Affiliation:
1Department of Cardiology, Hipolito Unanue National Hospital, Lima 15007, Peru
ORCID: https://orcid.org/0000-0001-5072-2632
Affiliation:
2Department of Nuclear Cardiology, National Institute of Cardiology Ignacio Chavez, Mexico City 14080, Mexico
ORCID: https://orcid.org/0000-0002-6888-9374
Affiliation:
1Department of Cardiology, Hipolito Unanue National Hospital, Lima 15007, Peru
ORCID: https://orcid.org/0000-0001-7626-0173
Affiliation:
2Department of Nuclear Cardiology, National Institute of Cardiology Ignacio Chavez, Mexico City 14080, Mexico
ORCID: https://orcid.org/0009-0006-6541-0504
Affiliation:
2Department of Nuclear Cardiology, National Institute of Cardiology Ignacio Chavez, Mexico City 14080, Mexico
ORCID: https://orcid.org/0009-0006-2702-9542
Affiliation:
2Department of Nuclear Cardiology, National Institute of Cardiology Ignacio Chavez, Mexico City 14080, Mexico
ORCID: https://orcid.org/0009-0008-2821-9692
Affiliation:
2Department of Nuclear Cardiology, National Institute of Cardiology Ignacio Chavez, Mexico City 14080, Mexico
ORCID: https://orcid.org/0000-0003-0677-9935
Affiliation:
1Department of Cardiology, Hipolito Unanue National Hospital, Lima 15007, Peru
ORCID: https://orcid.org/0000-0003-4379-3058
Affiliation:
2Department of Nuclear Cardiology, National Institute of Cardiology Ignacio Chavez, Mexico City 14080, Mexico
Email: niesza2001@hotmail.com
ORCID: https://orcid.org/0000-0002-4820-1325
Explor Cardiol. 2024;2:196–203 DOI: https://doi.org/10.37349/ec.2024.00033
Received: March 23, 2024 Accepted: August 05, 2024 Published: September 22, 2024
Academic Editor: Andrea Borghini, Institute of Clinical Physiology - National Research Council (IFC-CNR), Italy
Chagas disease is a systemic illness characterized by acute and chronic phases. If untreated, it can lead to dysfunction of vital organs, notably the heart, ultimately resulting in heart failure. Transmission primarily occurs through the feces of triatomine insects carrying the protozoan parasite Trypanosoma cruzi, either via a bite wound or intact mucous membranes. Diagnosis of Chagas disease involves serological tests, electrocardiographic findings, and imaging studies. A 58-year-old male patient from Peru with chronic dilated cardiomyopathy underwent evaluation at a tertiary care hospital. Given the uncertain etiology, a comprehensive diagnostic approach was adopted, emphasizing the pivotal role of cardiovascular magnetic resonance imaging and computed tomography angiography in managing chronic cardiomyopathy of Chagas disease. Leveraging these imaging modalities together could augment our ability to evaluate myocardial inflammation and tailor therapeutic strategies accordingly.
Chagas disease (CD) is caused by the protozoan Trypanosoma cruzi and is transmitted by triatomine insects in endemic areas such as Mexico, Central America, and South America. In 2023, Peru was considered a high-prevalence country, with 19 confirmed cases of CD reported [1, 2]. The infection leads to an acute phase lasting 4–8 weeks, with 20–30% of cases progressing to chronic CD, characterized by cardiomyopathy and megaviscera, ultimately leading to heart failure (HF) [3].
Recently, cardiovascular magnetic resonance (CMR) has gained significance as a noninvasive tool for identifying myocardial inflammatory activity (edema and myocardial hyperemia) at an early stage. These changes are identifiable before irreversible lesions such as necrosis and fibrosis develop. However, other tools have also emerged, such as computed tomography (CT), for the early recognition of tissue characterization. The combined use of these modalities can further enhance our ability to assess myocardial inflammation and guide therapeutic interventions [3].
A 58-year-old male from San Martín, Peru, with a history of valvular disease diagnosed in 2020 and HF (NYHA functional class II), sought medical care after two years of persistent and worsening respiratory symptoms. He has worked in the agriculture field over the past decades. A transthoracic echocardiogram revealed severe symptomatic mitral valve insufficiency. He was evaluated at a tertiary care hospital due to exacerbated dyspnea (NYHA functional class IV), orthopnea, prominent edema, chest pain, and syncope. Cardiovascular examination revealed jugular distension, hepatojugular reflux, and a holosystolic murmur at the mitral and tricuspid foci.
