Abstract
Mineral nanoparticles and osteoinductive biomaterials are essential in advancing bone regeneration by addressing skeletal conditions and injuries that compromise structural integrity and functionality. These biomaterials stimulate the differentiation of precursor cells into osteoblasts, creating biocompatible environments conducive to bone tissue regeneration. Among the most promising innovations, mineral-based nanoparticles and nanocomposite hydrogels have emerged as effective strategies for enhancing osteoinductive potential. This review explores the diverse types of osteoinductive biomaterials, including natural sources, synthetic compounds, and hybrid designs that incorporate mineralized nanoparticles. Emphasis is placed on polymeric hydrogels as delivery platforms for these materials, highlighting their dual role as structural supports and bioactive agents that promote osteogenesis. Challenges such as immune rejection, biodegradability, mechanical stability, and short in vivo residence time are critically discussed, alongside their impact on clinical translation. By presenting a comprehensive analysis of mechanisms, applications, and limitations, this review identifies opportunities for integrating osteoinductive biomaterials with emerging fields like immunology and biomechanics. Ultimately, this work aims to provide actionable insights and advance the development of novel, clinically relevant solutions that improve patient outcomes and address the growing global need for effective bone repair and regeneration.
Keywords
Osteoinductive biomaterials, hydrogel, bone tissue regenerationIntroduction
Osteoinductive biomaterials are fundamental to advancing bone healing applications, addressing a wide spectrum of skeletal conditions and injuries that compromise structural integrity and functionality. These materials are designed to stimulate the differentiation of progenitor cells into osteoblasts, fostering environments conducive to bone regeneration [1]. Such innovations are vital for restoring mobility and improving the quality of life for patients suffering from fractures, bone defects, and degenerative diseases [2]. The urgency for effective bone regeneration strategies is underscored by the increasing prevalence of bone-related disorders such as osteoporosis and traumatic injuries, particularly within aging populations. For example, in the United States alone, over 500,000 bone grafting surgeries are performed annually, at a cost exceeding $2.5 billion, a financial burden expected to double in the near future [3]. Current treatments, such as autografts and allografts, are effective but come with significant limitations, including donor site morbidity, limited availability, and risk of immune rejection [3]. Consequently, there is a pressing need for innovative, cost-effective solutions that not only enhance bone regeneration but also address these challenges [2, 4]. Among the most promising advancements, mineral nanoparticles, such as calcium phosphate, magnesium hydroxide, and bioactive glass nanoparticles, have garnered significant attention [1, 5]. These mineral-based nanoparticles exhibit intrinsic osteoinductive properties and can be incorporated into polymeric hydrogels and nanocomposites to create multifunctional systems [4, 6]. Polymeric hydrogels, in particular, offer a versatile platform for delivering these bioactive agents while providing structural support and mimicking the extracellular matrix (ECM) [7]. Injectable and sprayable hydrogels exemplify minimally invasive solutions that promote rapid recovery, making them particularly suitable for complex cases, including large bone defects and cancer-induced bone damage [8]. This review explores the role of mineral nanoparticles and nanocomposite hydrogels in bone regeneration, focusing on their mechanisms of action, clinical applications, and emerging strategies. Additionally, we discuss unresolved challenges and identify future research directions to bridge the gap between laboratory innovation and clinical implementation. By doing so, we aim to provide a comprehensive framework for advancing regenerative medicine and improving outcomes for patients with bone-related conditions.
Osteoinductive biomaterials
The process of bone healing is a complex biological mechanism that restores skeletal integrity following injury, trauma, disease, or surgical intervention. Over the past decades, osteoinductive biomaterials have emerged as a critical component in enhancing and accelerating the bone healing process. Osteoinductive biomaterials are materials that have the intrinsic ability to induce undifferentiated precursor cells to differentiate into osteoblasts, the cells responsible for bone formation [9, 10]. This property allows these materials to actively stimulate the formation of new bone tissue, rather than simply providing a passive scaffold for tissue growth [9, 10]. These biomaterials are engineered to recruit osteoprogenitor cells, facilitating their differentiation into osteoblasts, thereby promoting the regeneration of bone tissue. They can be derived from natural sources, synthesized from bioactive compounds, or designed as hybrid materials that combine both elements. Figure 1 summarizes the key approaches to bone regeneration, illustrating how osteoinductive biomaterials serve as both structural supports and bioactive agents to drive osteogenesis. This chapter delves into the diverse types of osteoinductive biomaterials, exploring their principles, mechanisms of action, and clinical applications. Through an extensive review of the literature and a critical evaluation of current trends, we aim to provide a comprehensive understanding of how these materials contribute to bone regeneration. In particular, we will discuss the limitations and challenges associated with their use, as well as potential directions for future research and development. By illuminating the capabilities of osteoinductive biomaterials, this chapter seeks to enhance the field of regenerative medicine, ultimately leading to better clinical outcomes for patients in need of bone repair and regeneration.
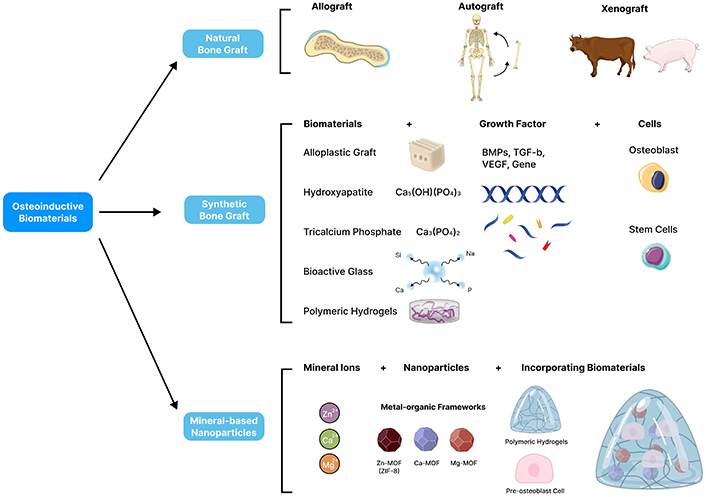
An illustration of different types of osteoinductive biomaterials for bone tissue regeneration. Natural bone grafts include autografts from the patient, allografts from human donors, and xenografts from other species. Synthetic bone grafts, composed of ceramics, polymers, or composites, provide alternative approaches. These synthetic biomaterials can be modified by incorporating osteogenic growth factors like BMPs, TGFβ, VEGF, and PDGF to promote bone formation and facilitate healing. Recent advances include mineral-based nanoparticles, such as calcium phosphate, hydroxyapatite, and bioactive glass nanoparticles. These nanoparticles enhance osteoinduction by providing a biomimetic mineral matrix that supports cell attachment, proliferation, and differentiation. Parts of the figure were adapted from pictures provided by Servier Medical Art, licensed under CC BY 4.0
Natural bone graft
Allograft
An allograft involves the transplantation of bone tissue from a donor to a recipient of the same species but with a different genetic background [11, 12]. In bone regeneration, allografts have gained popularity as they address key challenges such as the limited availability of donor sites, the morbidity associated with autografts, and the need for larger graft volumes in cases of massive bone defects [9, 12]. The main advantage of allografts lies in their ability to provide biological compatibility while offering structural support. Typically, allografts are processed to remove cellular content while preserving the ECM, which serves as a scaffold to promote bone regeneration [9]. However, several limitations exist with allograft use. There is a risk of immune rejection, even though processing reduces immunogenicity, and the possibility of disease transmission, though rare, persists despite rigorous screening and sterilization. Additionally, allografts have reduced osteoinductive potential as they lack the growth factors and living cells found in autografts, which can lead to slower or incomplete bone healing, particularly in large defects. Processing can also weaken the mechanical strength of allografts, making them less durable than autografts or native bone. Despite these challenges, ongoing advancements in tissue engineering and biomaterials are focused on improving the effectiveness and safety of allografts, offering the potential to enhance their role in bone regeneration.
