Abstract
Aim:
This study aimed to synthesize, characterize, and evaluate the antifungal efficacy of green-synthesized silver nanoparticles (AgNPs) against Verticillium dahliae Kleb., a soil-borne fungal pathogen that affects numerous crops.
Methods:
AgNPs were synthesized using Laurus nobilis L. (laurel) leaf extract. The synthesized AgNPs were characterized using UV-VIS spectroscopy, Fourier-Transform Infrared Spectroscopy (FTIR), zeta potential, particle size analysis (PSA), and scanning electron microscopy (SEM). In vitro antifungal assays were conducted to assess the impact of AgNPs on V. dahliae mycelial growth, and SEM was used to examine the morphological changes in treated mycelium.
Results:
UV-VIS spectroscopy confirmed AgNP synthesis with a characteristic SPR peak between 400–450 nm. FTIR analysis identified the presence of phenolic compounds on the nanoparticle surface. Zeta potential analysis (–27.7 mV) indicated stable dispersion. Zeta size analysis indicated an average diameter of approximately 100 nm and a polydispersity index (PdI) of 0.229. SEM imaging confirmed a predominantly spherical morphology and PSA revealed a size range of 14–34 nm, with an average diameter of 24 nm. In vitro antifungal assays showed significant inhibition of V. dahliae mycelial growth, with radial mycelial growth reduced to 2.75 cm compared to 4.8–6.4 cm in the control group after 14 days. SEM imaging of treated mycelium revealed pronounced morphological damage, including collapse and shrinkage of hyphae and spores.
Conclusions:
Green-synthesized AgNPs using L. nobilis leaf extract demonstrated significant antifungal activity against V. dahliae. The observed inhibition of mycelial growth and morphological damage suggests the potential of these AgNPs as a sustainable and eco-friendly alternative for managing this fungal pathogen. The antifungal mechanism may involve membrane disruption, increased permeability, oxidative stress, and the inactivation of cellular components.
Keywords
Silver nanoparticle, Verticillium dahliae, wilting disease, green synthesis, antifungal activityIntroduction
Nanotechnology has been rapidly advancing across diverse fields, including medicine, engineering, bioremediation, pharmacy, and cosmetics [1, 2]. Nanoparticles offer significant advantages, primarily due to their unique physicochemical properties, such as a high surface area-to-volume ratio [3]. These properties have facilitated the integration of numerous commercial nanoparticles into various industries. The global nanotechnology market was valued at $76 billion in 2020 and is projected to reach $170 billion by 2026 [4]. Among these, silver nanoparticles (AgNPs) are extensively employed, particularly for their remarkable antimicrobial properties. AgNPs can be synthesized via three main methods: chemical, physical, and biological. Among these, the biological approach, often termed green synthesis, is the most cost-effective and environmentally sustainable method [5]. Biological synthesis utilizes various natural resources, including plants, microorganisms, and algae, with plant extract-mediated synthesis being especially favored due to its simplicity, time efficiency, cost-effectiveness, and the absence of toxic by-products [6].
Before the discovery of the first antibiotic, penicillin, silver and colloidal silver were widely utilized to treat various infections and preserve crops, food, wine, and water [7]. The advancement of nanotechnology has facilitated the development of nanoscale silver particles, which demonstrate significantly enhanced chemical, physical, and biological properties [8]. Numerous studies have investigated the antimicrobial activity of AgNPs. For instance, AgNPs synthesized using Madhuca longifolia leaf extract demonstrated significant inhibitory effects on the growth of Bacillus cereus, Staphylococcus saprophyticus, Escherichia coli, and Salmonella typhimurium in a concentration-dependent manner [9]. Similarly, AgNPs synthesized using Salix viminalis leaf extract exhibited significant inhibitory effects against E. coli and Staphylococcus aureus [10]. Another study reported that green synthesized AgNPs effectively inhibited the growth of six different phytopathogenic fungi species, even at low concentrations [11]. Previously, green synthesized AgNPs via Laurus nobilis L. extract have presented an effective control for soft rot disease factor Pectobacterium carotovorum in pepper [12]. Collectively, these findings suggest that green synthesized AgNPs could serve as a new generation of antimicrobial agents.
