Affiliation:
1Department of Chemical and Product Safety, German Federal Institute of Risk Assessment (BfR), 10589 Berlin, Germany
†These authors contributed equally to this work.
Email: Ajay-Vikram.Singh@bfr.bund.de
ORCID: https://orcid.org/0000-0002-9875-7727
Affiliation:
1Department of Chemical and Product Safety, German Federal Institute of Risk Assessment (BfR), 10589 Berlin, Germany
†These authors contributed equally to this work.
Affiliation:
2Infosys LT, Hinjawadi Rajiv Gandhi Infotech Park, Hinjawadi, Pune 411057, India
†These authors contributed equally to this work.
Affiliation:
4Kamla Nehru Institute of Physical and Social Sciences (KNIPSS), Sultanpur 228001, India
Affiliation:
5Qatar University Young Research Centre (QUYRC), Qatar University, Doha 2053, Qatar
ORCID: https://orcid.org/0000-0002-1350-6153
Affiliation:
6Department of Translational Medicine, University of Ferrara, 44121 Ferrara, Italy
7Centre Haemostasis and Thrombosis, University of Ferrara, 44121 Ferrara, Italy
ORCID: https://orcid.org/0000-0001-8448-066X
Affiliation:
6Department of Translational Medicine, University of Ferrara, 44121 Ferrara, Italy
7Centre Haemostasis and Thrombosis, University of Ferrara, 44121 Ferrara, Italy
ORCID: https://orcid.org/0000-0001-6213-6120
Affiliation:
3Chair Vascular Diseases Center, University of Ferrara, 44124 Ferrara, Italy
ORCID: https://orcid.org/0000-0002-7107-888X
Explor BioMat-X. 2024;1:124–134 DOI: https://doi.org/10.37349/ebmx.2024.00009
Received: December 08, 2023 Accepted: March 14, 2024 Published: April 25, 2024
Academic Editor: Stephanie Willerth, University of Victoria, British
Nanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough understanding of its regulatory landscape. An in-depth discussion of regulatory considerations, including molecular safety assessment, harmonization of the regulatory landscape, and shaping the future of innovation, is presented in this discourse. The molecular safety assessment entails evaluating interactions between nanoparticles and biomolecules, ensuring compatibility at the molecular level. Harmonization involves developing international standards and guidelines for a consistent regulatory approach, while shaping innovations emphasizes integrating molecular safety assessments into early stages of development. Challenges encompass the need for standardized assessment methods, balancing innovation with safety, and addressing unique features of novel molecular designs. As the nanomedicine landscape evolves, effective regulatory strategies must navigate the intricate interplay of molecules and technologies, ensuring both patient access and product safety.
The realm of nanomedicine has ushered in a new era of healthcare, where the convergence of nanotechnology and medicine has given rise to innovative approaches that hold immense promise for improving diagnosis, treatment, and overall patient outcomes [1]. At the heart of this transformation lies the intricate interplay of key molecular players that govern the design, functionality, and therapeutic efficacy of nonmedical interventions [2]. The fusion of nanoscale science with medicine has opened up avenues previously thought to be confined to the realm of science fiction. Nanomedicine, with its tailored nanoparticles, targeted drug delivery, and precision molecular interactions, has redefined medical paradigms [3]. This introduction serves as a prelude to unraveling the pivotal role that specific molecules play in propelling these innovations [4].
The genesis of nanomedicine can be traced back to the realization that materials and devices at the nanoscale possess unique properties that can be harnessed to address longstanding challenges in medicine [5]. The reduced dimensions at this scale result in distinctive behaviors, from enhanced surface area-to-volume ratios to quantum effects. These attributes have been strategically manipulated to engineer multifunctional nanoparticles capable of navigating biological barriers, homing in on disease sites, and releasing therapeutic payloads with precision [6, 7].
The versatility of nanomedicine extends beyond drug delivery. Molecular imaging techniques, such as quantum dots and contrast agents, have revolutionized diagnostic accuracy by offering molecular-level insights into cellular and tissue processes [8]. Furthermore, the emergence of RNA-based nanomedicine, including small interfering RNA (siRNA) and messenger RNA (mRNA) therapeutics, has brought gene silencing and editing to the forefront of disease intervention [9]. The Table 1 below presents a molecular toolbox for nanomedicine, including molecules that harness immune responses, modulate genetic expression, and mimic natural signaling pathways. The molecules listed are associated with their respective applications in the context of nanomedicine, as supported by the provided search results.