Autograft
An autograft is a natural biomaterial widely used for reconstructing large bone segment defects, especially in cases of significant bone loss from trauma, tumor resection, or congenital abnormalities [11, 13]. Harvested from the patient’s own body; commonly from the iliac crest, fibula, or ribs, and autografts offer unmatched biocompatibility, eliminating risks of immune rejection or disease transmission that can arise with allografts or synthetic materials [13]. Their biological properties are critical to bone healing, providing osteogenesis (the formation of new bone from osteoprogenitor cells), osteoinduction (stimulating precursor cells to differentiate into bone-forming osteoblasts), and osteoconduction (acting as a scaffold for new bone growth and integration) [9]. Autografts also possess the unique ability to remodel and integrate over time, fusing with the surrounding bone to restore both mechanical strength and functionality [14]. Despite these advantages, they come with certain limitations, including potential donor site morbidity, limited graft quantity, and the need for a secondary surgical procedure to harvest the tissue. Nevertheless, their proven effectiveness in promoting bone regeneration and minimizing complications makes autografts the preferred choice for treating critical-sized bone defects, particularly in complex reconstructive surgeries.
Xenograft
A xenograft is a type of graft derived from a donor of a different species, widely used in medical and research applications such as tissue engineering, reconstructive surgery, and transplantation studies [15]. Xenografts are commonly used in born treatments, dental surgeries, and various reconstructive procedures due to their wide availability and ability to support healing [14]. Typically sourced from animals like pigs (porcine) or cows (bovine), xenografts are frequently used for soft tissue repair, bone reconstruction, and skin grafts. To reduce the risk of immune rejection, these grafts undergo extensive processing, including decellularization and sterilization, to remove cellular components and antigens that could trigger an immune response in the recipient [16]. Xenografts offer several advantages, primarily due to their availability in larger quantities compared to autografts (from the patient) or allografts (from human donors) [16]. These grafts serve as a scaffold that supports tissue regeneration, promoting new tissue growth until they are gradually resorbed or replaced by the patient’s own tissue [16]. In bone grafting, xenografts provide osteoconductive properties, meaning they act as a framework for new bone formation, although they lack the osteogenic (bone-forming cells) and osteoinductive (growth factor-stimulating) qualities seen in autografts [17]. One of the significant challenges with xenografts is the potential for immune rejection, as the recipient’s immune system may recognize the graft as foreign [18]. However, advances in graft processing techniques, such as decellularization and crosslinking, have greatly reduced the immunogenicity of xenografts while preserving their structural integrity [18]. These improvements have made xenografts safer and more effective, especially in cases where autografts or allografts are not viable options.
Synthetic bone graft
Alloplastic graft
Alloplastic grafts are synthetic materials used for bone regeneration, particularly in dental treatments, offering a versatile alternative to human or animal donor tissue [19]. These grafts are made from minerals, synthesized compounds, or a combination of both. A major benefit of alloplastic grafts is that they eliminate the need for tissue harvesting, making procedures less invasive and avoiding issues related to donor sourcing [19]. Common materials include hydroxyapatite (HA), a naturally occurring mineral that forms a major component of bone, and bioactive glass [19]. However, despite these advantages, alloplastic grafts come with certain limitations. One significant drawback is the variable resorption rate of certain materials. Synthetic calcium carbonate grafts, for example, are less commonly used due to their high rate of resorption, which can weaken bone integrity and increase the risk of fractures [20]. This rapid breakdown of the material can undermine the stability of the graft, particularly in areas where long-term support is required. In contrast, combinations of tricalcium phosphate (TCP) and HA are designed to balance osteoconduction with controlled resorbability, but achieving the optimal balance between these properties remains a challenge [19]. These grafts must degrade at a rate that allows natural bone to regenerate without compromising structural integrity. Another limitation of alloplastic grafts is their lack of osteoinductive properties. Unlike natural bone grafts, which contain growth factors and living cells that stimulate new bone formation, synthetic grafts lack these biological signals. This can lead to slower healing and less efficient integration with the host bone, especially in cases where extensive bone regeneration is needed [21]. While advances are being made to incorporate bioactive agents or surface modifications to enhance cellular response, alloplastic grafts still fall short of fully mimicking the biological complexity of natural bone tissue [21]. Therefore, continued research is focused on improving the performance of these synthetic materials to better match the regenerative capabilities of natural bone. The next section will provide a detailed exploration of common synthetic bone grafts, focusing on their properties, clinical applications, and their role in advancing bone regeneration strategies.
Hydroxyapatite
HA has been widely utilized in hard tissue engineering, primarily due to its chemical similarity to the mineral composition of human bones and teeth [22]. It is a naturally occurring form of calcium apatite with the chemical formula Ca10(PO4)6(OH)2, indicating that its crystal structure contains two repeating units [22]. This mineral plays a crucial role in providing strength and support in natural bone and tooth structures. In bone regeneration, HA is one of the most used biomaterials, known for its excellent biocompatibility, osteoconductivity, and close resemblance to the mineral content of human bone. As a scaffold for cell attachment and proliferation, HA facilitates the regeneration of damaged or deficient bone tissue by creating a supportive framework that encourages new bone growth [22]. Its bioactive properties further enhance its role in bone healing by promoting interfacial interactions between the graft and surrounding tissue, aiding in faster and more efficient healing. HA’s ability to integrate with natural bone makes it a vital component in bone repair and regenerative therapies.
