Abstract
Aim:
There is a lack of studies that analyzed factors influencing on feasibility of coronary flow velocity reserve (CFVR) during exercise stress echocardiography (SE). The aim of the study was to define the feasibility of assessment of CFVR during exercise through SE depending on experience, techniques, and clinical factors.
Methods:
This is a single-center study. SE was performed using three generations of echo systems in five consecutive cohorts of patients by experienced and novice specialists. All patients performed a supine bicycle testing. CFVR was calculated in the middle/middle-distal parts of the left anterior descending artery (LAD). Three different adjustment settings were used for LAD visualization.
Results:
The study included 3,014 patients (59 years old ± 11 years old, 54% males). Age [odds ratio (OR) 0.98, 95% confidence interval (CI) 0.96–0.99, P < 0.01], body mass index (BMI; OR 0.95, 95% CI 0.91–0.98, P < 0.003), rest heart rate (OR 0.98, 95% CI 0.97–0.99, P < 0.0005) and doctor’s experience (OR 2.7, 95% CI 1.57–4.53, P < 0.0003) were independent factors that influence on feasibility. The feasibility of CFVR assessment during exercise SE in the whole population by experienced doctors was 89.4%. The feasibility of CFVR assessment of LAD in obese patients performed by experienced doctors using modern echo machines and new techniques was high (86.0%).
Conclusions:
Coronary artery velocity reserve during supine exercise SE is a feasible, non-invasive available tool. The new generation echo machine and the new techniques provide a good feasibility of CFVR assessment, even in novice doctors. Despite a lower level of possibility to assess CFVR in obese patients or with a higher resting heart rate, this method is feasible in a great majority of such patients.
Keywords
Coronary flow reserve, exercise stress echo, exercise coronary reserve, coronary artery ultrasoundIntroduction
Stress echocardiography (SE) is a great part of patients’ examination in chronic coronary syndromes and beyond coronary artery disease (CAD). Exercise stress tests are the only true physiological test and by far the safest. Thus, if the patient is able to exercise, then exercise stress is considered the test of choice [1, 2]. A lot of pilot and multicenter studies have proved an independent prognostic and diagnostic value of coronary flow velocity reserve (CFVR) in the left anterior descending artery (LAD) during SE [3–8]. The finding supports recent recommendations that coronary flow reserve should be measured more routinely in clinical practice [2]. Until recently it was considered that CFVR could be measured only in pharmacological tests. The recent multicenter study proved the feasibility of CFVR measuring during all types of SE [9], also matching data with the adverse outcome. However, there is a lack of studies that analyzed factors influencing this feasibility. The aim of the study was to define the feasibility of assessment of CFVR during exercise through SE depending on experience, techniques and clinical factors.
Materials and methods
Study population/analyzed groups
This is a single-center study included 3,014 patients. The inclusion criteria were that all the consecutive patients referred to SE for diagnostic/prognostic aims, as a clinical decision made by their doctors, and as well as their willingness to give their written informed consent to allow the scientific utilization of observational data, abiding respectfully to their privacy rights. All the phases of these studies were approved by the local ethical committees. There were five cohort of consecutive patients.
Group 1
Novice specialists with the “old coronary flow visualization” technique performed SE using an old generation echocardiography system (OGES). The patients were recruited from November 2011 to September 2012.
Group 2
Experienced specialists with the “old coronary flow visualization” technique performed SE in group 2 using OGES. The patients were recruited from March 2015 to December 2017.
Group 3
Experienced specialists with the “old coronary flow visualization” technique performed SE in group 3 using a new generation echocardiography system (NGES). The patients were recruited from February 2018 to December 2021.
Group 4
Experienced specialists with the “new coronary flow visualization” technique performed SE in group 4 using NGES. The patients were recruited from September 2022 to July 2023.
Group 5
A novice specialist with the “new coronary flow visualization” technique performed SE in group 5 using NGES. The patients were recruited from September 2022 to July 2023.
A specialist is considered to be a “Novice” if he/she has performed less than 500 SE tests with CFVR or having less than 1 year of practice.
A specialist is considered to be “Experienced” if he/she has performed more than 500 SE tests with CFVR whilst having more than 1 year of practice.
