Affiliation:
1Division of Public Health, Infectious Diseases and Occupational Health, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
2Department of Cardiovascular Medicine, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
3Department of Internal Medicine, University of Miami Miller School of Medicine/Jackson Memorial Hospital, Miami, FL 33136, USA
Email: quinteromartinez.juan@mayo.edu
ORCID: https://orcid.org/0000-0002-0785-9868
Affiliation:
1Division of Public Health, Infectious Diseases and Occupational Health, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
Affiliation:
2Department of Cardiovascular Medicine, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
Affiliation:
4Division of Clinical Trials and Biostatistics, Department of Quantitative Health Sciences, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
Affiliation:
6Department of Oral and Maxillofacial Medicine, School of Clinical Dentistry, The University of Sheffield Faculty of Medicine, Dentistry and Health, S10 2TA Sheffield, UK
ORCID: https://orcid.org/0000-0003-0681-4083
Affiliation:
1Division of Public Health, Infectious Diseases and Occupational Health, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
Affiliation:
2Department of Cardiovascular Medicine, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
Affiliation:
2Department of Cardiovascular Medicine, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
Affiliation:
1Division of Public Health, Infectious Diseases and Occupational Health, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
2Department of Cardiovascular Medicine, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
Affiliation:
1Division of Public Health, Infectious Diseases and Occupational Health, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
2Department of Cardiovascular Medicine, Mayo Clinic College of Medicine and Science, Rochester, MN 55905, USA
ORCID: https://orcid.org/0000-0002-4473-7077
Explor Cardiol. 2024;2:204–216 DOI: https://doi.org/10.37349/ec.2024.00034
Received: March 22, 2024 Accepted: July 26, 2024 Published: September 24, 2024
Academic Editor: Andrea Borghini, Institute of Clinical Physiology-National Research Council (IFC-CNR), Italy
Aim: The association of echocardiographic findings and subsequent risk of left-sided native valve endocarditis (LS-NVE) is undefined. The aim of this study was to determine if transthoracic echocardiography (TTE) measurements are associated with the subsequent development of LS-NVE in patients without cardiac predisposing conditions.
Methods: Institutional databases were evaluated for adults diagnosed with LS-NVE from 2008 to 2020. Patients with prosthetic valves, cardiovascular implantable electronic devices, intracardiac devices, injection drug use, and predisposing cardiac conditions were excluded. Only patients who had a TTE performed 6 months to 3 years before the development of LS-NVE were included as cases. Controls were patients within the same Mayo location with a TTE report and were matched in a 1:3 ratio according to age, gender, Charlson comorbidity index, and echocardiography date.
Results: There were 148 cases and 431 matched controls. As compared to controls, infective endocarditis (IE) cases had a higher prevalence of diabetes mellitus (46.6% vs. 30.4%) and chronic kidney disease (46.6% vs. 28.1%) (P < 0.001). Left ventricular outflow tract velocity (P = 0.017), left ventricular ejection fraction (P = 0.018), and E:e’ ratio (P = 0.050) were associated with LS-NVE.
Conclusions: Echocardiographic measurements were associated with subsequent LS-NVE development in this pilot study. A larger cohort of LS-NVE patients, however, is needed to validate these findings.
Infective endocarditis (IE) is a potentially life-threatening syndrome with an in-hospital mortality rate of approximately 20% despite optimal medical and surgical management [1–3]. Echocardiography is an essential tool in the diagnosis of IE, but also in the identification of heart conditions that predispose to its development [4–7]. The designation of a predisposing heart condition is nonspecific for left-sided native valve endocarditis (LS-NVE) and may include a myriad of congenital and acquired conditions such as congenital abnormalities, rheumatic heart disease, hypertrophic cardiomyopathy, bicuspid aortic valve, or mitral valve prolapse [8–13].