Tricalcium phosphate
TCP is a prominent biomaterial used in bone healing applications due to its excellent biocompatibility and controlled bioresorbability [23]. Its chemical composition, Ca3(PO4)2, closely mimics the mineral phase of natural bone, making it highly suitable for bone grafts and tissue regeneration [23]. TCP is available in two primary forms α-TCP and β-TCP differentiated by their crystallinity and resorption rates, with β-TCP being preferred for most clinical applications because of its slower and more controlled degradation [23]. In bone regeneration, TCP acts as an osteoconductive scaffold, providing a framework for the attachment and growth of new bone cells [24]. Over time, TCP is resorbed and replaced by newly formed bone, offering temporary structural support while allowing for natural bone healing [25]. Its ability to degrade at a controlled rate aligns with the body’s bone regeneration processes, minimizing the risk of premature graft failure or incomplete bone formation [25]. TCP is available in various forms, including granules, porous scaffolds, and injectable pastes, offering versatility across a wide range of medical applications [20]. It is widely used in dental bone grafts, spinal fusion surgeries, and the treatment of large bone defects, providing essential support to the body’s natural bone healing processes [20]. Furthermore, TCP is being explored for use in 3D-printed scaffolds, enabling customized bone regeneration solutions for more complex clinical cases [26]. However, TCP has certain limitations, particularly its lack of osteoinductive properties [27]. While it supports bone growth, it does not actively stimulate new bone formation without additional bioactive molecules or growth factors. This limitation often necessitates combining TCP with other biologically active components to enhance its regenerative potential [27, 28]. Despite these challenges, TCP remains a crucial material in bone healing due to its optimal balance between osteoconduction and resorption, as well as its versatility in various bone regeneration techniques.
Bioactive glass
Bioactive glasses, synthetic materials primarily composed of silicon, calcium, phosphorus, and sodium, have emerged as a promising option in osteoinductive biomaterials [21]. These materials exhibit exceptional bioactivity, allowing them to form strong bonds with living tissues [21]. Upon implantation, bioactive glasses initiate surface reactions that result in the development of an HA-like layer, closely mimicking the mineral composition of natural bone [21]. This reaction creates a scaffold that supports the adhesion and proliferation of bone cells, facilitating the healing process. The osteoconductive properties of bioactive glasses make them ideal for promoting bone growth. They not only provide structural support but also release essential ions, such as calcium and phosphate, which further enhance bone regeneration and remodeling [29]. These bioactive ions provide a favorable environment for cellular activity, accelerating the formation of new bone tissue [29]. Furthermore, bioactive glasses can be available in various forms, including particles, scaffolds, and implant coatings, and are widely used in medical applications, particularly in orthopedic and dental procedures [24, 30]. Their ability to integrate with natural bone and promote rapid healing makes them valuable in repairing bone defects [30]. However, challenges remain, such as refining the degradation rate and improving mechanical properties, which are critical to optimizing their performance in clinical applications [30]. Despite these limitations, bioactive glasses continue to show great potential as a key material in advancing bone regeneration therapies.
Mineral-based biomaterials
Minerals play a central role in the intricate process of bone regeneration, providing essential components that are vital for the development, sustenance, and restoration of bone tissue [1]. As a dynamic and living organ, bone relies heavily on its mineral composition to uphold both its structural resilience and functional capacity [1]. Minerals contribute to the structural integrity of bone by supporting the formation of HA crystals, essential for reinforcing bone tissue during fracture repair [31]. Moreover, these minerals play key roles in activating osteoblasts, the cells responsible for bone formation, and supporting collagen synthesis, which provides strength and flexibility to bones as shown in Figure 2 [31–33]. The intricate dance of bone regeneration involves a delicate interplay of biological and chemical factors, with minerals playing a central role in fortifying the structural integrity of the skeletal system [1]. In recent years, the incorporation of nanoparticles into this intricate mix has ignited a transformative wave in bone tissue engineering and regeneration, opening new avenues for enhancing the effectiveness and precision of therapeutic interventions [31, 34, 35]. This integration paves the way for the development of advanced biomaterials and therapeutic interventions that can mimic the natural mineral composition of bone while providing exceptional control and specificity in promoting tissue regeneration. Therefore, effective delivery of essential minerals is paramount for bone healing, as these minerals are integral to various physiological processes vital for the regeneration and maintenance of bone tissue [31]. The following sections will demonstrate how various key minerals promote bone healing and why their delivery is crucial for successful outcomes in bone healing process.