GE Vivid 7 Dimension ultrasound system (GE Healthcare, Liestal, Switzerland) was defined in the study as an OGES. GE Vivid 9 (GE Healthcare, USA, manufactured in Horten, Norway), Vivid E95 (GE Healthcare, USA, manufactured in Horten, Norway) were defined as a NGES.
“Old coronary flow visualization” was defined as if it used only coronary preset on ultrasound system. The “new technique” was defined as it was described in section coronary artery flow visualization. The groups 1, 2, 3 and 4 were examined by the same doctors.
SE
SE was performed according to previous and current guidelines as previously detailed [1, 10]. We used commercially available ultrasound machines:
GE Vivid 7 Dimension connected to a standard M4S transthoracic transducer.
GE Vivid E9 connected to a standard M5S transthoracic transducer.
GE Vivid E95 connected to a standard M5S transthoracic transducer.
All patients performed a supine bicycle testing with the conventional visual assessment of wall motion abnormalities using e-Bike EL&BP (General Electric, USA). The initial power output was 50 W, followed by increases of 25 W every 2 min until standard endpoints were reached. Electrocardiogram (ECG) and arterial pressure were monitored during the test. All studies were continuously recorded on digital video disc (DVD), internal and external hard-drives for off-line analysis.
Images were obtained in the apical four- and two-chamber views, and the long- and short-axis parasternal views. Echocardiographic images were acquired at rest and at the peak of exercise. Post-stress images, analogous to those at rest, were obtained as soon as possible after stopping the exercise, and not later than 60 s. The images at rest, during stress and after stopping were compared side by side in a cine-loop display. Anti-anginal drugs were usually suspended before testing.
Coronary flow visualization
CFVR was calculated in the middle/middle-distal parts of the LAD. The LAD was examined from a low left parasternal position to a modified apical two-, four-, or five-chamber positions at varying levels using views in the anterior interventricular groove. Examination of the anatomical course of the coronary arteries was performed using color Doppler mapping. The ultrasound beam was aligned parallel to the vessel flow as much as possible. There were two similar approaches for coronary flow color Doppler visualization.
Old coronary flow visualization technique
We used only the manufacturer preset “Coronary” with the embedded system settings. The velocity scale of color Doppler usually was set to 0.23–0.41 m/s at rest and the peak of exercise. Pulsed wave Doppler registered blood flow velocity patterns using a sample volume (2.0–3.0 mm) placed on the color signal (Figure 1A).
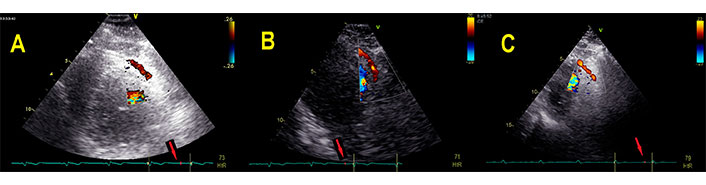
Three types of adjustment settings. A. The manufacturer preset “Coronary” with the embedded system settings. The velocity scale of color Doppler usually was set to 0.26 m/s; B. “Coronary” preset, color flow Doppler, modified two-dimensional (2D) mode to reduce the frequency, the “tissue priority” was adjusted to zero, the velocity scale of Nyquist limit was set to 0.20; C. “Cardiac” preset modification, color flow Doppler with decreased depth, Nyquist limit was adjusted to 0.23, “tissue priority” was reduced to zero. Red arrows point the time of diastolic coronary flow, near ECG P-waves. Red arrows: the time of recording coincides with a P-wave on ECG
New coronary flow visualization technique
If the standard “Coronary” preset could not provide optimal visualization, we used some special setting modifications to apply a better acquisition.
In order to visualize a longer length of LAD, we used a different scheme:
Switch “Coronary” preset.
Switch color flow Doppler.
Modify 2D mode to down frequency.
Down “tissue priority” to zero “0”.
Down velocity scale of Nyquist limit to 0.15–0.25 m/s adjusting the optimal visualization (Figure 1B).
If this scheme could not provide the optimal view. We used the standard manufactured “Cardiac” preset modification:
Switch “Cardiac” preset.
Switch color flow Doppler.
Decrease the depth.
Modify Nyquist limit to 0.2–0.23 m/s.
Down “tissue priority” to zero “0” (Figure 1C).