Many plant species are highly susceptible to a diverse array of pathogens, including viruses, bacteria, fungi, and other microorganisms, which can severely compromise plant growth and significantly reduce crop yield [13]. Traditional management strategies to combat these pathogens have primarily relied on pesticide application, which has been widely adopted due to its effectiveness in disease control. However, increasing concerns regarding the environmental and health impacts of pesticide use—such as soil degradation, water contamination, and adverse effects on non-target organisms—have prompted the search for more sustainable and eco-friendly alternatives [14]. Recent advancements in nanotechnology and biopesticides present promising opportunities for improved disease management. The development of plant-based nanomaterials and natural antimicrobial agents provides innovative solutions that mitigate the adverse effects associated with traditional pesticide use [15].
Verticillium dahliae, a notorious soil-borne pathogen, inflicts severe damage on numerous plant species by causing wilting disease. Remarkably, this pathogen can persist in the soil for prolonged periods, even in the absence of a host plant [16]. Once it infects a plant, V. dahliae disrupts the water transport system by blocking the xylem vessels, which results in wilting and subsequent tissue necrosis [17]. Despite its significant impact on crop health and yield, there are currently no effective chemical control measures available for managing this disease. This highlights the critical need for alternative strategies, including the development of resistant plant varieties and innovative biological control approaches. Green synthesized AgNPs have demonstrated significant potential in agricultural applications. For instance, AgNPs derived from Melia azedarach leaf extracts have exhibited strong antifungal properties, effectively targeting V. dahliae and enhancing growth in Solanum melongena [18]. Similarly, a paracetamol-silver nanocomplex achieved over 80% inhibition against various fungal pathogens, further emphasizing the potential of silver-based nanomaterials as sustainable alternatives for pest management and crop improvement [19].
Recent research has increasingly highlighted the importance of integrated pest management strategies, which combine biological control, cultural practices, and the use of resistant plant varieties to reduce reliance on chemical pesticides. In this study, AgNPs were green synthesized using L. nobilis leaf extract. The synthesized AgNPs were characterized through UV-VIS spectroscopy, FTIR spectroscopy, SEM, and zeta potential and particle size analysis (PSA). The in vitro antifungal activity of the AgNPs was evaluated against V. dahliae, the causative agent of wilting disease. This research indicates that AgNPs have the potential to play a crucial role in the development of novel antifungal formulations, offering a promising alternative to traditional chemical treatments.
Materials and methods
Synthesis and characterization of AgNPs
L. nobilis leaves were collected from the Ege University Botanical Garden (Izmir-Turkey, Herbarium No: EGE 23943), and cleaned with tap water followed by distilled water to remove dust and debris. The leaves were then ground with liquid nitrogen, and 10 g of the powdered material was added to 100 mL of distilled water. This mixture was stirred at 60°C for 10 minutes. The homogenate was filtered through Whatman No. 1 paper and centrifuged at 4,500 g at +4°C for 10 minutes.
For the synthesis of AgNPs, 1 mL of the laurel leaf extract was gradually added to 99 mL of a 1 mM silver nitrate (AgNO3) solution (28-1350, Sigma-Aldrich, USA) under constant stirring at room temperature for 48 hours. The reaction was then halted by cooling the mixture to +4°C. AgNPs were stored at +4°C for further analyses.