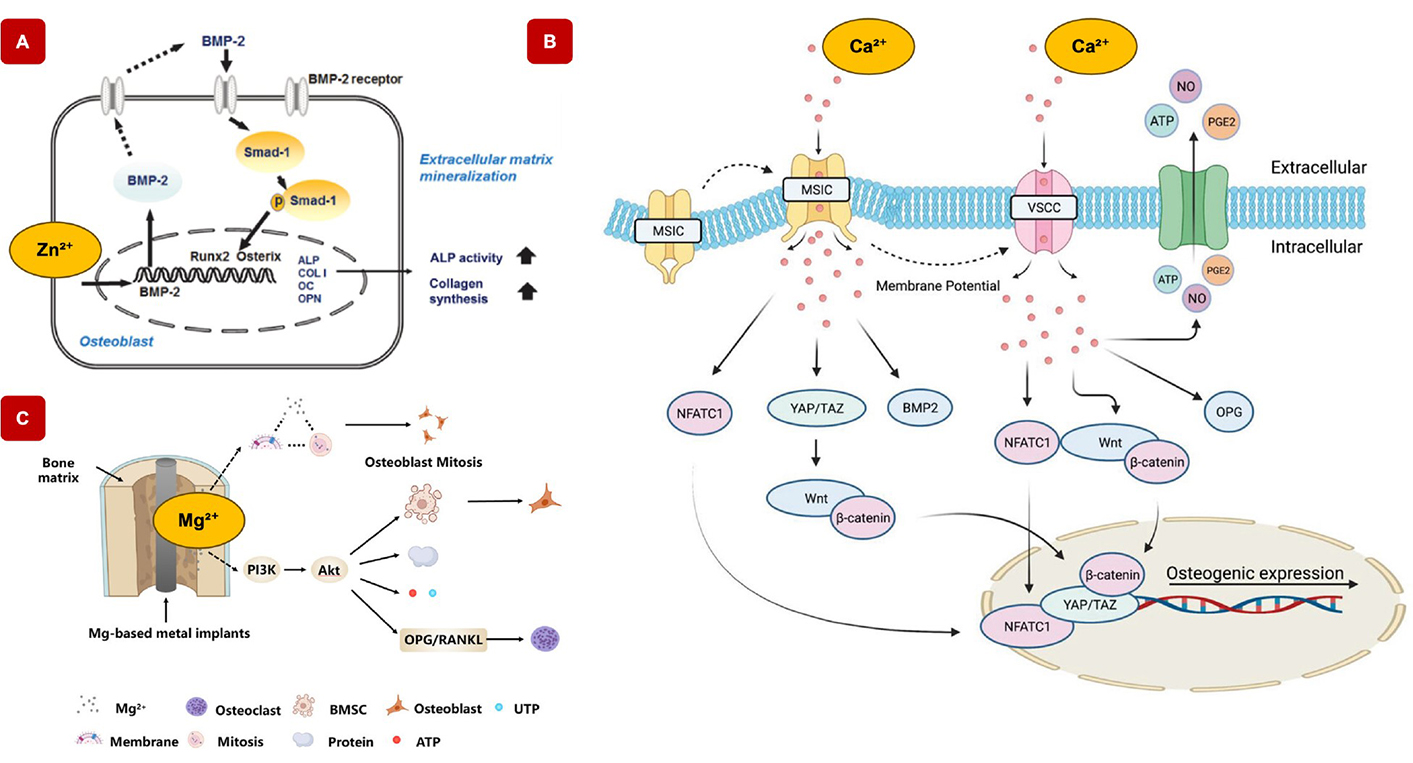
Mechanisms of various mineral ions in bone regeneration. A) Contribution of zinc ions (Zn2+) to osteogenic gene expression. Increasing Zn2+ concentrations in the culture medium enhances the expression of RUNX2 and activates Smad-1, indicating that Zn2+ promotes RUNX2 expression through the canonical BMP-2 signalling pathway. This suggests that zinc plays a critical role in osteogenesis by modulating key molecular components involved in bone formation [36]. Adapted from [36]. CC-BY 3.0. B) Mechanisms of calcium ions (Ca2+) in bone regeneration. Ca2+ play a pivotal role in regulating key signaling pathways that drive osteogenic differentiation and bone regeneration. Ca2+ influences pathways such as the Yes-associated protein/transcriptional coactivator with YAP/TAZ pathway, which is critical for cell proliferation. Additionally, Ca2+ modulates the canonical Wnt/β-catenin signaling pathway, a central regulator of bone formation and remodeling. Through these mechanisms, calcium ions enhance the expression of osteogenic markers, promote bone matrix deposition, and facilitate the differentiation of MSCs into osteoblasts, contributing to the overall process of bone regeneration [37]. Adapted with permission from [37]. © 2023 Wiley-VCH GmbH. C) Role of magnesium ions (Mg2+) in bone regeneration. Mg2+ enhances osteogenic differentiation by activating key signaling pathways such as PI3K-Akt and Wnt, which are crucial for promoting bone formation. Mg2+ promotes osteogenic differentiation via the PI3K-Akt and Wnt signaling pathways, while inhibiting osteoclastic differentiation through the OPG/RANK/RANKL signaling pathway [38]. Adapted from [38]. CC-BY 4.0
Zinc
Zinc ions (Zn2+), an essential trace element, play an essential role in bone healing [39, 40]. Numerous studies have demonstrated that zinc ions (Zn2+) promote cell proliferation, alkaline phosphatase (ALP) activity, osteogenic differentiation, and calcium deposition in both primary and established mesenchymal stem cell (MSC) lines by activating key signaling pathways involved in bone formation [39, 41]. Moreover, zinc stimulates the expression of osteogenic markers, such as ALP and osteocalcin (OC), which leads to increased mineralization, collagen synthesis, and bone matrix deposition [32]. In the early stages of osteogenic differentiation, extracellular Zn ions upregulate the expression of specific osteogenic genes in MSCs, including ALP, runt-related transcription factor 2 (RUNX2), and type 1 collagen [32]. As differentiation progresses into the middle and late stages, zinc supplementation further enhances the expression of late osteogenic markers, such as OC and osteopontin. Importantly, the regulatory effects of zinc on cell function are dose-dependent [32]. Zinc concentrations exert a dual impact on bone marrow-derived mesenchymal stem cells (BMSCs); while optimal levels stimulate osteogenesis, promoting bone formation and mineralization, excessively high concentrations can inhibit or even negatively affect cellular activity. This delicate balance highlights the need for precise control of Zn supplementation to ensure effective bone regeneration.
Calcium
Calcium ions (Ca2+) are essential in bone regeneration as they participate in crucial physiological processes within the body, particularly in bone metabolism and mineralization [42]. They contribute to bone regeneration primarily through mineralization, where they combine with phosphate ions to form HA, the main mineral component of bone matrix providing strength and rigidity [42]. Additionally, calcium ions serve as signaling molecules, stimulating osteoblast activity and collagen production essential for bone formation [37]. They also act as secondary messengers in signaling pathways that regulate bone cell differentiation, proliferation, and function [37]. Furthermore, calcium ions facilitate cell adhesion to the ECM, supporting interactions necessary for bone formation and remodeling. Indirectly, calcium ions play a role in muscle contraction, which supports bone health by exerting mechanical forces during movement, stimulating bone remodeling and regeneration [37]. In essence, ensuring adequate calcium intake and proper calcium signaling is crucial for maintaining bone health and facilitating bone regeneration processes [42]. Ca-based nanoparticles, particularly HA nanoparticles, mimic the natural mineral composition of bone and serve as excellent scaffold materials for bone tissue engineering [43]. They provide essential structural support while promoting cell adhesion, proliferation, and differentiation, which together foster new bone formation.