To identify coronary artery flow we use the profile of flow, that was diastolic predominant. The time of the maximal velocity in sinus rhythm was observed nearly P-wave on ECG (red arrows, Figure 1).
Coronary artery flow velocity reserve assessment
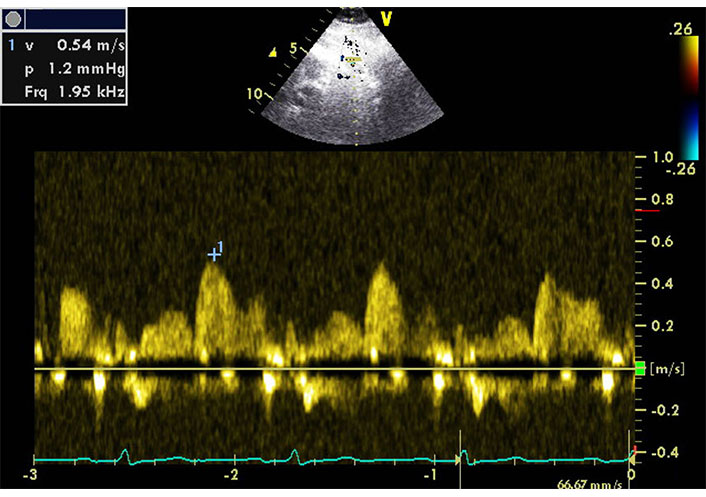
Coronary flow Doppler images were acquired before and during exercise in the same part of the artery without moving the hand or with slight adjustment till just before the peak of exercise. If it was necessary to acquire the different parameters or loops with wall motion, we nearly visualized the same section of LAD after acquiring. The diastolic peaks of coronary flow velocities were measured (Figure 2). The ratio of peak stress velocity to rest velocity was calculated as a CFVR. We consider the study to be technically successful if the Doppler signal could be registered till the peak of exercise.
Statistical analysis
Continuous data are expressed as mean ± standard deviation or median (lower-upper quartile) depending on variable distribution, categorical data is expressed in percentages. A comparison of proportions was performed with Pearson’s Chi-square (χ2) test and Fisher’s exact test, as appropriate. Continuous variables were compared by paired-samples t-test. The following categorical and continuous data were accounted for in the regression analysis: age, sex, body mass index (BMI), presence of arterial hypertension, diabetes mellitus, left bundle branch block (LBBB), previous myocardial infarction (MI), previous percutaneous coronary intervention (PCI), previous coronary bypass surgery, heart rate, blood pressure, exercise capacity, doctor’s experience, ejection fraction (EF), presence of ischemia during SE. A significance of 0.05 was required for a variable to be included in the multivariate model. Hazard ratio (HR) with the corresponding 95% confidence interval (CI) were estimated. P < 0.05 was considered statistically significant. The Statistica statistical package version 12.0 (Stat Soft Inc., Tulsa, Oklahoma, USA) and MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium) were used for statistical analysis.
Results
Patient cohorts
The main clinical characteristics, exercise, echocardiography parameters and LAD velocity data of the 3,014 study patients are described in Table 1. The patients were referred to SE with known (1,077 patients, 36%) or suspected CAD (1,937 patients, 64%).
