties [49, 53, 54]. An initial study by Mekhail et al. [49] indicated that GDP crosslinked scaffolds using pH 5 chitosan solutions behaved the stiffest (0.867 ± 0.0931 MPa in C6PH5), which could be explained by the characteristic increase in protonated amine groups allowing for additional crosslinking sites [49]. However, as the modulus of elasticity values amongst the groups were relatively low, this labels them as categorically soft biomaterials, meaning that within in vivo bone applications, the implementation of a fixator to bear some of the mechanical load would be necessary [49, 54]. To account for this limitation, additional modulus testing was conducted with the alternative purine crosslinker, ADP. This new ADP crosslinked chitosan scaffold had a modulus of elasticity of 0.0046 ± 0.0013 MPa and incorporation of two bio ceramics, HA and β-TCP, resulted in a further increase in this parameter (0.0128 ± 0.0024 MPa in CS75HA and 0.0134 ± 0.0018 MPa in CS75TCP) [54].
In addition to compressive mechanical properties, further work focused on examining the material’s viscoelasticity using rheology. Examination of C6PH5 sponges with GDP crosslinking demonstrated a combination of elastic and viscous components indicating viscoelasticity of the material, that was further confirmed in tan(δ) values below one [53]. Results also showed no crossover between the elastic and shear modulus, thus suggesting the presence of a stable internal scaffold structure [53, 54]. These trends were not changed with the addition of osteoinductive mediators, including pyrophosphatase, and bioceramics, or with the change of crosslinker from GDP to ADP [53, 54].
This scaffold’s rapid gelation property further encouraged the hypothesis that it could serve well as a biomaterial for drug encapsulation since it limits solution diffusion into surrounding tissue areas. The entrapment efficiency of neurotrophin factor 3 was thus preliminarily assessed in a study examining the scaffold’s potential for targeting remyelination after spinal cord injuries, with results showing similarly high efficiencies (around 74 %) in both C3PH5 and C6PH6 scaffolds [52]. Additional work by Nayef et al. [19] demonstrated that the encapsulation of the osteogenic mediator, BMP7, could be completed with an 84.3% efficiency compared to only 23.8% efficiency in liposomes, thereby clearly demonstrating the beneficial effect of the sponge on entrapment. Observation of scaffold release kinetics were also conducted in the same study, with results showing a release of around 7% at day 1, suggesting no significant bulk release, and only around 50% after 15 days in culture [19].
As the protein release profile has been established in the literature to correlate with degradative properties, this was equally important to assess in these designed crosslinked scaffolds through the addition of lysozymes [223, 224]. This degradation technique is meant to be representative as to what the material will face in vivo and was replenished daily throughout the course of the experiment [204, 225]. Jahan et al. [54] was able to demonstrate the profile of ADP crosslinked scaffolds, where by day 7, all scaffold groups were at least 50% degraded. However, the addition of the bioceramics appeared to decrease this degradation rate, suggesting that the apatites have a protective effect on the chitosan subunits [54].
One of the interesting findings for this scaffold was specifically in the formation of byproducts because of degradation. It is well known that these products need to be an additional consideration in scaffold design criteria as they should be non-toxic thereby limiting any undesired immunogenic responses [37, 38]. In early work on non-loaded scaffolds, the introduction of purines as crosslinkers was shown to result in the production of large quantities of pyrophosphate, due to their enzymatic cleavage as the scaffold degrades [19]. This pyrophosphate unit is known to significantly inhibit mineralization activities, thereby limiting the potential of the chitosan-based GDP crosslinked scaffold for bone regenerative abilities [19, 55, 226–231]. Thus, more recent work has examined the incorporation of pyrophosphatase (PPTase), which encourages the breakdown of pyrophosphate into phosphate ions, hence promoting mineralization and allowing the purine crosslinker to act as a reservoir for phosphate ions [19, 53, 55].