Molecular toolbox for nanomedicine
| Molecules | Immune responses | Genetic expression | Signaling pathways |
|---|---|---|---|
| Superparamagnetic iron oxide nanoparticle probes | Used in immune cell labeling and tracking | Can modulate genetic expression when functionalized with specific ligands | Can mimic natural signaling pathways when designed to target specific receptor |
| Nanoscale delivery systems | Can be engineered to modulate immune responses for targeted therapy | Have the potential to modulate genetic expression for therapeutic purpose | Can be designed to mimic natural signaling pathways for targeted drug delivery |
| Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas system | Used for molecular detection in immune-related research | Can be employed to modulate genetic expression through gene editing | Utilized to study and manipulate natural signaling pathways within cells |
Central to the success of nanomedicine are the key molecules that orchestrate its intricate dance. From lipids and polymers forming the foundation of nanoparticle carriers to antibodies and ligands guiding their specific interactions, these molecules function as the architects of therapeutic precision [10, 11]. The narrative of nanomedicine is woven from the threads of molecular recognition, binding kinetics, and cellular uptake—each influenced by the intricate chemistry and physics governing these entities [12].
This commentary embarks on a journey to decipher the molecular intricacies driving nanomedicine’s progress. It investigates the multifaceted interactions between nanoparticles and biomolecules, highlights the molecular design principles underpinning personalized therapies, and explores the evolving regulatory landscape governing these molecularly tailored interventions [13].
In the following sections, this perspective paper will navigate through the molecular landscapes of nanoparticle carriers, bioconjugation strategies, and the dynamic interface between nanotechnology and molecular biology [14]. Through this exploration, research community hopes to shed light on the essence of nanomedicine as an interdisciplinary endeavor where the synergy of molecules at the nanoscale converges with the intricacies of human biology, forging a path towards transformative medical solutions [15]. The Figure 1 presents a molecular toolkit where artificial intelligence (AI) could be instrumental for future nanomedicine designed for grasping the bio-physicochemical characteristics at the interface between nanomaterials and biology [16]. This model aims to enhance understanding by predicting key nanomaterial properties that influence interactions with biological systems. Once suitable nanomaterial properties are chosen, it is crucial to employ real experimental data for the development, fine-tuning, and validation of machine learning (ML) models before one can make predictions about unfamiliar nanomaterial properties or their interactions with biological entities. The radial outlines on the right panel signify the areas where AI and ML techniques can be applied to forecast unfamiliar properties, enabling one to predict toxicity and health effects in the field of nanomedicine.
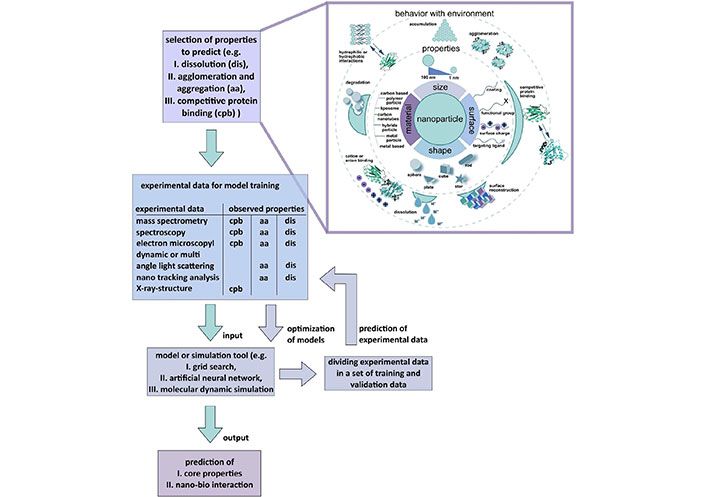
Schematic Illustration an AI toolbox for comprehending the nano-bio interface’s bio-physicochemical identity. The model predicts nanomaterial properties affecting nano-bio interactions. Real experimental data is crucial for developing, optimizing, and validating ML models before predicting unfamiliar nanomaterial properties and their interactions, particularly in nanomedicine’s toxicity and health effects [17]
Note. Reprinted from “Artificial intelligence and machine learning empower advanced biomedical material design to toxicity prediction,” by Singh AV, Rosenkranz D, Ansari MHD, Singh R, Kanase A, Singh SP, et al. Adv Intell Syst. 2020;2:2000084 (https://onlinelibrary.wiley.com/doi/full/10.1002/aisy.202000084). CC BY.
Nanoparticles, as carriers of therapeutic payloads, represent a cornerstone of modern nanomedicine. These minute entities, often measured in nanometers, have redefined drug delivery by harnessing the power of molecules and materials at the nanoscale. This section researches into the intricate world of nanoparticle carriers, highlighting their design, functionality, and impact on therapeutic outcomes [18].