Magnesium
Magnesium ions (Mg2+) play an essential role in bone healing, exhibiting osteoinductive properties that stimulate MSCs to differentiate into osteoblasts, thereby promoting bone formation. Mg2+ actively influences several critical signaling pathways, including the Wnt/β-catenin pathway, to enhance osteogenic differentiation and facilitate mineralization [38]. Additionally, Mg2+ promotes osteogenesis through the activation of the PI3K-Akt and Wnt signaling pathways [38]. At the same time, it inhibits osteoclastic differentiation by regulating the OPG/RANK/RANKL signaling axis, thereby reducing bone resorption. Through these mechanisms, magnesium contributes to both the promotion of bone formation and the suppression of bone degradation, making it a key factor in the bone regeneration process [38]. It also plays a role in stabilizing bone structure and improving the mechanical properties of the bone matrix [44, 45]. Additionally, magnesium is vital for angiogenesis, the formation of new blood vessels at the bone regeneration site [46]. By upregulating the expression of angiogenic factors and encouraging endothelial cell proliferation and migration, magnesium-based nanoparticles help establish a vascular network essential for supplying nutrients and oxygen to regenerating bone tissue. Furthermore, magnesium’s anti-inflammatory properties can reduce inflammation, speeding up the healing process and enhancing the overall environment for bone regeneration.
Metal-organic frameworks
Metal-organic frameworks (MOFs) are nanoscale structures composed of metal ions or clusters coordinated with organic ligands, forming highly porous, crystalline frameworks [47, 48]. These versatile materials have found applications across various fields, including biomedicine [47, 48]. The first report on MOFs was published by Kinoshita and colleagues in 1959, but interest in these materials surged in the 1990s when Hoskins and Robson applied systematic approaches to their “reticular” design and synthesis [49]. MOFs are particularly valued for their versatile architecture, large internal surface area, and the ease with which their configurations can be tuned by modifying either the metal ions or organic ligands. These properties make MOFs ideal candidates for drug delivery systems, imaging, and sensors. Among them, mineral-based MOF nanoparticles stand out due to their distinctive properties, making them promising candidates for biomedical use, particularly in bone regeneration [50]. These MOFs can be fabricated using essential bioactive cations like calcium, zinc, and magnesium—key minerals that play a crucial role in the bone healing process [49]. Additionally, mineral-based MOFs can promote bone regeneration through properties like osteoconductivity (supporting bone growth), osteoinductivity (stimulating bone formation), and antibacterial activity, all of which further their potential in bone healing applications. Their tunable pore sizes and surface chemistries allow for precise control over the delivery kinetics of bioactive agents, offering customized therapeutic molecules for effective bone healing [51]. Especially, Zeolitic Imidazolate Framework-8 (ZIF-8) is a type of Zn-based MOF, that belongs to a subclass of reticular structures derived from tetrahedral four-connected networks found in zeolites and minerals [52]. Due to its unique structural properties, ZIF-8 has garnered significant attention across various biomedical applications. These frameworks consist of transition-metal ions (Zn, Co, and Cu) and imidazolate-type linkers, offering precise control over pore size and shape, surface area, and functionality [52]. The synthesis of ZIF-8 involves the coordination of Zn2+ with 2-methylimidazole (C4H6N2), resulting in a robust and stable crystalline framework [53]. This reaction results in the formation of the ZIF-8 structure, where Zn2+ ions are intricately linked through the nitrogen atoms of the 2-methylimidazole molecules, leading to a robust and stable framework. Overall, mineral-based MOFs, like ZIF-8, hold great promise for advancing bone regeneration through their combination of innovative, tunable materials, controlled drug delivery, and enhanced biocompatibility.
Emerging and novel strategies to deliver osteoinductive biomaterials
Polymeric hydrogels
Polymeric hydrogels, known for their high-water content and interconnected polymer networks, have become essential biomaterials in tissue engineering, particularly for bone regeneration [6, 14, 54]. Their unique properties have established them as key components in the biomedical field, significantly contributing to pharmaceutical research and development while enabling a wide range of invasive and minimally invasive administration routes [55]. Additionally, this high-water content not only mimics the natural ECM but also creates an optimal environment for the diffusion of oxygen, nutrients, and other small molecules essential for cell growth and proliferation [6]. Furthermore, the interstitial spaces within hydrogel networks can accommodate various drug molecules, facilitating their diffusion into the biological medium and allowing hydrogels to function as reservoirs for controlled release applications [55]. Consequently, polymeric hydrogels effectively support crucial cellular processes such as adhesion, proliferation, and differentiation, which are vital for successful bone healing [6, 55, 56]. Moreover, their versatility allows for diverse applications, such as three-dimensional bioprinting, injectable systems, preformed implantable scaffolds, sprayable forms, and coatings for implants [35]. Figure 3 highlights these key unique properties of polymeric hydrogels make them highly adaptable for diverse applications. This adaptability not only improves biofunctionality but also enables targeted drug delivery, facilitates cell encapsulation, and enhances traditional methods, while allowing for customization to meet the specific requirements of different treatment areas [35, 56]. Despite their promise, hydrogels face several critical challenges that must be addressed to enhance their clinical efficacy, along with strategies to overcome these limitations. Immune rejection remains a significant hurdle, as even biocompatible hydrogels can provoke adverse inflammatory responses [57]. This can be addressed by incorporating immune-modulating agents, such as anti-inflammatory cytokines or bioactive peptides, and engineering hydrogels with stealth properties to reduce immune recognition and promote seamless integration with host tissues [58]. Biodegradability is another concern, as mismatched degradation rates can disrupt natural bone regeneration [59]. This challenge can be tackled by designing hydrogels with tunable degradation profiles through advanced crosslinking strategies, enzymatically degradable linkers, and bioresorbable materials that synchronize with the tissue remodeling timeline [57, 59]. Limited in vivo retention is also problematic, as hydrogels often exhibit short residence times, reducing their therapeutic impact [4]. Solutions include incorporating nanoparticles, optimizing network density for structural reinforcement, and developing adhesive hydrogels that adhere strongly to surrounding tissues for enhanced stability [4, 60]. Finally, their mechanical properties frequently fall short of the demands of physiological loads in bone repair [61]. Hybrid strategies, such as reinforcing hydrogels with mineral nanoparticles, bioactive glass, or polymer fibers, can significantly enhance mechanical strength without compromising bioactivity, enabling hydrogels to better withstand physiological stresses while supporting tissue regeneration [36, 61]. As a result, polymeric hydrogels represent a highly innovative and flexible platform for advancing medical therapies in bone tissue regeneration. The following sections will delve into the versatility and chemical properties of polymeric hydrogels in bone healing applications.