The formation of AgNPs was monitored by measuring absorbance between 300 and 700 nm using a UV-VIS spectrophotometer (840-208200, Thermo Scientific, USA). The aqueous laurel extract and synthesized AgNPs were analyzed by FTIR (Spectrum Two, Perkin Elmer, USA) to identify functional groups. The surface potential and particle size distribution of the nanoparticles were assessed using a zetasizer (Nano ZS, Malvern, UK). The green synthesized AgNPs were then dried on an aluminum layer at 60°C overnight, and their size and morphology were examined using SEM (Apreo S, Thermo Scientific, USA).
In vitro antifungal activity of AgNPs against V. dahliae
The highly virulent Vd11 isolate (non-defoliating-SS4) of V. dahliae was cultured on potato dextrose agar (PDA) at 25°C for 14 days to obtain a young fungal culture. Mycelial growth inhibition assay was performed to assess the antifungal activity. In this assay 15 mL of AgNPs were mixed into autoclaved PDA medium while it was still warm and then poured into petri dishes, which were left to cool overnight at +4°C. Mycelial discs (5 mm in diameter) were excised from the growing margin of the 14-day-old V. dahliae Vd11 culture (diluted to 1 × 107 CFU/mL) and placed in the center of the petri dishes using glass perforators. Petri dishes containing water instead of AgNPs were served as controls. The dishes were incubated at 25°C for 14 days. Fungal growth was measured by radial mycelial expansion using a digital caliper, and the width and length of colonization were assessed for both control and treatment groups. To examine mycelium morphology, small samples from the 14-day-old Vd11 culture were dried for 1 day, then critical point dried under high vacuum and gold-coated using a sputter coater (EM ACE600, Leica, Germany). The effects on mycelial morphology were observed using SEM.
Results
Synthesis and characterization of AgNPs
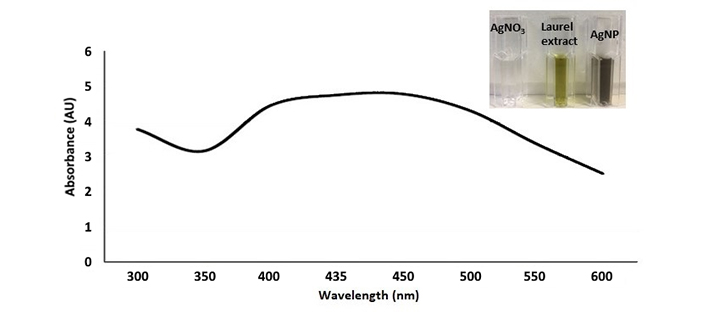
The formation of AgNPs was monitored by observing the color change of the reaction mixture from bright yellow to brown, which occurred after 48 hours of incubation. This color change indicates that phytochemical compounds in the laurel leaf extract acted as reducing agents, facilitating the reduction of silver ions to AgNPs. The formation of AgNPs was further validated by UV-VIS spectroscopy analysis, which revealed a surface plasmon resonance (SPR) peak characteristic of AgNPs (Figure 1). The laurel extract-mediated synthesis and capping of AgNPs were confirmed by the SPR peak observed between 400 and 450 nm, consistent with the typical optical properties of AgNPs.
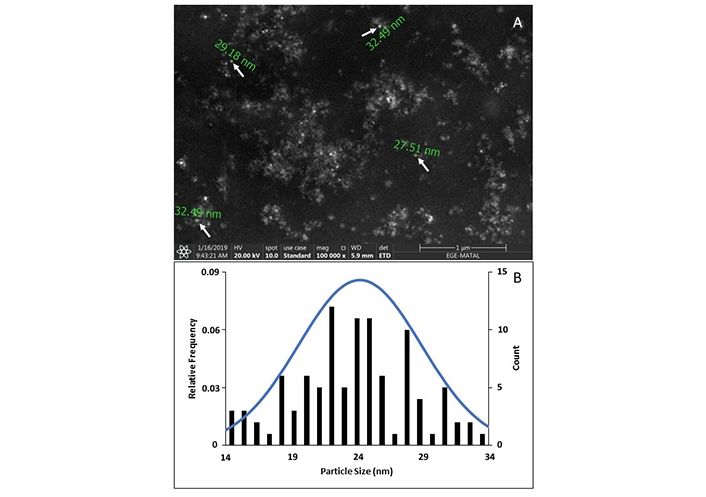
The shape of the AgNPs was examined using SEM (Figure 2). SEM analysis revealed that the AgNPs are predominantly spherical and uniform in shape. The PSA revealed that the synthesized AgNPs exhibited a size range of 14–34 nm, with an average diameter of 24 nm based on over 100 individual measurements.