Lipid-based nanoparticles have emerged as versatile carriers in the nanomedicine landscape. Comprising lipids and phospholipids, these nanoparticles mimic cellular membranes and offer a unique molecular interface for drug encapsulation and delivery [19, 20]. Liposomes, for instance, bilayered lipid vesicles, have been instrumental in delivering a diverse range of therapeutics, from chemotherapeutic agents to genetic materials [21, 22]. The molecular arrangement of lipids enables controlled release, enhancing the therapeutic window and minimizing off-target effects [23, 24].
Polymer-based nanoparticles have revolutionized drug delivery by affording precise control over their size, shape, and surface properties [25]. The meticulous selection of polymers and their molecular architecture governs drug loading, release kinetics, and interactions with biological systems. Co-polymeric structures, such as micelles and dendrimers, exhibit tunable properties, enabling the formulation of therapeutics that exhibit extended circulation times and preferential accumulation in disease sites. The molecular diversity of polymers allows for tailoring nanoparticles for specific disease contexts and patient profiles [26].
Inorganic nanoparticles, often composed of metals, semiconductors, or metal oxides, bring molecular properties to the forefront. Quantum effects, plasmonic resonance, and surface-enhanced properties are harnessed to create nanoparticles with unique optical and electronic signatures [27]. Gold nanoparticles, for instance, exhibit surface plasmon resonance that can be tuned to absorb and scatter light, enabling applications in both diagnostics and therapeutics [28]. Their interactions with biomolecules are governed by molecular affinity, shaping their behavior in biological environments [29].
As nanomedicine journeys through the molecular landscapes of these nanoparticle carriers, it becomes evident that their functionality is underpinned by the intricate dance of molecules at the nanoscale [30]. Whether it’s the molecular assembly of lipids, the tailored architecture of polymers, or the molecular properties of inorganic materials, the interplay of molecules orchestrates the delivery of therapeutics with unprecedented precision [31, 32]. This molecular symphony, conducted on the nanoscale, exemplifies the convergence of scientific disciplines that defines the essence of nanomedicine [33].
In the intricate world of nanomedicine, the ability to precisely guide nanoparticles to their intended destinations within the body is a testament to the remarkable interplay of molecules. This section unveils the strategic art of bioconjugation and targeting strategies—integral facets that bridge the molecular precision of nanoparticles with the dynamic landscape of cellular interactions [34]. On the role of key molecules in driving nanomedicine innovations, it is essential to underscore the pivotal role played by molecular design in shaping the transformative landscape of nanomedicine. Key molecules, such as nanoparticles and biomolecules, serve as the foundation for the development of innovative drug delivery systems, diagnostic tools, and therapeutic interventions [12]. The judicious selection and engineering of these key molecules enable precise targeting, controlled release, and enhanced therapeutic efficacy. Moreover, understanding the intricate interactions between nanoparticles and biomolecules is crucial for ensuring molecular safety and efficacy. Advances in nanomedicine hinge on the ability to harness the unique properties of key molecules, emphasizing the need for a thorough exploration of their roles in driving innovations [2]. This nuanced perspective enables a more comprehensive understanding of the molecular intricacies that underpin the progress of nanomedicine and aligns with the overarching theme of navigating regulatory challenges in the field.
Ligand functionalization stands as a prime example of molecular ingenuity in nanomedicine. By tethering specific molecules—ligands—to the surface of nanoparticles, researchers have unlocked the potential for selective targeting. These ligands, often antibodies, peptides, or aptamers, bind to receptors on the surface of target cells with exquisite specificity. This molecular interaction not only enhances cellular internalization but also offers a means to tailor therapeutic interventions for individual patients or disease subtypes [35]. The molecular compatibility between ligands and receptors is the linchpin in this strategy, dictating the success of nanoparticle homing.
Antibody-drug conjugates (ADCs) epitomize the fusion of molecular recognition and therapeutic delivery. Here, monoclonal antibodies serve as molecular vehicles, selectively binding to antigens on cancer cells. Once bound, the ADCs internalize, releasing potent cytotoxic payloads that specifically target cancerous cells [36]. The synergy of molecular recognition and therapeutic payload delivery minimizes collateral damage to healthy tissues, making ADCs a paradigm-shifting approach in oncology. The precision of this strategy lies in the specific pairing of antibodies with their antigenic targets.
It’s important to note that the regulatory landscape for nanomedicine is continually evolving as the field progresses, and the challenges mentioned above represent just a snapshot of the complex considerations involved in ensuring the safe and effective use of nanomedicine products as mentioned above in Table 2.
Summarizes the key aspects related to regulatory considerations in the field of nanomedicine
| Regulatory aspect | Description |
|---|---|
| Molecular safety assessment |
|
| Harmonizing regulatory landscape |
|
| Shaping future nanomedicine innovations |
|
| Regulatory challenges |
|
Peptides, short sequences of amino acids, have proven themselves as molecular keys that unlock cellular entry for nanoparticles. These bioactive sequences hold the potential to interact with specific cellular receptors, facilitating internalization via different exposure routes [38, 39]. Furthermore, peptides can be tailored to interact with enzymes present in disease microenvironments, triggering localized therapeutic release. The molecular interactions between peptides and their cognate receptors are fundamental to their targeting efficiency and therapeutic efficacy [40].