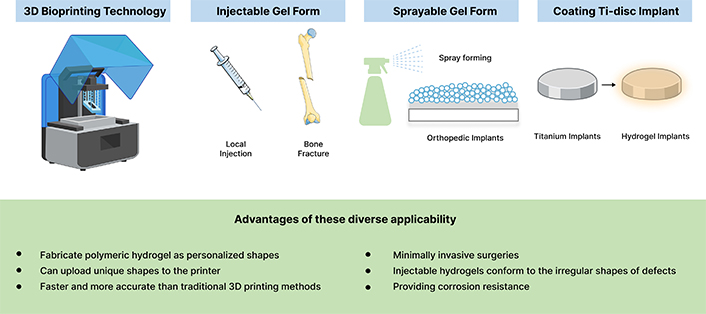
Adaptability of polymeric hydrogels in bone healing applications: key properties and versatile uses. The versatility of polymeric hydrogels is evident in their wide range of applications, spanning injectable systems, three-dimensional bioprinting, preformed scaffolds, sprayable coatings, and implantable materials. These adaptable forms allow hydrogels to be precisely customized for diverse clinical needs, including the fabrication of complex shapes, enabling minimally invasive procedures, and the injection into irregular defect sites. Additionally, hydrogels can protect orthopedic implants by serving as supportive coatings. This remarkable adaptability makes polymeric hydrogels a promising solution for advancing bone healing strategies, enhancing both functionality and therapeutic effectiveness. Parts of the figure were adapted from pictures provided by Servier Medical Art, licensed under CC BY 4.0
Osteoinductive biomaterials into polymeric hydrogels
The incorporation of nanoparticles into polymeric hydrogels has emerged as a key innovation in biomedical research, biomaterials, especially for bone healing applications [35, 62–64]. By incorporating osteoinductive nanoparticles within crosslinked polymeric networks, these nanocomposite hydrogels combine the advantageous properties of both components, resulting in enhanced biological functionality and versatility [35, 62–65]. In particular, introducing mineral-based nanoparticles, such as MOFs, into polymeric hydrogels marks a significant advancement into the development of biomaterials (Figure 4). This integration leverages the flexibility and tunability of polymer networks while incorporating the osteoconductive and bioactive properties of minerals, which are essential for bone regeneration [35]. These nanocomposite hydrogel systems not only provide mechanical support but also enable the controlled release of therapeutics, including growth factors or antibiotics [35, 66]. This sustained release ensures that therapeutic agents are delivered precisely where and when needed, optimizing the healing process and minimizing the risk of overdosing [35, 66]. The addition of mineral-based nanoparticles offers several crucial benefits for bone healing [35]. By mimicking the inorganic composition of natural bone tissue, these mineral-based nanoparticles enhance the integration of the hydrogel with the host bone, promoting the differentiation of stem cells into osteoblasts, which are critical for new bone formation [35]. Furthermore, these nanocomposite hydrogels exhibit improved mechanical strength and structural integrity, making them ideal for applications requiring robust support, such as orthopedic surgeries and bone defect repairs [67]. This combination of polymeric hydrogels with mineral-based nanoparticles results in a multifunctional material with great potential for orthopedic, dental, and craniofacial applications [35, 67]. These nanocomposite hydrogels are particularly valuable for complex bone defects where enhanced biofunctionality is required. The following sections will explore the fabrication techniques, properties, and applications of mineral-based polymeric hydrogels, showcasing their pivotal role in advancing bone regeneration therapies.
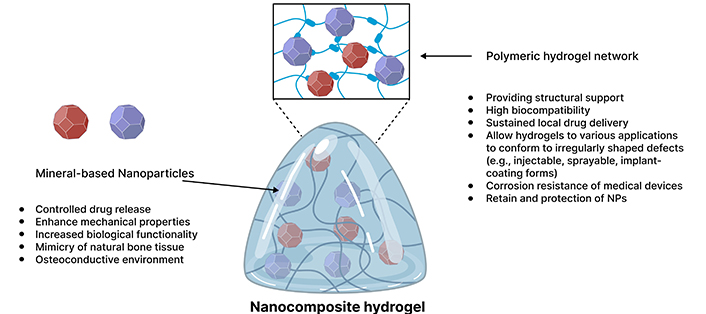
Illustration depicting the incorporation of mineral-based nanoparticles into polymeric hydrogels. The schematic highlights the uniform distribution of nanoparticles within the crosslinked polymer network, which enhances the structural integrity and mechanical properties of the hydrogel. This integration imparts osteoconductive and bioactive characteristics, effectively mimicking the natural bone matrix. The figure emphasizes the dual functionality of the polymeric network, serving as a reservoir for the controlled release of therapeutic agents and minerals, thereby promoting efficient drug delivery and supporting bone regeneration. Key components, including polymer chains, and nanoparticles, are labelled to illustrate their contributions to improved biofunctionality in bone healing applications
Implementable hydrogels
Implantable hydrogels in bone regeneration are advanced biocompatible polymer-based materials specifically engineered to integrate directly into bone defects or injury sites, playing an essential role in promoting the bone healing process [35, 68]. 3D bioprinting of bone tissue grafts can be optimized using MRI or CT imaging, with 3D CAD modeling providing accurate dimensional data for large bone defects, which can then be directly imported into the 3D bioprinter (Figure 5A). A variety of hydrogels, both natural and synthetic, can be utilized for printing. These hydrogels closely mimic the natural ECM of bone tissue, providing essential structural support while creating an optimal environment for critical cellular activities such as adhesion, proliferation, and differentiation, all key to successful bone regeneration [21, 35]. Their moldable nature allows them to conform precisely to the unique contours of the defect site, ensuring a custom fit that promotes more efficient healing [35]. A major advantage of implantable hydrogels is their biodegradability; they gradually degrade as new bone tissue forms, eliminating the need for follow-up surgeries to remove the material [35]. Additionally, these hydrogels can act as controlled-release systems, delivering bioactive molecules like growth factors or therapeutic drugs directly to the injury site in a precise and sustained manner [35, 69]. This controlled delivery prevents overdosing and ensures a steady release of therapeutic agents over time, accelerating the healing process and improving treatment efficacy [69, 70]. With tunable mechanical properties that can be tailored to match the surrounding bone tissue, implantable hydrogels support seamless integration of the regenerated bone with existing tissue [68]. This combination of structural reinforcement, biological functionality, and degradability makes them an invaluable tool in bone regeneration, significantly enhancing treatment outcomes by promoting the growth of functional, healthy bone tissue [35].