In recent years, the implementation of this scaffold has been heavily investigated for promoting bone regeneration using both in vitro and in vivo methodologies [19, 53–55, 57]. With respect to cellular biocompatibility, the pre-osteoblast MC3T3 cells have been the front-runner cell source for this application as they are widely used in biomaterials research, and thus the results can easily be compared to other studies [204, 232, 233]. Preliminary testing of GDP and ADP crosslinked chitosan sponges with these MC3T3 cells revealed a significant difference in terms of metabolic activity, cellular density, and general morphology amidst the two crosslinkers [56]. The usage of GDP in scaffolds produced a more favorable environment for the cells, which demonstrated an elongated fibroblast morphology, whereas those cells cultured on ADP crosslinked scaffolds exhibited low attachment with a spheroid like morphology (Figure 6A) [56]. Incorporation of additives such as HA, BMP7 or PPTase successfully encouraged more pronounced ALP activity over control scaffolds, therefore supporting the diamond concepts notion that the addition of osteogenic mediators is necessary for enhanced osteogenic differentiation [19, 53–55]. Nayef et al. [19] also demonstrated the beneficial effect of PPTase encapsulation on the formation of mineralized nodules, with mineralization levels similar to those of direct BMP7 injection. A later study further confirmed this benefit through Von Kossa staining, showing that the highest mineralization could be observed when both HA and PPTase were included in the sponge [55].
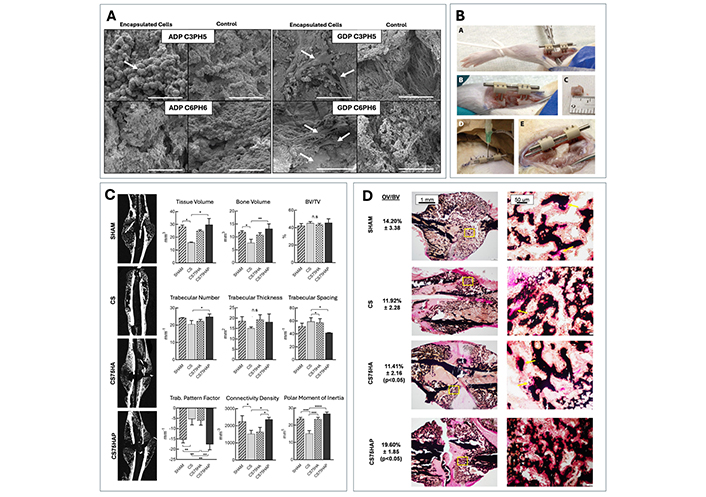
Presentation of previous in vitro and in vivo work conducted on the purine-cross-linked chitosan scaffold by the Tabrizian Laboratory. (A) Comparison of pre-osteoblastic MC3T3 cell viability, proliferation and attachment on scaffolds crosslinked with GDP (GDP C3PH5 and C6PH6) vs. ADP (ADP C3PH5 and C6PH6) using SEM. Reproduced from Ref. [56] with permission from the Royal Society of Chemistry. (B) Demonstration of the scaffolds injectability properties in a rat model, where the scaffold can effectively take the shape of the defect. Reproduced from Ref. [19] with permission from Elsevier. (C) Examination of the callus morphology using μ-CT imaging and quantification at day 17 post-surgery for a mouse tibial fracture model with different test groups: SHAM, chitosan/GDP scaffold (CS), chitosan/GDP scaffold with hydroxyapatite (CS75HA), and chitosan/GDP scaffold with hydroxyapatite and pyrophosphatase (CS75HAP). (D) Von Kossa and Van Gieson staining of the same callus to look at the formation of osteoid in each treatment group. (C, D) Reproduced from Ref. [55] under CC-BY conditions from Springer Nature
To assess the feasibility of this injectable material in bone defect regenerative applications in vivo, a 6 mm rat model was used, and the appropriate solutions were injected through a double barrel syringe with a mounted double lumen needle system [19]. Examination of the defect site post injection revealed the presence of the scaffold within the shape of the defect, indicating successful rapid gelation (Figure 6B) [19]. Further work by Jahan et al. [55] used a rod fixated tibial fracture model as means to thoroughly investigate the potential of the cell-free designed GDP crosslinked scaffold for bone healing. Results from this study showed a significant increase at day 17 post-surgery with respect to the total bone and tissue volume parameters for scaffolds with both PPTase and HA (CS75HAP) additives compared to control scaffolds (Figure 6C) [55]. The incorporation of Von Kossa/Van Gieson staining additionally supported the μ-CT results, with a significantly higher amount of osteoid observed in the CS75HAP scaffold and SHAM groups (Figure 6D) [55]. Together, these trends echo the findings from in vitro studies, thus further confirming the benefit of additives to the scaffold’s function as a biomaterial for bone applications.