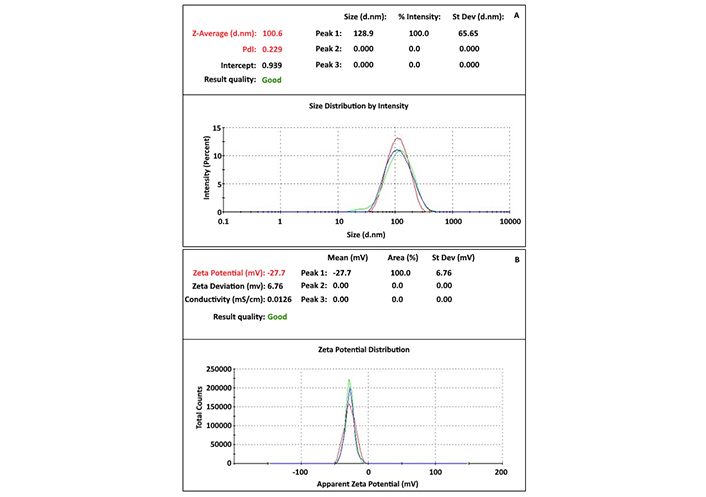
Particle distribution and surface potential of AgNPs were assessed using zeta size and zeta potential analysis (Figure 3). Zeta size analysis indicated that the particles had an average diameter of approximately 100 nm, with a polydispersity index (PdI) value of 0.229, reflecting a moderate level of size variation. Zeta potential analysis revealed that the particles are negatively charged, with a zeta potential of –27.7 mV. These results confirm that the AgNPs are stable and maintain colloidal dispersion in solution.
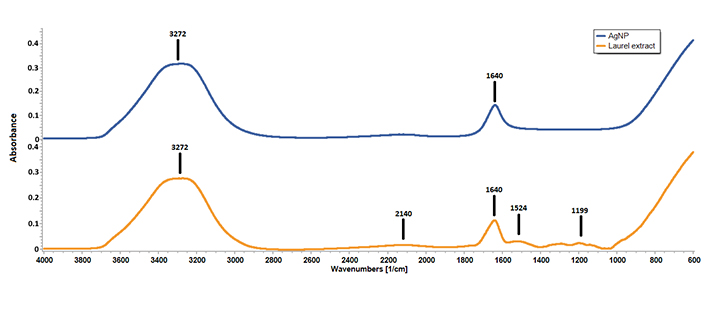
The chemical composition of AgNPs was analyzed using FTIR spectroscopy (Figure 4). The FTIR spectra revealed the absence of peaks at 1,524 cm–1, 1,291 cm–1, and 1,199 cm–1 in the AgNPs spectrum, which were present in the laurel leaf extract. On the other hand, peaks at 3,272 cm–1, 2,140 cm–1, and 1,640 cm–1, which were observed in the laurel leaf extract, were retained in the AgNPs spectrum.
In vitro antifungal activity of AgNPs against V. dahliae
The laurel leaf extract-capped AgNPs significantly inhibited the mycelial growth of V. dahliae over a 14-day incubation period compared to the control group. Radial mycelial growth was quantified by measuring the diameter of fungal colonies in two dimensions 14 days post-inoculation. In the control group, the average colony length and width were 4.8 cm and 6.4 cm, respectively. In contrast, colonies treated with green synthesized AgNPs exhibited substantially reduced growth, with average dimensions of 2.75 cm for both length and width (Figure 5).