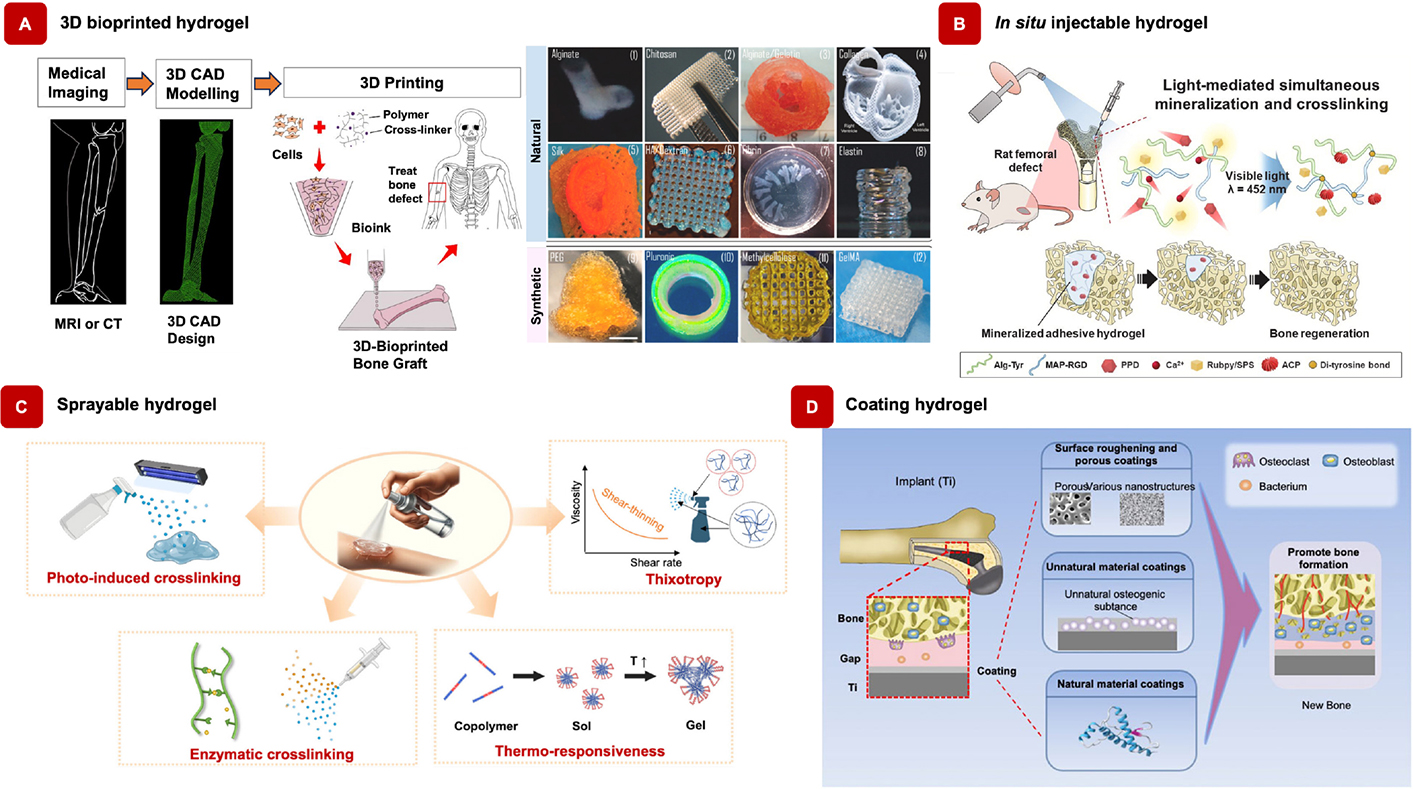
Case study on the versatility of polymeric hydrogels in advancing biomedical applications. A) Strategies for 3D bioprinting and the fabrication of 3D-bioprinted scaffolds using natural and synthetic hydrogels [71, 72]. Adapted from [71, 72], CC-BY 4.0. B) In situ injectable hydrogels crosslinked within a rat femoral tunnel defect, promoting light-mediated mineralization and bone regeneration [73]. Adapted with permission from [73]. © 2024 Elsevier Ltd. C) Sprayable hydrogels enabling photoinduced and enzymatic cross-linking, as well as thermoresponsive systems, for in situ hydrogel formation [74]. Adapted with permission from [74]. © 2025 American Chemical Society. D) Osteogenic coatings on titanium implants enhance bone integration by promoting new bone formation, while the elastic coating expands to fill interface gaps for a secure fit [75]. Reprinted from [75], CC-BY 4.0
Injectable form of hydrogels for bone repair
Hydrogel can be injectable, marking a significant advancement in the realm of regenerative medicine, particularly in the context of bone regeneration [6]. This unique property enables hydrogels to be delivered directly to target sites via minimally invasive procedures, offering a promising solution for treating bone defects and injuries [76]. Injectable hydrogels, known for their ability to transform from a liquid to a gel-like state upon injection, represent a significant advancement in regenerative medicine [6, 76]. Their injectability is enabled by distinctive physical and chemical attributes, facilitating easy administration into defect sites through minimally invasive procedures like syringe delivery [6]. According to Yun et al. [73], in situ injectable hydrogels effectively induced simultaneous crosslinking and amorphous calcium phosphate (ACP) formation within the hydrogel matrix, further enhancing new bone formation (Figure 5B). This emerging technology, capable of sustaining prolonged drug release with a single injection, is expected to provide patients and medical professionals with a compelling blend of cost-effectiveness and time efficiency. Injectable hydrogels offer diverse applications in promoting bone regeneration within the realms of bone tissue engineering and regenerative medicine. They serve as versatile tools in several ways: Firstly, as fillers for bone defects, where they can be directly injected into sites affected by trauma, disease, or surgical procedures [6]. Here, the hydrogel fills the void within the defect, providing structural support and facilitating the growth of new bone tissue. Secondly, as delivery vehicles for cells such as MSCs or osteoblasts [77]. These cells are mixed with the hydrogel before injection, allowing for controlled delivery to the defect site [77]. Once injected, the hydrogel provides an optimal environment for cell survival, proliferation, and differentiation into bone-forming cells, thus facilitating tissue regeneration [78]. Thirdly, they facilitate localized drug delivery by encapsulating bioactive molecules like growth factors, cytokines, or small molecules [78]. These molecules are released in a controlled manner at the defect site, stimulating cellular activities essential for bone formation and remodeling. Additionally, they can enhance the properties of solid scaffold materials used in bone tissue engineering by modifying mechanical properties, promoting cell infiltration, and creating a conducive environment for tissue regeneration [6, 78]. Furthermore, their injectability enables minimally invasive procedures, reducing the need for open surgeries and associated risks. Surgeons can deliver the hydrogel directly to the defect site using syringes or catheters, facilitating faster recovery times. Moreover, they can be combined with other biomaterials, scaffolds, or therapeutic modalities to create synergistic effects for bone regeneration, thus addressing various challenges associated with bone defects and injuries [78]. Lastly, hydrogels can be customized to mimic the natural ECM of bone tissue, allowing researchers to design hydrogels with specific properties optimized for bone regeneration in different clinical scenarios. In conclusion, the versatility and effectiveness of injectable hydrogels make them promising tools for promoting bone regeneration and addressing various challenges associated with bone defects and injuries.