Previous literature findings have clearly demonstrated the importance of Wnt signaling in the early stages of fracture healing and bone formation, thus suggesting a new potential avenue for drug and therapeutic targeting [234–237]. As mentioned earlier, binding of Wnt proteins to appropriate receptors activates the canonical Wnt pathway, whereby the destruction complex is disbanded, leading to increased levels of stabilized β-catenin that can translocate and upregulate target genes [84, 87, 93]. In the absence of this stabilization, the differentiation of stem cells into the osteoblastic lineage is inhibited, and thus bone healing is negatively affected [234].
Many publications in the literature have used this knowledge to suggest the targeting of cytoplasmic protein, GSK3, inhibition through small molecules, as an alternative technique to mimic the function of Wnt related proteins and improve the osteoinductive potential of bulk or composite biomaterials (Table 3) [234, 235, 238, 239]. Indeed, work by Comeau-Gauthier et al. [240] successfully demonstrated that the embedding of GSK3 inhibitor, Tideglusib within a surgifoam collagen scaffold in a mouse femoral cortical window defect model yielded a significant increase in the bone volume fraction compared to controls (Figure 7A). This finding was accompanied by an observable increase in ALP activity, which together confirm the benefit of GSK3 inhibitors on the initial stages of osteoblast differentiation [240].
Summary of bone related effects of GSK3 inhibitors, AZD2858, BIO, and LiCl in pre-clinical fracture and defect models. Reproduced from Ref. [234] with permission from Elsevier
| Species | Models | Treatment | Dose | Healing and Strength | Major Findings | Refs |
|---|---|---|---|---|---|---|
| Mice | Femur Fracture | AZD2858 controlled releaseAZD2858 injection | 0.168 mg | ↑ BV/TV, strength↔ BV/TV | Nanoparticles from controlled release over 9 days showed greater accumulation in fracture bone and accelerated fracture healing (4 weeks)At same dose, free AZD failed to accelerate bone healing | [241] |
| Femur Fracture | 6BIO controlled release6BIO injection | 6.9 nmol/kg | ↑ BV/TV↔ BV/TV | Micellar delivery of 6BIO controlled release over 7 days showed accelerated fracture healing compared with free 6BIO and control (3wks)At same dose, free 6BIO failed to accelerate bone healing | [242] | |
| Femur Gap Defect | 6BIO controlled release6BIO injection | 198 μm | ↔ BV/TV↔ BV/TV | Polymeric nanoparticles showed greater accumulation in fractured bone but failed to improve bone healing (3 wks)At same dose, free 6BIO failed to accelerate bone healing | [243] | |
| Tibia Fracture | 6BIO injection | 0.75 mg/kg | ↔ callus volume | Free 6BIO failed to accelerate bone healing (3 wks) | [244] | |
| Tibial Fracture | LiCl PO | 200 mg/kg | ↑ BV/TV | Lithium impaired bone healing when started before fracture and enhanced repair when started 4 days after the injury | [245] | |
| Tibial Fracture | LiCl injections | 100 mg/kg | ↔ strength | No effect on bone strength (2 wks) | [79] | |
| Femur Fracture | LiCl PO | 200 mg/kg | ↑ BV/TV, strength | Accelerated bone healing and bone strength (3 wks) | [246] | |
| Femur Fracture | LiCl | 20 mg/kg | ↑ BMD, strength | Maximal healing and strength occurred with a low dose (20 mg/kg) dose given for a longer time (2 wks) with a 7d of onset (4 wks) | [247] | |