As part of the medical evaluation, an electrocardiogram revealed sinus rhythm with left anterior fascicular block and low QRS voltage (Figure 1); a chest X-ray showed moderate to severe cardiomegaly. Subsequently, transthoracic echocardiography revealed a significant reduction in left ventricular systolic function with a reduced left ventricular ejection fraction (LVEF 24%), global hypokinesia associated with a suggestive image of an interatrial aneurysm, four-chamber dilation, and severe mitral and tricuspid insufficiency (Figure 2). Mortality risk stratification was performed using the Rassi score, which yielded a high-risk score of 17/20, considering the NYHA functional class IV, cardiomegaly, global wall-motion abnormality, male sex, and low QRS voltage [4].
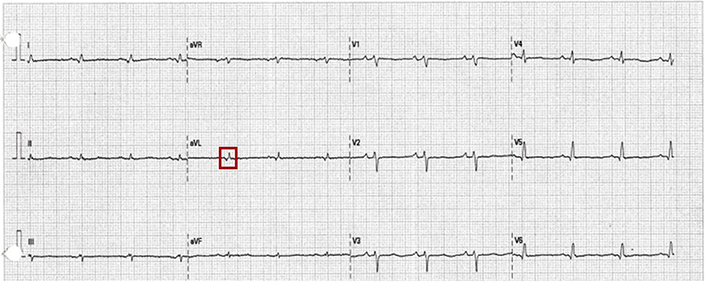
12-lead electrocardiogram (ECG). The ECG shows sinus rhythm, frontal plane axis left deviation of 60°, in lead avL qR pattern and R-peak time of 60 ms is observed (red square), QRS duration of 80 ms, and generalized low voltage
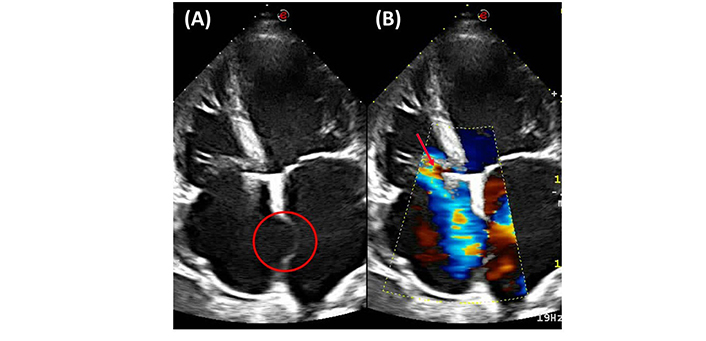
Four-chamber view transthoracic echocardiography. (A) Global hypokinesia associated with a suggestive image of an interatrial aneurysm (red circle). (B) Color-Doppler echocardiography evidencing four-chamber dilation with severe tricuspid valve insufficiency (red arrow)
Due to suspicion of chronic ischemia, patient age, and findings of mitral insufficiency on echocardiography, a coronary computed tomography angiography (CTA) was conducted, along with high-sensitivity cardiac troponin T test (hs-cTnT) and C-reactive protein (CRP) levels. The imaging test did not reveal abnormalities, and the hs-cTnT level upon admission was 6 ng/L, with no significant changes during hospitalization, ruling out the probability of obstructive subepicardial coronary artery disease (Figure 3). Additionally, the CRP levels were within the normal range at 0.55 mg/dL. Iodine contrast enhancement confirmed inferolateral and apical aneurysmatic images, revealing transmural enhancement patterns in aneurysmatic segments (Figure 4).