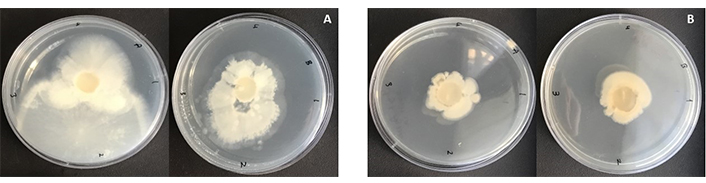
Colonies of Verticillium dahliae grown on PDA medium for 14 days. A) control group without any treatment, B) treated with AgNPs
SEM images revealed morphological and structural damage to V. dahliae mycelium caused by the green synthesized AgNPs (Figure 6). Significant collapse and shrinkage of hyphae and spores were observed. In contrast, SEM images of the mycelium from the control group showed no morphological damage, indicating that the hyphae were healthy and intact.
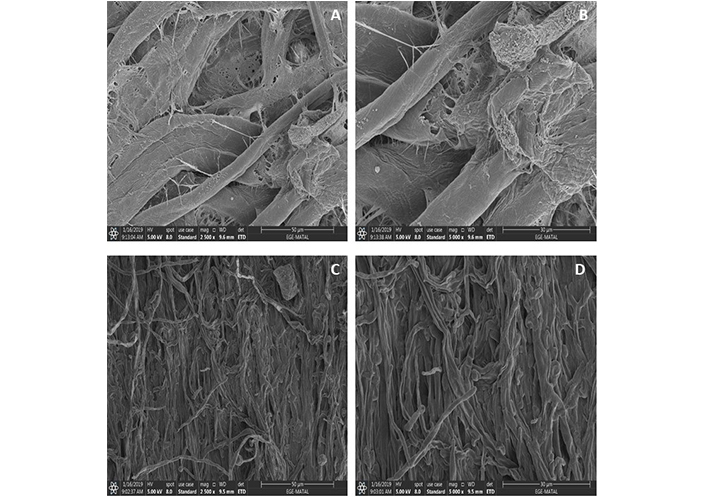
SEM images of (A, B) control group without any treatment, (C, D) AgNPs treated 14 days incubated Verticillium dahliae colonies
Discussion
In the present study, the aqueous leaf extract of laurel facilitated the reduction of silver ions from AgNO3 to AgNPs. Over the past two decades, AgNPs have been utilized as antimicrobial agents, demonstrating notable efficacy against bacterial species [20]. However, their effectiveness against fungal species appears to be comparatively lower, which may be attributed to the protective role of chitin in the fungal cell wall, which offers a defense mechanism against elemental silver (Ag0). The reduction of AgNO3 to AgNPs using L. nobilis leaf extract follows a complex mechanism primarily driven by the plant’s phenolic compounds. These compounds, including flavonoids, tannins, and other polyphenolic substances, possess strong reducing properties due to their hydroxyl groups, which can donate electrons to silver ions (Ag+). This electron transfer reduces Ag+ to elemental silver (Ag0), leading to the formation of nanoparticles [21]. Concurrently, the phenolic compounds act as stabilizing agents, preventing nanoparticle aggregation by capping the surface of the formed AgNPs. The hydroxyl groups of phenols form a protective layer around the nanoparticles, thus maintaining their stability and uniformity in size.
UV-VIS spectroscopy revealed characteristic SPR peak between 400 and 450 nm, indicative of the SPR of AgNPs. This observation, along with the color change of the reaction mixture, confirms the reduction of Ag+ ions to Ag0 facilitated by the natural phytomolecules in L. nobilis leaf extract [9, 22]. Additionally, no significant color change or agglomeration of the green synthesized AgNPs was observed even after ten weeks, indicating their stability over time.