| Rats | Femur Fracture | LiCl | 20 mg/kg | ↑ strength | Best regimen corresponds to a low dose of 20 mg/kg given at 7d of onset for 2 wks duration (4 wks) | [80] |
| Tibial Gap Defect | Li2CO3 (localized) | 10 mM | ↑ strength | Accelerated bone healing (2 wks) | [81] | |
| Femur Fracture | AZD2858 PO daily | 30 μmol/kg | ↑ BMD, strength | Accelerated healing through intramembranous repair without the formation of cartilage (3 wks) | [82] |
The examination of other GSK3 inhibitors such as lithium chloride [75–81], AZD2858 [82, 83], and 6-bromoindirubin-3'-oxime (BIO) [88, 89, 243, 244, 248–251] in bone applications have all also demonstrated similar positive effects on early markers of osteogenic differentiation such as an increase in ALP levels or RUNX2 gene expression. Interestingly, a study conducted by Li et al. [89] on stem cell like myoblastic C2C12 cells was able to illustrate increased BMP2 induced ALP activity in BIO treated groups compared to those of other GSK3β inhibitors (Figure 7B) [89]. These findings and the adjacent null results presented in cultures of pre-osteoblastic MC3T3 cells suggest a potential additional benefit in a GSK3β independent mechanism at early stages of differentiation [89]. Within the same study, the authors were further able to confirm the significant reductive effect of BIO on the phosphorylation of extracellular signal regulated kinase 1/2 (ERK1/2) in the MAP kinase pathway but not on p38 phosphorylation, thus concluding a combinatory influence of BIO on both the Wnt and BMP signaling pathway [89].
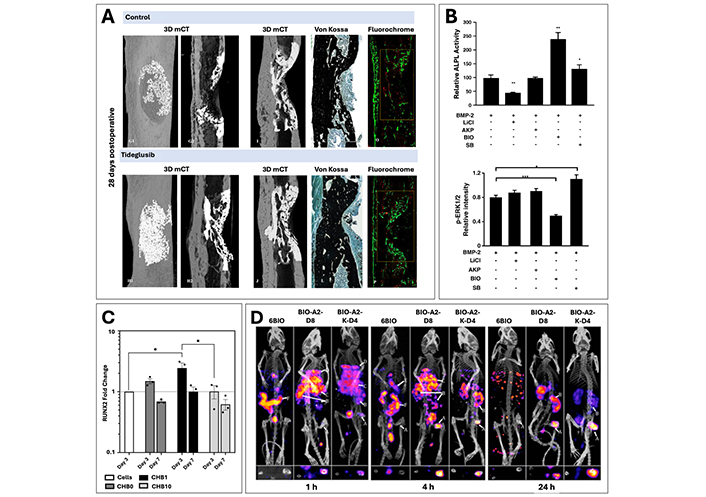
Examples of therapeutic strategies using GSK3 inhibitors for targeting of Wnt signaling cascade. (A) μ-CT imaging and histological staining (Von Kossa and Calcein/Alizarin Red) of femoral window defect treated with Tideglusib loaded collagen scaffold or a respective collagen control after 28 days. Reproduced from Ref. [240] with permission from Elsevier. (B) Comparison of ALP activity and p-ERK1/2 intensity for BMP2 induced C2C12 cells with GSK3 inhibitors, BIO, lithium chloride (LiCl), 1-azakenpaullone (AKP), and SB216763 (SB), showing an additive effect of BIO in conjunction with BMP signaling. Reproduced from Ref. [89] with permission from Elsevier. (C) RUNX2 gene expression profile at days 3 and 7 for chitosan-based crosslinked scaffolds with BIO at doses of 0 (CHB0), 1 (CHB1), and 10 (CHB10) μM, showing a dose dependent beneficial response. Reproduced from Ref. [252] under CC-BY conditions from Elsevier. (D) Imaging using SPECT/CT after 1, 4 and 24 hours to examine the biodistribution of linear (BIO-A2-D8) and branched (BIO-A2-K-D4) micelles with BIO compared with free BIO injections (6BIO) to the fracture site (labelled arrow A) and the kidneys (labelled arrow B). Reproduced from Ref. [253] with permission from the American Chemical Society