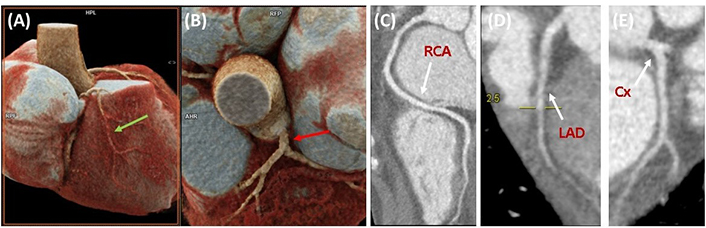
Coronary computed tomography angiography. (A and B) 3D cardiac reconstruction of the RCA (green arrow) and LCA (red arrow). (C–E) Coronary computed tomography angiography showing adequate blood flow, absence of luminal abnormalities, ruling out coronary disease. RCA: right coronary artery; LCA: left coronary artery; LAD: left anterior descending artery; Cx: circumflex artery
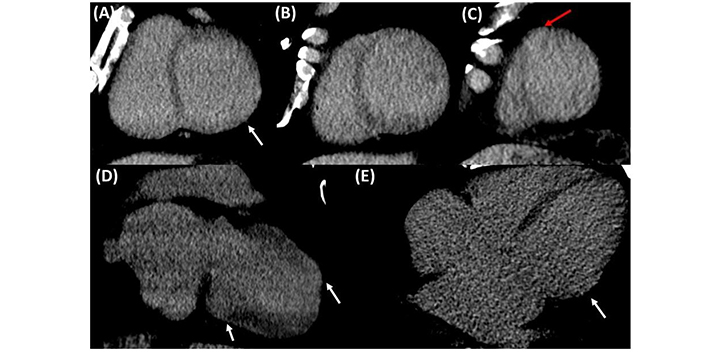
Computed tomography showing delayed enhancement with iodine, protocol was implemented with low 80 kV and maximum milliampere at 700 to enhance the iodine. Images were acquired at 7 min post-contrast. (A–C) Short axis view showing inferolateral (white arrows) and apical (red arrows) aneurysmatic segment with transmural enhancement. (D and E) Two-chamber view showing transmural enhancement at the level of the aneurysmatic segments (white arrows)
Systematically, the most common causes of non-ischemic dilated cardiomyopathy were excluded, including toxic, endocrinological, nutritional, and autoimmune factors. Notably, given the endemic nature of CD vectors in Peru, suspicion was raised, prompting the performance of an indirect chemiluminescent immunoassay to detect specific IgG antibodies against excretory-secretory antigens of T. cruzi, as well as an enzyme-linked immunosorbent assay for IgG, both of which returned positive results.
CMR was performed to quantify myocardial fibrosis and assess disease severity. T1 mapping revealed prolonged global relaxation times (1,100 ms), indicating diffuse myocardial fibrosis (Figure 5). The examination also revealed a 32% extracellular volume (ECV) fraction in post-contrast-enhanced T1 mapping, suggesting edema (Figure 6). Furthermore, contrast-enhanced gadolinium images confirmed a transmural pattern of late gadolinium enhancement (LGE), revealing 16 grams of fibrosis mass in aneurysmal segments (Figure 7).
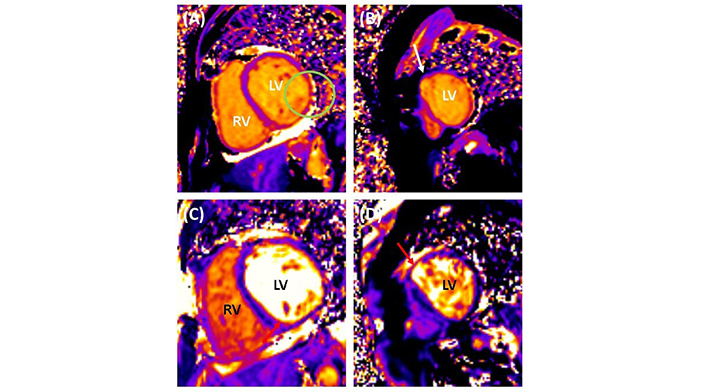
Short axis view CMR. (A) T1 mapping CMR evidencing shortened global relaxation time (1,100 s) showed by areas with increased signal (green circle), indicative of diffuse myocardial fibrosis. (B) T1 mapping CMR evidencing prolonged global relaxation time (1,091 s) and presence of aneurysmatic segment (white arrow). (C and D) T2 mapping CMR evidencing increase signal areas (red arrows) with increased T2 values corresponding to late gadolinium enhancement demonstrating presence of edema. CMR: cardiovascular magnetic resonance
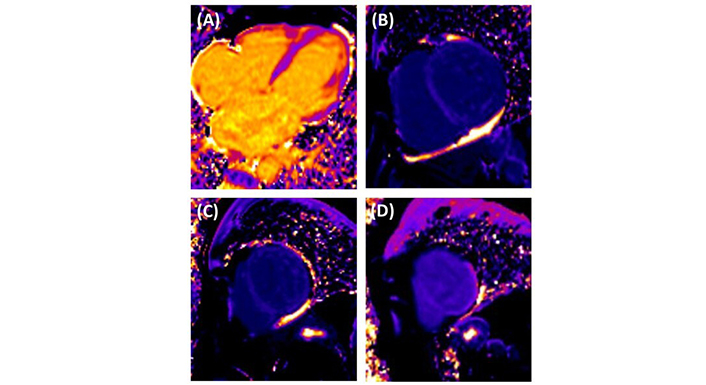
Post-contrast enhanced short T1 mapping cardiovascular magnetic resonance. (A) Four-chamber view providing a comprehensive assessment of the heart chambers. (B–D) Basal third, middle third and apical third of the LV indicating fibrotic changes according to ECV (32%). LV: left ventricle; ECV: extracellular volume
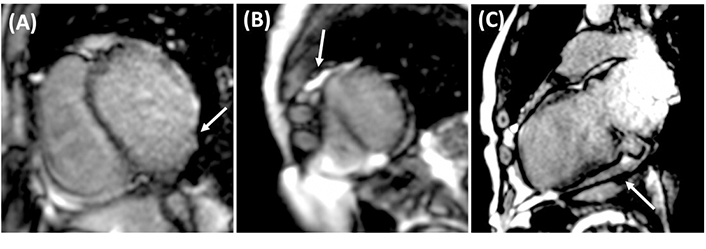
CMR with gadolinium. (A) Short axis view CMR with aneurysmal segments in the LV free wall, evidenced with LGE (white arrow). (B) Short axis view CMR evidencing LGE transmural pattern with a fibrosis mass (white arrow) of 16 grams in the apical aneurysmatic segments located in the LV. (C) Vertical long axis view CMR evidencing basal aneurysmatic segment with presence of fibrosis (white arrow). CMR: cardiovascular magnetic resonance; LV: left ventricle; LGE: late gadolinium enhancement