Patients’ clinical, echocardiography, SE and coronary flow velocity characteristics
| Variables | Overall (n = 3,014) | Group 1 (n = 903) | Group 2 (n = 1,204) | Group 3 (n = 693) | Group 4 (n = 113) | Group 5 (n = 101) | P value |
|---|---|---|---|---|---|---|---|
| Age | 59 ± 11 | 56 ± 9 | 59 ± 11 | 61 ± 11 | 60 ± 11 | 58 ± 13 | < 0.0001 |
| Male sex | 1,651 (54.8%) | 582 (64.4%) | 599 (49.8%) | 357 (51.5%) | 65 (57.5%) | 48 (47.5%) | < 0.0001 |
| BSA (m2) | 1.96 ± 0.21 | 1.98 ± 0.21 | 1.96 ± 0.20 | 1.96 ± 0.22 | 1.95 ± 0.23 | 1.93 ± 0.24 | 0.103 |
| BMI (kg/m2) | 28.6 ± 4.5 | 28.9 ± 4.6 | 28.6 ± 4.6 | 28.6 ± 4.3 | 27.4 ± 3.9 | 27.8 ± 4.6 | < 0.006 |
| Hypertension, n (%) | 2,326 (77.2%) | 717 (79.4%) | 835 (69.4%) | 600 (86.6%) | 98 (86.7%) | 76 (75.2%) | < 0.0001 |
| Diabetes, n (%) | 389 (12.9%) | 127 (14.1%) | 134 (11.1%) | 105 (15.2%) | 13 (11.5%) | 10 (9.9%) | 0.090 |
| LBBB, n (%) | 130 (4.3%) | 50 (5.5%) | 40 (3.3%) | 31 (4.4%) | 7 (6.2%) | 2 (2.0%) | 0.057 |
| PCI, n (%) | 587 (19.5%) | 83 (9.2%) | 290 (24.1%) | 169 (24.4%) | 31 (27.4%) | 14 (13.9%) | < 0.0001 |
| CABG, n (%) | 173 (5.7%) | 31 (3.4%) | 65 (5.4%) | 68 (9.8%) | 8 (7.1%) | 1 (1.0%) | < 0.0001 |
| Previous MI | 838 (27.8%) | 348 (38.5%) | 282 (23.4%) | 169 (24.4%) | 23 (20.4%) | 16 (15.8%) | < 0.0001 |
| HtR (bpm) rest | 72.5 ± 12.2 | 73.2 ± 12.6 | 71.6 ± 12.0 | 73.2 ± 12.5 | 70.5 ± 11.1 | 74.0 ± 12.4 | < 0.004 |
| SBP (mmHg) | 132.4 ± 17.3 | 132.5 ± 17.2 | 134.9 ± 17.4 | 128.8 ± 17.9 | 129.8 ± 12.8 | 129.9 ± 11.8 | < 0.0001 |
| EF at rest (%) | 63.0 ± 9.0 | 62.3 ± 11.2 | 63.9 ± 7.8 | 62.8 ± 8.5 | 61.9 ± 6.8 | 65.9 ± 7.4 | < 0.0004 |
| AF during test, n (%) | 96 (3.2%) | 19 (2.1%) | 35 (2.9%) | 32 (4.6%) | 5 (4.4%) | 5 (5.0%) | < 0.05 |
| Exercise capscity, Wt | 100 (75–125) | 100 (75–150) | 100 (75–125) | 75 (75–125) | 75 (50–125) | 75 (50–125) | < 0.0001 |
| Ischemia, n (%) | 1,546 (51.3%) | 473 (52.4%) | 646 (53.7%) | 312 (45.0%) | 55 (48.7%) | 60 (59.4%) | < 0.003 |
| HtR (bpm) peak | 125.8 ± 20.8 | 129.6 ± 20.3 | 124.7 ± 21.4 | 122.8 ± 20.3 | 121.9 ± 18.4 | 130.4 ± 19.2 | < 0.0001 |
| LAD velocity at rest, cm/s | 32.5 ± 13.0 | 34.7 ± 13.1 | 33.1 ± 13.3 | 29.7 ± 12.1 | 28.4 ± 9.4 | 31.7 ± 15.2 | < 0.0001 |
| LAD velocity at peak, cm/s | 61.3 ± 21.1 | 64.7 ± 25.3 | 62.6 ± 19.9 | 57.0 ± 19.2 | 54.7 ± 19.8 | 59.5 ± 11.8 | < 0.0001 |
| CFVR | 1.99 ± 0.67 | 1.94 ± 0.71 | 2.01 ± 0.68 | 2.00 ± 0.64 | 2.00 ± 0.51 | 2.09 ± 0.47 | 0.198 |
BSA: body surface area; CABG: coronary artery bypass surgery; HtR: heart rate; SBP: systolic blood pressure; AF: atrial fibrillation; Wt: watt
The achieved double product of heart rate and SBP were divided into quartiles. The CVFR increased significantly depending on the quartiles (1.51 ± 0.49 vs. 1.91 ± 0.58 vs. 2.26 ± 0.55 vs. 2.45 ± 0.50, P < 0.0001, respectively).
The prevalence of ischemia tests and CFVR obtained by novice vs. experienced operators were not significantly different: 53% vs. 51%, P = 0.17; 1.96 ± 0.68 vs. 2.00 ± 0.66, P = 0.12, respectively.