Indirubins and their derivatives, such as the semi-synthetically derived cell-permeable indirubin, BIO have been more recently established as gold standard agonists of Wnt signaling, along with lithium chloride and are known to derive from edible gastropod mollusks as well as shrubs [237, 254, 255]. Multiple studies exploring the usage of BIO on cell cultures have clearly indicated the improvement of osteogenic differentiation parameters in its presence, yet the importance of dosage on the observation of these positive effects is consistently seen throughout the literature [88, 248, 251]. Indeed, Zhao et al. [88] observed that the addition of BIO to co-cultured bone marrow derived MSCs (BMSC) in doses past 2.5 μM resulted in significant morphological changes to the cells along with a general inhibition of proliferative activity. Further work within the same study incorporating lower doses of 0.5 and 1 μM were successful in increasing the number of ALP positive cells, as well as an enhancement of gene expression levels for early osteogenic markers, COLI and RUNX2 [88]. Another study using the GDP crosslinked chitosan scaffold [252] has also exhibited the dose dependency of BIO incorporation, with findings revealing a marked elevation in both ALP secretion levels as well as RUNX2 gene expression (Figure 7C) for 1 μM doses compared to 10 μM. BIO’s dose dependency is highly expected as it is characteristically known to have cytotoxic effects at higher concentrations, thereby explaining previous literature findings [243].
The general translation of the promising osteoblastic differentiation results to in vivo models has, however, been limited with results showing mixed healing outcomes [234]. Application of these activators in a systemic manner, often used in animal studies, generally faces obstacles due to the non-specificity of the Wnt signaling cascade [234, 237]. This could explain why systemic treatments of these therapeutics tend to result in a relatively poor biodistribution and an increase in the possibility of off-target side effects [234, 236]. These limitations have therefore shifted the focus of bone tissue engineering research in this domain towards the design of localized targeting methods such as drug loading into delivery carriers such as liposomes and nanoparticles, or encapsulation in biomaterial scaffolds and hydrogels (Figure 8) [236].
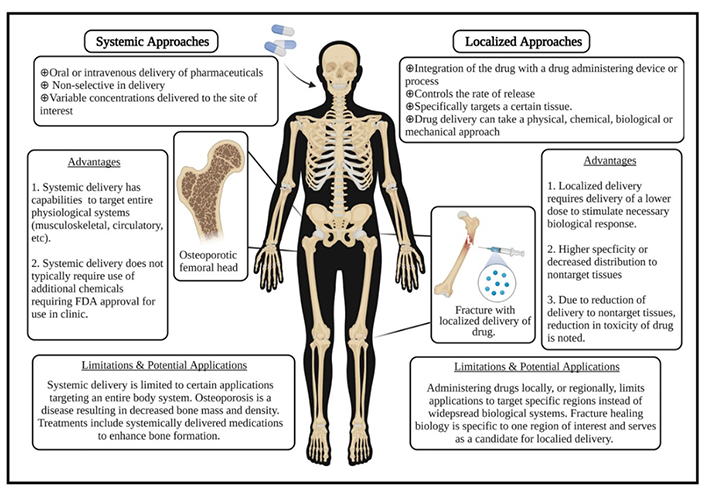
Description of the advantages and limitations for systemic vs. localized drug treatment delivery. Reproduced from Ref. [236] under CC-BY conditions from John Wiley and Sons
A recent study by Low et al. [242, 252] effectively demonstrated the benefit of localized treatment by comparing two BIO incorporated aspartic acid octapeptide micellar delivery systems (branched and linear) to a direct injection of free BIO through the tail vein. Results showed that by adding the targeting moiety to these two groups, BIO was able to accumulate within the fractured bone region, prior to subsequent clearance by the kidneys (Figure 7D) [253]. In comparison, the free BIO injection yielded a systemic biodistribution with almost no accumulation visible in the fracture area (Figure 7D arrow A), thereby confirming the benefit of the targeting moiety [253]. Further work by the same research group additionally demonstrated that the implementation of the bone specific targeting mechanism allowed for a significant increase in bone density, volume fraction and trabecular thickness compared to respective free BIO control within their mouse femoral fracture model [242]. Together, these results clearly indicate the importance of a localized treatment method, which allows for the benefits of Wnt signaling agonists, while effectively reducing the necessary drug dosages and limiting unwanted off-target accumulation [234, 236, 237].