A 24-hour Holter study revealed sinus rhythm with a mean heart rate of 65 bpm. Additionally, it showed isolated supraventricular extrasystoles, including bigeminy and trigeminy patterns, without supraventricular tachycardia. Due to the high mortality risk associated with chronic cardiomyopathy of Chagas disease (CCCD), an implantable cardioverter-defibrillator (ICD) was recommended for the prevention of sudden cardiac death (SCD). The patient was discharged without complications following the placement of the ICD.
The diagnosis of CD involves conventional and non-conventional serological tests. According to the World Health Organization, confirmation requires two positive results. Common electrocardiographic abnormalities include complete right bundle branch block and left anterior fascicular block [3, 5].
Echocardiography plays a crucial role in assessing CCCD, enabling the prediction of mortality from early changes to advanced stages with left ventricular enlargement and impaired systolic function. CCCD patients presenting with atypical pain may undergo CTA to rule out obstructive subepicardial coronary artery disease. Additionally, CT can be used for the early characterization of myocardial fibrosis through late enhancement assessment and evaluate biventricular systolic function, complementing echocardiography [6].
Holter monitoring aids in detecting cardiac arrhythmias for routine CCCD assessment, facilitating the diagnosis of conditions such as sinus node dysfunction and supraventricular tachyarrhythmias [3].
Long-term mortality risk stratification using the Rassi score aids in directing therapy based on individual survival probabilities determined by the existence of six clinical features: NYHA class III or IV, cardiomegaly on chest radiography, segmental or global wall-motion abnormalities on echocardiography, non-sustained ventricular tachycardia on Holter monitoring, low QRS voltage on electrocardiography, and male sex. Patients at high risk of mortality may benefit the most from aggressive therapeutic interventions, including ICD, heart transplantation, and cardiac resynchronization [4].
Comprehensive evaluation of CCCD also involves CMR, which correlates myocardial fibrosis with disease severity, ventricular arrhythmias, and cardiovascular events. CMR assesses biventricular systolic function, T2 mapping for edema, and LGE for regional fibrosis detection. Myocardial T1 mapping measures ECV for interstitial fibrosis assessment, and global T1-weighted enhancement aids in detecting hyperemia and inflammation. ECV fraction has a prognostic role in early-stage cardiomyopathy. The late enhancement technique in CMR serves as a crucial predictor for severe cardiovascular events, independently forecasting total death, cardiovascular death, and sustained ventricular tachyarrhythmias. The European Association of Cardiovascular Imaging and the SBC Cardiovascular Imaging Department recommend CMR for quantifying myocardial fibrosis extension, assessing SCD risk, and potentially influencing implantable ICD indications [3, 7].
With a confirmed diagnosis of non-ischemic HF, a LVEF of 24%, and NYHA class IV, the individual met class I Level B evidence criteria for an ICD according to the 2012 American Heart Association guidelines. The ICD effectively addresses potential ventricular arrhythmias, preventing SCD, and offers a promising approach to enhancing overall survival [8, 9].
The clinical case presented demonstrates how patients can benefit from a multimodality-based strategy in CCCD, aiding in the selection of the most appropriate therapeutic management based on specific findings (Table 1). While iodine late enhancement is an innovative technique that can assist in guiding management, it does not replace gadolinium late enhancement for the determination of fibrosis and disease severity.