Considering the: (i) nonspecific classification of predisposing cardiac conditions associated with the development of LS-NVE; (ii) fact that 40% of patients with NVE do not have a structural abnormality or valve damage prior to the development of IE [14]; and (iii) evolution of technical advances in echocardiography over recent years, it seems appropriate to evaluate echocardiography as a potential tool in assessing the likelihood of the subsequent development of LS-NVE. Thus, the primary objective of the current investigation was to determine whether transthoracic echocardiography (TTE) measurements were associated with the subsequent development of LS-NVE in patients with no predisposing cardiac conditions.
A case-control study design was conducted. Cases and controls were adult (≥ 18 years) patients from Mayo Clinic sites (Rochester, Florida, Arizona, and the Health Systems) from 2008 to 2020. To determine the cases, institutional databases of patients with an initial diagnosis of LS-NVE were queried. Medical charts were reviewed, and IE cases were identified according to the modified Duke criteria [10], and categorized into either possible or definite LS-NVE. Only patients who had a TTE performed 6 months to 3 years before the development of IE were considered for study inclusion, and every individual was only included once. Controls were selected from a large retrospective cohort of adult patients with at least one TTE report over a similar period to that of the cases. Patients with prosthetic valves, cardiovascular implantable electronic devices (CIEDs), intracardiac devices, previous IE, injection drug use, and predisposing heart conditions (congenital abnormalities, rheumatic heart disease, hypertrophic cardiomyopathy, bicuspid aortic valve, and mitral valve prolapse) were excluded among cases and controls.
Case and controls were matched using age, sex, Charlson comorbidity index (CCI), and date of echocardiogram. Our matching algorithm attempted to sample all LS-NVE cases and 3 times as many controls as cases, matching them exactly on sex and CCI category, and closely on age (±3 years) and timing of echocardiography (±1 year).
Details characterizing the degree of valve abnormality and other echocardiographic measurements were then collected for both matched cases and controls. Clinical characteristics, medical history, procedures, and comorbid conditions (at the time of the patients’ echocardiography) were also collected from the electronic health record. The CCI was calculated as described previously [15]. This study was approved by the Mayo Clinic Institutional Review Board and included only patients with institutional research authorization.
A registered cardiac sonographer conducted the echocardiographic examination following the American Society of Echocardiography guidelines [16–18]. Data were extracted from the echocardiography reports for all patients. Mid-segmental linear measurements of the left ventricular (LV) internal diastolic and systolic diameters (LVIDD and LVISD), LV posterior wall diastolic thickness (LVPWDT), and interventricular septum diastolic thickness (IVSDT) were performed in a parasternal long-axis view. LV ejection fraction (EF) (LVEF) was calculated using the modified Quinones equation or biplane method of disks (Simpson). For the hemodynamic measurements, the velocity-time integral (VTI) and peak velocity of the left ventricular outflow tract (LVOT) were measured using pulsed wave Doppler in an apical long-axis or five-chamber view. LVOT diameter was measured using a parasternal long-axis view during systole. The LVOT cross-sectional area and VTI were multiplied to obtain the stroke volume [19]. Cardiac output was derived from (stroke volume × heart rate) and then indexed according to the patient’s body surface area. Transmitral flow was acquired using pulsed-wave Doppler for E-wave and tissue Doppler for E-wave. Both measurements were obtained from the septal side of the mitral annulus during the same heartbeat using a four-chamber view. The E-wave to e’ ratio was used to determine LV filling pressures. Native valvular regurgitation for the aortic and mitral valves was classified according to the American Society of Echocardiography guidelines into normal, trivial, mild, moderate, and severe [20].
To test for unadjusted associations between the case-control group and each of the clinical and echocardiographic variables, we used unmatched methods (e.g., Wilcoxon rank-sum tests, Pearson χ² tests) to avoid deletion of matched sets with partially missing data. However, the comparisons were also repeated on the matched sets using matched methods (univariable conditional logistic regression models) and checked for agreement. The results of these bivariate analyses were not used in selecting variables to include in the prognostic model of LS-NVE.