The time spent on additional scanning of coronary artery flow before SE was approximately 60–90 s.
Feasibility
The feasibility of CFVR assessment during exercise stress echo of the overall study population was 81.3%. Some examples of CFVR are in the Figure 3.
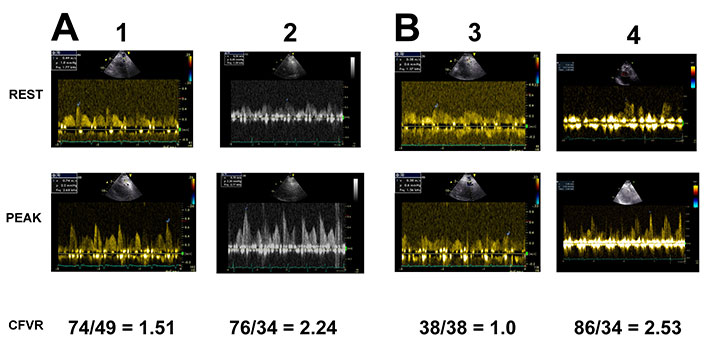
Examples of different CFVR. Matched pairs of patients with a low exercise capacity (A) and a medium exercise capacity (B) on the similar heart rate. Depressed (patients 1, 3) and normal (patients 2, 4) CFVR during exercise
Among all the above-mentioned parameters, age, BMI, resting heart rate and doctor’s experience were independent factors that influence on feasibility, Table 2.
Significant independent parameters influencing on feasibility of CFVR assessment
| Variables | Odds ratio | Multivariate analysis HR (95% CI) | P value |
|---|---|---|---|
| Age | 0.9795 | 0.9643–0.9950 | 0.0096 |
| BMI | 0.9470 | 0.9137–0.9816 | 0.0029 |
| Rest HtR | 0.9779 | 0.9657–0.9902 | 0.0005 |
| Doctor experience | 2.6697 | 1.5735–4.5298 | 0.0003 |
HtR: heart rate
The differences between the groups feasibilities presented in Table 3. It was 65% vs. 89% (P < 0.0001) for doctors who performed less than 500 SE tests and experience of less than 1 year, in comparison with doctors who performed more than 500 SE and a practice more than 1 year. The feasibility of CFVR LAD assessment in the whole population by experienced doctors was 89.4%.
The differences between the group feasibilities of CFVR assessment
| Variables | Group 1 (n = 903) | Group 2 (n = 1,204) | Group 3 (n = 693) | Group 4 (n = 113) | Group 5 (n = 101) | P value |
|---|---|---|---|---|---|---|
| Feasibility | 63.5% | 89.6% | 88.7% | 91.2% | 76.2% | < 0.0001 |
| Feasibility in obesity vs. not obese patients | 61.9% vs. 65.0% (P = 0.36) | 86.5% vs. 91.3% (P < 0.01) | 86.2% vs. 90.2% (P = 0.11) | 84.4% vs. 93.0% (P = 0.11) | 65.5% vs. 80.6% (P = 0.11) | - |
Group 1 vs. group 2, P < 0.0001; group 2 vs. group 3, P = 0.55; group 3 vs. group 4, P = 0.44; group 4 vs. group 5, P < 0.003; group 1 vs. group 5, P < 0.02. -: blank cell
The feasibility of CFVR assessment of LAD in obese patients performed by experienced doctors was 86.0% using NGES.
Discussion
This study has shown that the assessment of CFVR during supine bicycle exercise tests is a highly feasible method. Indeed, most SE can be added by measuring of coronary flow velocity even in cases when the doctor is a newcomer. If the doctor has experience in conducting more than 500 tests with coronary flow assessment and has more than 1 year of practice, the feasibility of this method is near 90% even with the old generation echo systems. The new techniques and new generation echo systems made the visualization of coronary artery simpler and more accessible. The use of multifrequency matrix single crystal phased array probe transducers with second tissue harmonics provide an estimation of both distal and proximal-mid LAD parts. The new manufacturer presets help to ease the coronary artery visualization. It plays a crucial role in training doctors. In the study there is a significant difference between feasibility for novice doctors with using the new techniques.
The aim of CFVR assessment was proved in a large number of studies including pilot and multicenter ones [3–9]. It was found that, across a broad range of pathologies and patient cohorts, an impaired CFR was associated with an increased hazard of all-cause mortality and major adverse cardiovascular events [11].