Sprayable hydrogels
Sprayable hydrogels are a unique and innovative approaches in bone regeneration, offering unique advantages for both patients and healthcare providers (Figure 5C). These hydrogels can be applied as a fine mist or spray, allowing for even distribution over irregular and hard-to-reach defect sites [79–82]. This method enhances the adaptability and effectiveness of hydrogels in promoting bone regeneration by filling microvoids and adhering well to complex surfaces. The use of spray devices ensures uniform application, providing a consistent layer that conforms to the defect’s shape and dimensions [81]. Like injectable hydrogels, sprayable hydrogels enable minimally invasive treatments, reducing the need for extensive surgical procedures, decreasing patient recovery time, and minimizing the risk of complications associated with open surgeries [82]. Moreover, sprayable hydrogels can be coated onto medical implants to enhance their biofunctionality, offering protection against corrosion and improving the integration of implants with surrounding tissue [83]. This coating also reduces implant-related complications, promoting longer-lasting and more effective results [83]. In addition, the properties of sprayable hydrogels can be finely tuned by modifying their chemical composition, mechanical properties, and degradation rates, allowing for optimization based on specific clinical needs and bone defect types [82]. This versatility is particularly advantageous in orthopedic and craniofacial surgeries, where irregular and complex defect sites are frequently encountered. The customizable nature of sprayable hydrogels enables the development of innovative, patient-specific solutions, significantly enhancing treatment outcomes in bone regeneration therapies.
Coating orthopedic implants
Orthopedic implants are essential in modern healthcare, providing effective solutions for treating musculoskeletal conditions and greatly enhancing the quality of life for individuals affected by injuries or orthopedic disorders [84]. These implants are designed to replace and support fractured bones, aid in bone union and regeneration, and enhance mechanical stabilization. However, despite their importance, orthopedic implants often face complications that can hinder their long-term success [84]. One of the main challenges is the lack of biofunctionality, which is critical for supporting cell migration, proliferation, and integration with the surrounding tissues [85]. Additionally, implant-related infections due to bacterial colonization pose a significant risk, leading to implant failure. When an implant is introduced into the body, it is particularly vulnerable to bacterial contamination, especially during the initial hours post-surgery [85]. Bacteria can quickly adhere to both natural and synthetic surfaces, using various strategies to ensure survival and proliferation. This can lead to severe infections at the implant site, known as surgical site infections (SSIs), which are a leading cause of implant failure [86, 87]. To address these challenges and enhance implant outcomes, advanced surface coating techniques have been developed [88, 89]. Surface coatings are crucial for improving the performance, durability, and biocompatibility of orthopedic implants. These specialized coatings are designed to reduce the incidence of SSIs, enhance osseointegration (the integration of the implant with bone), and ultimately prevent implant failure [89]. By mimicking natural physiology, these coatings promote better tissue integration, reduce immune responses, and increase the implant’s resistance to wear and corrosion, thereby extending its lifespan. Moreover, surface coatings enhance bone bonding, prevent infections through antimicrobial properties, and reduce friction and wear, contributing to smoother implant function [89]. Figure 5D demonstrates the types and effects of osteogenic coatings on titanium implants, emphasizing their potential to enhance new bone formation. These coatings can also be customized to release therapeutic agents or optimize surface roughness, acting as barriers to minimize metal ion release and reducing the risk of allergic reactions. Overall, the application of these coatings significantly improves implant safety, functionality, and patient outcomes, ensuring long-term success and better quality of life for patients.
Conclusions
Osteoinductive biomaterials, including synthetic, natural, and hybrid types, represent a critical advancement in addressing the growing need for effective bone healing applications. These materials stimulate osteogenesis by promoting the differentiation of precursor cells into osteoblasts, essential for bone regeneration and remodeling. The integration of polymeric hydrogels has further expanded their potential, providing a multifunctional platform that offers structural support, mimics the ECM, and enhances the delivery of bioactive molecules, therapeutic agents, and cells. While these systems demonstrate remarkable promise, challenges such as immune rejection, biodegradability, mechanical strength, and limited in vivo retention must be addressed to realize their full clinical potential. Innovations in material design, including the incorporation of mineral nanoparticles and hybrid approaches, are critical for overcoming these limitations. Furthermore, interdisciplinary collaboration and long-term preclinical studies are essential to bridge the gap between laboratory research and clinical application. As bone-related disorders continue to rise, the development of these biomaterial-hydrogel systems not only holds the potential to improve patient outcomes but also contributes to the advancement of regenerative medicine. By addressing current limitations and exploring novel strategies, these systems pave the way for more effective, personalized, and scalable solutions in bone repair and beyond.
Abbreviations
| ALP: | alkaline phosphatase |
| ECM: | extracellular matrix |
| HA: | hydroxyapatite |
| MOFs: | metal-organic frameworks |
| MSC: | mesenchymal stem cell |
| OC: | osteocalcin |
| SSIs: | surgical site infections |
| TCP: | tricalcium phosphate |
| ZIF-8: | Zeolitic Imidazolate Framework-8 |
Declarations
Author contributions
CCE: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. AP: Conceptualization, Writing—review & editing, Supervision. The authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Arghya Paul is thankful for the funding and support from Canada Research Chairs Program of the Natural Sciences and Engineering Research Council (NSERC) of Canada (CRC-2018-00028), NSERC Discovery Grant and Canadian Institutes of Health Research Operating Grant (CIHR – IMHA, Grant no: 185629). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.