For echocardiographic data, measurements on 46 variables were collected. Of them, 20 were missing in > 15% of patients and were therefore removed from further evaluation. Due to the paucity of severity categories, the ordinal variables for mitral and aortic stenosis were removed. In contrast, those for mitral and aortic regurgitation were retained but were dichotomized as presence (mild, moderate, or severe) or absence (none or trivial) to preserve degrees of freedom (df). Next, the remaining 24 echocardiographic variables were reduced to the 12 most clinically relevant ones. Lastly, we examined data redundancy among the remaining variables and removed two more variables: LV mass index (due to overlapping information with the other variables) and LV end-diastolic dimension (LVEDD) [due to collinearity with LV end-systolic dimension (LVESD)]. Hence, the final model was fitted with 10 echocardiographic variables (13 df), including 5 anatomical-related measures—IVSDT, LVESD, LVPWDT, and mitral and aortic regurgitation, and 5 hemodynamic-related measures—LVEF, LVOT velocity, LV cardiac index, E:e’ ratio, and aortic valve systolic peak (AVSP) velocity. Because significantly higher rates of chronic kidney disease and diabetes mellitus were found in the LS-NVE cases versus controls (despite matching on CCI), a post hoc analysis was conducted by repeating the original logistic regression with the inclusion of these 2 additional covariates.
Continuous variables were modeled with 3-knot restricted cubic splines to assess the default assumption of nonlinearity. For those variables with little to no evidence of a nonlinear effect, the nonlinear terms were removed, and the variables were refitted as linear in the final model. To preserve the total sample size in the modeling despite missing data (the rate of missingness among echocardiographic variables ranged from 0% to 13.6%), multiple imputations were employed with 25 imputation sets based on predictive mean matching. Model estimates were depicted with partial effects plots to show how the predicted probabilities of IE changed over the range of observed values for each continuous variable. The relative contribution of variables to the model tested individually or as a group, was quantified with adjusted χ² statistics, whereby subtracting the df from the Wald partial χ² values, the contributions of variables can be compared on the same scale. The model was internally validated for discrimination and calibration using bootstrap resampling. Predictive discrimination was quantified by the C-statistic and other model indexes. At the same time, calibration was demonstrated with a smooth nonparametric calibration curve comparing predicted and observed probabilities of IE for both the original and bootstrapped models.
If not otherwise indicated, patient characteristics presented with median and interquartile ranges (IQR) for continuous variables and numbers and percentages for categorical or ordinal variables. A P-value of < 0.05 was considered statistically significant. Analyses were performed using the statistical programming language R, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Overall, a total of 579 patients were included in the analysis: 148 LS-NVE cases [124 definite (83.8%) and 24 possible] and 431 matched controls (Figure 1). The median age was 63.8 (IQR: 53.6–74.6) years, and 329 (56.8%) patients were males (Table 1). The patients’ race was predominantly white in both cases (91.8%) and controls (92.2%). LS-NVE cases had a higher prevalence of diabetes mellitus (46.6% vs. 30.4%) and chronic kidney disease (46.6% vs. 28.1%) when compared with controls (P < 0.001 for both comparisons). The median CCI score was 3 (IQR: 0–6) for cases and 2 (IQR: 0–6) for controls.