Role of cardiac imaging for the diagnosis and prognosis of CCCD [3]
| Cardiac study | Initial indications | Repeated testing | Typical features |
|---|---|---|---|
| 12-lead ECG | Initial diagnostic and prognostic assessment of every individual with positive serology for CD, enabling the clinical classification. | Perform an ECG annually if there are multiple specific changes or every two years if the ECG is normal or has nonspecific (isolated) changes. | Most frequent and defined alterations are atrioventricular conduction blocks, right bundle branch block, left anterior fascicular block, ventricular repolarization alterations, and ventricular ectopic beats. |
| Association of 2 or more abnormalities in the same electrocardiographic tracing, characterizes severe cardiomyopathy. | |||
| Chest X-ray | Initial diagnostic and prognostic assessment of every individual with CCCD. To diagnose cardiovascular impairment. | Perform when there is clinical evidence of pulmonary or systemic congestion. | An enlarged cardiac area with minimally congested pulmonary fields is often present. In addition, right ventricle enlargement signs are common on posteroanterior and lateral views. Signs of right pleural effusion secondary to systemic congestion can also be evidenced. |
| Echocardiography | Initial assessment and follow-up of patients with CD. Assessment of every individual with CCCD. | Reassess periodically: every 3 to 5 years for preserved ejection fraction cases and no previous segmental contractility changes.Reassess every 1 to 2 years for cases with global or segmental left ventricular dysfunction. | Left ventricular enlargement, segmental and/or diffuse hypokinesia, ventricular aneurysms , and left ventricle dysfunction. |
| Suspicion of CCCD arises from history, clinical examination, or electrocardiographic changes. | Alteration in myocardial relaxation is the first change visible, as it progresses, it can worsen to a typical restrictive pattern. | ||
| CMR | When there is suspicion of concurrent CCCD and coronary artery disease or other non-ischemic cardiomyopathy, CMR is utilized to assess the etiology and extent of myocardial fibrosis. | Not established. | Allows for morphological and functional assessment, globally and segmentally, as well as the detection of intracardial thrombi, overcoming technical limitations of echocardiography. |
| In non-endemic areas where initial clinical suspicion of CD is absent, CMR can strongly indicate probable CCCD diagnosis based on LGE patterns, prompting further serological tests for etiological diagnosis confirmation. | Identification of myocardial inflammatory activity (edema and myocardial hyperemia) at an early stage before the development of irreversible lesions, such as necrosis and fibrosis. They can aid in risk stratification and therapeutic decision-making. | ||
| T2-weighted images and T2 mapping should be included for assessment of myocardial edema in addition of LGE to detect gross regional myocardial fibrosis. T1 mapping should be included to calculate the myocardial extracellular volume (interstitial and diffuse fibrosis measure). | |||
| Computed tomography angiography | Non-invasive evaluation of coronary anatomy in patients with CCCD and a high likelihood of obstructive subepicardial coronary artery disease. | Not established. | Characterization of normal myocardial tissue/fibrosis using late enhancement as an alternative to CMR. |
| Assessment of biventricular systolic function, along with echocardiography, for patients with contraindications to CMR. |
CCCD: chronic cardiomyopathy of Chagas disease; ECG: electrocardiogram; CD: Chagas disease; CMR: cardiovascular magnetic resonance; LGE: late gadolinium enhancement
CCCD: chronic cardiomyopathy of Chagas disease
CD: Chagas disease
CMR: cardiovascular magnetic resonance
ECV: extracellular volume
HF: heart failure
ICD: implantable cardioverter-defibrillator
SCD: sudden cardiac death
LVY: Project administration, Resources. JJHR: Writing—original draft, Writing—review & editing, Investigation, Visualization, Supervision. MGC, PMD, ECG, and JAF: Resources. RVC: Investigation, Writing—review & editing, Validation. SLA: Resources, Writing—review & editing. NEZ: Resources, Writing—review & editing, Project administration. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The research described in this case report has been conducted in accordance with the ethical guidelines established by the World Medical Association’s Declaration of Helsinki for research involving human subjects. Given the nature of this research—specifically, the retrospective analysis of anonymized clinical data—the Ethics Committee of the National Institute of Cardiology Ignacio Chavez has determined that specific ethical approval is not required.
Informed consent to participate in the study was obtained from the participant.
Informed consent to publication was obtained from relevant participant.
The data is not publicly available due to ethical restrictions and legal constraints. But the data analyzed during the current case report is available from the corresponding author on reasonable request.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