As nanomedicine progresses into these molecular intricacies, it becomes clear that the success of nanomedicine hinges on the precise orchestration of molecular recognition events. The choice of ligands, the affinity of antibodies, and the specificity of peptides—each decision is a molecular brushstroke on the canvas of targeted therapeutic interventions [41]. This section underscores the notion that molecular interactions, guided by design and propelled by understanding, lie at the core of nanomedical achievements, shaping the future of medicine with unprecedented accuracy [42].
The journey through the intricate realm of nanomedicine has unveiled a landscape where molecules, both in their individual capacities and orchestrated collaborations, play a central role in redefining the boundaries of medical innovation [17]. This exploration of key molecular players in nanomedicine has shed light on the fundamental mechanisms that underpin the transformative potential of this field. As nanomedicine stands at this juncture, it is evident that the synergy of nanotechnology and molecular biology holds the promise of revolutionary advancements in healthcare [43]. Looking forward, several avenues beckon research community to further expand the horizons of nanomedicine, including robotics, AI and technological innovations [44].
The future of nanomedicine lies in the convergence of molecular insights with patient-centric care. Customized therapies, crafted with molecular precision, will harness genetic and molecular profiles to design interventions that are uniquely suited to individual patients [45, 46]. The era of one-size-fits-all treatments will give way to personalized approaches, where the interplay of molecules guides therapy selection, dosing, and duration assisted by digital revolution, AI and ML [47, 48].
Advances in molecular imaging and diagnostics will continue to empower clinicians with the ability to visualize disease processes at the molecular level. This molecular understanding will drive the development of theranostic strategies, where diagnostics and therapeutics are seamlessly integrated into a unified approach. Nanoparticles armed with both imaging agents and therapeutic payloads will enable real-time monitoring and adaptive interventions, maximizing treatment efficacy while minimizing side effects, molecular imaging [49, 50].
The exploration of nanomedicine’s future frontiers brings to light the potential of novel molecules and materials. From aptamers that mimic antibodies to emerging nanomaterials with unique properties, the palette of molecules available for nanomedical designs will diversify. The molecular toolbox will expand to include molecules that harness immune responses, modulate genetic expression, and mimic natural signaling pathways [51].
The potential of nanomedicine’s future hinges on interdisciplinary collaboration between doctors, data engineer to boost the advanced application of AI and ML [6, 17, 35, 52]. The cross-pollination of molecular biology, materials science, engineering, and clinical medicine will propel towards groundbreaking solutions [53]. In this era of convergence, experts from various domains will collectively unravel the molecular intricacies, creating a holistic understanding that accelerates innovation [54]. In conclusion, the interplay of molecules, driven by insights from nanotechnology and molecular biology, will continue to redefine the landscape of healthcare [55]. As research community chart a course into the future, it is imperative that researchers, clinicians, and policymakers come together to harness the potential of these key molecular players [56]. Through this collaboration, researchers can unravel the full spectrum of possibilities offered by nanomedicine, and in doing so, pave the way for a new era of precision medicine that is both molecularly informed and patient-centric [57–59].
To further enhance the molecularly tailored nanomedicine core concept, a nuanced emphasis on the dynamic role of emerging molecules in nanomedicine could be beneficial. The evolving landscape introduces not only innovative materials but also novel molecular entities, such as aptamers and advanced nanomaterials, each contributing uniquely to the expanding molecular toolbox [17]. Delving deeper into these emerging molecules and their specific applications could provide a more comprehensive understanding of the diverse avenues opening up in nanomedical designs. Additionally, future research highlighting how these molecules address current challenges or pave the way for innovative therapeutic modalities would strengthen the molecular nanomedicine emphasis on pioneering future advances. By offering a more detailed exploration of the potential applications of these emerging molecules, the conclusion of research viewpoint paper provides a richer perspective on the intricate interplay of molecules driving the ongoing revolution in nanomedicine.
ADCs: antibody-drug conjugates
AI: artificial intelligence
ML: machine learning
AVS, PB, and AKU equally contributed to: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. AP, JU, and JB: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. VT, DG, and MT: Validation, Writing—review & editing, Supervision. RM and PZ: Supervision, Project administration. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The data supporting the findings of this study are available upon request.
This study was funded by the application of multivalent biomineralized nanostructures as a modular strategy for targeting colorectal cancer [CDIRCC-2024-468] by Qatar University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