The necessity for a localized delivery system to target the Wnt signaling pathway highlights the significant potential of GSK3β inhibitors as osteoinductive mediators in composite scaffold designs. Unlike single bulk biomaterials, composite scaffolds offer the advantage of precise tuning of material properties such as porosity, mechanical strength, and biodegradability. This customization allows the designed scaffolds to more closely mimic the natural bone microenvironment, which ultimately improves their biocompatibility and ability to integrate effectively in the surrounding tissue. These tailored scaffolds can be further designed to incorporate bioactive agents that activate the Wnt signaling pathway, which has been well-established as a key regulator of osteogenesis. By enabling a sustained and localized release of these osteoinductive signals, composite scaffolds provide continuous exposure of the cells in the surrounding tissues to osteogenic cues, that promote both osteoblastic differentiation and mineralization. The prolonged osteoinductive effects, achieved through the encapsulation of these factors in composite scaffolds, can effectively enhance bone formation and have the potential to significantly improve healing outcomes.
The design of an optimal biomaterial for this complex application necessitates a carefully thought-out approach that incorporates the principles of the diamond concept, which is essential for successful healing outcomes in critical size bone defect treatment. As research within this field continues to evolve, future work should continue to focus on developing composite and co-polymeric biomaterials that more accurately resemble the native internal architecture and functionality of the bone tissue they intend to regenerate. Recent studies have increasingly suggested the potential of targeting Wnt signaling through incorporation of specific GSK3β agonists as osteoinductive mediators during the biomaterial design stages. Introduction of a localized approach through scaffold encapsulation facilitates a precise targeting action to the specific tissue of interest and mitigates associated risks of systemic delivery systems. Initial findings in the literature indicate that this targeting strategy has the potential to significantly enhance both osteogenic differentiation and bone formation, further underscoring its promise to achieve more favorable healing outcomes. Thus, the integration of these advancements into the biomaterial design process could improve the material’s osteoinductive potential and lead to the development of a more effective clinical treatment for critical size bone defects.
ADP: adenosine diphosphate
ALP: alkaline phosphatase
BCP: biphasic calcium phosphates
BGs: bioactive glasses
BIO: 6-bromoindirubin-3'-oxime
BMP: bone morphogenetic proteins
CaP: calcium phosphates
COLI: collagen type I
GDP: guanosine diphosphate
GSK3: glycogen synthase kinase 3
HA: hydroxyapatite
LEF: lymphoid-enhancer binding factor
LRP5/6: lipoprotein receptor-related protein 5 and 6
MMT: montmorillonite
MSC: mesenchymal stem cells
PCL: polycaprolactone
PDGF: platelet derived growth factors
PGA: poly(glycolic) acid
PLA: polylactic acid
PLGA: poly(lactic-co-glycolic) acid
PPTase: pyrophosphatase
RUNX2: runt related transcription factor 2
SEM: scanning electron microscopy
TCF: T-cell specific transcription factor
TCP: tri-calcium phosphate
μ-CT: micro-computed tomography
The authors wish to acknowledge the financial support of the CIHR and NSERC Collaborative Health Research Program, as well as the Canada Research Chair – Tier 1 in Regenerative Medicine and Nanomedicine, and the FRQS funding. However, the funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. Schematic figures were made using a paid subscription to the Biorender Software and graphs were created using Microsoft Excel.
CJA: Conceptualization, Methodology, Formal analysis, Writing—original draft, Writing—review & editing, Visualization. BMW: Conceptualization, Methodology, Writing—review & editing, Supervision. ARB: Writing—review & editing. MT: Conceptualization, Methodology, Writing—review & editing, Supervision.
Maryam Tabrizian is Editor-in-Chief of Exploration of BioMat-X, and Aldo Roberto Boccaccini is an Associate Editor of the journal. They were not involved in the decision-making or the review process of this manuscript. The remaining authors declare no conflict of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