The FTIR spectrum revealed significant peaks, with the band at 3,272 cm–1 corresponding to O–H stretching, indicative of hydrogen bonds commonly found in natural compounds such as alcohols, flavonoids, or phenols [23]. The band at 2,140 cm–1 indicates the presence of alkyne groups [24], while the peak at 1,640 cm–1 corresponds to C=C stretching in aromatic compounds [25], suggesting that the phytochemical components of L. nobilis extract capped the AgNPs. Furthermore, the bands at 1,524 cm–1 (C–H stretching in alkenes and aromatic compounds) and 1,199 cm–1 (C–O stretching in phenolic compounds) were absent in the AgNP spectrum, confirming that AgNO3 was effectively reduced without aggregation, due to the presence of phenolic compounds in the laurel extract [26].
Zeta-size and potential analyses were employed to determine the size, distribution, and stability of the nanoparticles. The PdI value confirmed that the AgNPs were monodispersed. Additionally, the zeta-potential analysis indicated the stabilization of negatively charged AgNPs. A zeta-potential value lower than –14 mV suggests effective prevention of agglomeration, ensuring the stabilization of AgNPs in solution [27]. Zeta-size analysis typically measures larger particle sizes than SEM due to its capacity to analyze millions of particles, providing more robust data on size distribution and PdI.
Green synthesized AgNPs demonstrated significant inhibition of V. dahliae mycelial growth, showing marked suppression compared to the control group. Similarly, green synthesized AgNPs using M. azedarach leaf extract have been reported to inhibit V. dahliae growth [18]. Our findings are consistent with previous studies reporting inhibition of other plant pathogens, including Macrophomina phaseolina, Alternaria alternata, Rhizoctonia solani, and Fusarium oxysporum [28, 29]. The biosynthesis of AgCl nanoparticles using Aspergillus terreus demonstrated strong antimicrobial activity against pathogenic microorganisms such as Fusarium oxysporum and V. dahliae [30]. On another study, AgNPs synthesized from Citrus maxima peel extract exhibited significant antioxidant properties and effectively inhibited the growth of V. dahliae [31]. The antifungal mechanism of AgNPs likely involves disruption of membrane integrity [32], allowing silver ions to penetrate the intracellular matrix [33]. This mechanism of AgNPs may also involve electrostatic interactions between silver ions and negatively charged cell membranes, leading to increased membrane permeability, oxidative stress, and the inactivation of enzymes and ribosomal complexes, as well as disruption of mitochondrial and chromatin structures [34]. AgNPs may also interfere with intracellular structures, potentially altering genome-wide transcription [35]. While the antifungal properties of AgNPs are well-documented, the precise mechanisms remain to be fully elucidated due to the complexity of fungal systems.
Conclusions
In conclusion, the present in vitro study demonstrates that green synthesized AgNPs exhibit significant antifungal activity against V. dahliae, a pathogen known for causing considerable damage to various crops. The results suggest that these nanoparticles hold promise as a highly effective, eco-friendly, and easily accessible antifungal agent. Their ability to inhibit mycelial growth underscores the potential of AgNPs as a sustainable alternative to traditional chemical treatments. Given their natural synthesis and environmental compatibility, AgNPs could play a pivotal role in integrated pest management strategies for controlling fungal pathogens like V. dahliae in agriculture. Further research is warranted to explore the precise mechanisms underlying their antifungal action and assess their efficacy in field applications.
Abbreviations
| AgNPs: | silver nanoparticles |
| PDA: | potato dextrose agar |
| PdI: | polydispersity index |
| PSA: | particle size analysis |
| SPR: | surface plasmon resonance |
Declarations
Acknowledgments
Verticillium dahliae isolate (Vd11) was kindly gifted by Prof. Dr. Oktay Erdoğan from Pamukkale University, Denizli, Turkey.
Author contributions
AC, MA, and YK: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. PG: Investigation, Writing—original draft. LYA: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.