First, it was demonstrated for pharmacological tests. Indeed, the CFVR is independent, one of the major diagnostic and prognostic parameters during stress tests. However, the exercise stress tests are considered more physiological than pharmacological stress tests and include the prognostically important finding of the patient’s exercise capacity. According to current guidelines, if a patient can exercise, this should be the preferred stress modality [1, 2]. The Feasibility of CFVR assessment during exercise was demonstrated, first, in a pilot study [12, 13] in 2016, then in a multicenter study, proving its diagnostic and prognostic values [8, 9]. Currently, the CFVR is an essential valuable independent parameter, evaluated during all types of SE. Now it is an integral part of the modern ABCDE protocol [2, 14]. We did not find papers in literature on the direct comparison of CFVR values obtained through different exercise and pharmacological stressors in the same patients. Previously single and multicenter studies had shown that CFVR in LAD can be obtained during all forms of SE protocols, but the feasibility is significantly higher with rapid cardiac pacing and pharmacological tests as compared with exercises [9, 15]. However, exercise CFVR assessment was achievable for the majority of patients. Besides, it is possible to repeat the test through other attempts of CFVR evaluation, knowing its safe useful physiologic nature.
The main factors that influence on feasibility were age, BMI, resting heart rate and doctor’s experience. Previously the sceptics doubted in possibility of CFVR assessment in obese patients generally. Takeuchi and co-authors [16] had demonstrated that the noninvasive measurement of CFVR during SE was feasible (92%) even in a relatively obese population with high and low frequencies of obesity. Their study had shown to measure CFVR for obese patient during pharmacologic SE. Our study also confirmed a possibility of this assessment during exercise, although with a lower feasibility. The new generation of echo machines help to achieve a good coronary artery visualization during exercise tests in obese patients. Despite the influences of BMI on coronary artery visualization, it is possible in a great majority of patients that the feasibility of CFVR assessment is approximately 86% for experienced doctors. The resting heart rate is also a known factor that influences the coronary artery flow assessment [17]. It is connected with a shorter diastole, when the main coronary flow is better visualized. However, this method is feasible in a great majority of patients with more higher heart rate also.
Understanding the difficulties of coronary artery visualization in patients during exercise, we consider that 500 exams conducted by an echocardiographer to be the minimal level of experience. Earlier, a one year-experience was considered adequate. However, the new generation echo systems with developed methods of coronary artery visualization provide a good feasibility of CFVR assessment in novice specialists even during the first 100 exams.
Limitations
This is a single center study with cohorts of different periods. The study population consisted of five consecutive patient cohorts, there was a high prevalence of ischemic tests in these cohorts.
Groups 4 and 5 had a smaller number of patients that could influence to P value between subgroups of patients with and without obesity. There was not the whole information about smoking status to analyze its influence on feasibility and optimal techniques for coronary flow assessment. We had no coronary angiography data about LAD stenoses for the study cohorts. We did not compare the difference of the visualization methods between each other.
In conclusion, coronary artery velocity reserve during supine exercise SE is a feasible, non-invasive and readily available tool. The new generation echo machine and the new techniques provide a good feasibility of CFVR assessment in novice doctors. Despite a lower level of possibility to assess CFVR in obese patients or in a higher resting heart rate, this method is feasible in a great majority of such patients. It suits for every day practice.
Abbreviations
| BMI: |
body mass index |
| CFVR: |
coronary flow velocity reserve |
| CI: |
confidence interval |
| ECG: |
electrocardiogram |
| LAD: |
left anterior descending artery |
| NGES: |
new generation echocardiography system |
| OGES: |
old generation echocardiography system |
| OR: |
odds ratio |
| SBP: |
systolic blood pressure |
| SE: |
stress echocardiography |
Declarations
Author contributions
AZ and OH: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervision. EP and IB: Validation, Writing—review & editing. EK: Investigation, Writing—original draft, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All the phases of these studies were approved by the local ethical committee of Research Cardiology Center “Medika”.
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Not applicable.
Availability of data and materials
The data supporting the results of the article can be obtained by directly contacting the corresponding author (zag_angel@yahoo.com).
Funding
Not applicable.
Copyright
© The Author(s) 2024.