Demographics and comorbid conditions by IE case status
| Characteristic | N | Cases (N = 148) | Controls (N = 431) | P-value |
|---|---|---|---|---|
| Time of echo | 579 | 2015.9 (2011.7–2018.3) | 2015.9 (2011.6–2018.4) | 0.8371 |
| Male gender | 579 | 84 (56.8%) | 245 (56.8%) | 0.9852 |
| Age at time of echo | 579 | 63.7 (53.7–74.5) | 63.8 (53.6–74.6) | 0.9051 |
| Race: white | 572 | 135 (91.8%) | 392 (92.2%) | 0.8772 |
| Chronic kidney disease | 579 | 69 (46.6%) | 121 (28.1%) | < 0.0012 |
| Hemodialysis | 579 | 14 (9.5%) | 1 (0.2%) | < 0.0012 |
| Hypertrophic cardiomyopathy | 579 | 2 (1.4%) | 3 (0.7%) | 0.4572 |
| Myocardial infarction | 579 | 27 (18.2%) | 88 (20.4%) | 0.5672 |
| Hypertension | 579 | 88 (59.5%) | 238 (55.2%) | 0.3702 |
| Heart failure | 579 | 51 (34.5%) | 139 (32.3%) | 0.6212 |
| Cerebral stroke | 579 | 3 (2.0%) | 13 (3.0%) | 0.5262 |
| Diabetes mellitus | 579 | 69 (46.6%) | 131 (30.4%) | < 0.0012 |
| Charlson index | 579 | 3 (0–6) | 2 (0–6) | 0.4211 |
| Charlson index levels | 579 | - | - | 0.8191 |
| 0 | - | 43 (29.1%) | 129 (29.9%) | - |
| 1 | - | 7 (4.7%) | 21 (4.9%) | - |
| 2 | - | 19 (12.8%) | 56 (13.0%) | - |
| 3 | - | 17 (11.5%) | 47 (10.9%) | - |
| 4–6 | - | 26 (17.6%) | 75 (17.4%) | - |
| 7–10 | - | 23 (15.5%) | 68 (15.8%) | - |
| > 10 | - | 13 (8.8%) | 35 (8.1%) | - |
Values represent the median (quartile 1 to quartile 3) for continuous variables and frequency (percentage) for categorical variables. N is the number of non-missing values. P values are calculated by 1 Wilcoxon rank sum, or 2 Pearson χ². -: no data. IE: infective endocarditis; echo: echocardiography
Echocardiographic variables that were routinely (i.e., in > 80% of patients) measured in cases and controls are presented in Table 2. In these unadjusted analyses, anatomical echocardiographic measurements were different in patients with LS-NVE than those without IE, as evidenced by higher IVSDT, LVPWDT, and LV mass index (P ≤ 0.01 for all comparisons). LS-NVE cases also exhibited different hemodynamics compared with controls based on higher LVOT velocity, LV cardiac index, mitral E and A wave, E:e’ ratio, and AVSP velocity (P ≤ 0.002 for all comparisons).
Echocardiographic measures by LS-NVE case status
| Measurement | N | Cases (N = 148) | Controls (N = 431) | P-value |
|---|---|---|---|---|
| Heart rate (bpm) | 515 | 72.0 (63.0–81.8) | 72.0 (61.0–83.0) | 0.6431 |
| IVSDT (mm) | 535 | 12.0 (10.0–13.0) | 11.0 (10.0–12.0) | < 0.0011 |
| LVOT systolic TVI (cm) | 522 | 23.9 (20.8–27.0) | 21.0 (18.0–24.0) | < 0.0011 |
| LVOT velocity (m/s) | 526 | 1.2 (1.0–1.3) | 1.0 (0.9–1.2) | < 0.0011 |
| LV cardiac output (L/min) | 504 | 6.6 (5.3–7.9) | 5.8 (5.1–6.9) | < 0.0011 |
| LV cardiac index [L/(min·m2)] | 502 | 3.3 (2.8–3.8) | 3.0 (2.6–3.5) | 0.0021 |
| LVEDD (mm) | 547 | 49.0 (44.5–54.0) | 48.0 (44.0–53.0) | 0.2801 |
| LVESD (mm) | 503 | 31.0 (28.0–35.0) | 31.0 (28.0–35.0) | 0.6861 |
| LV mass (g) | 531 | 200.0 (163.8–264.0) | 181.0 (144.0–227.0) | 0.0041 |
| LV mass index (g/m2) | 529 | 102.0 (83.0–122.2) | 93.0 (77.0–114.0) | 0.0101 |
| LVPWDT (mm) | 535 | 11.0 (10.0–12.0) | 10.0 (9.0–11.0) | < 0.0011 |
| LV stroke volume (mL) | 516 | 94.5 (79.0–108.0) | 82.0 (69.0–99.0) | < 0.0011 |
| LV systolic stroke volume index (mL/m2) | 509 | 47.0 (40.0–53.0) | 43.0 (37.0–49.0) | < 0.0011 |
| MV E-wave peak velocity (m/s) | 526 | 0.9 (0.7–1.1) | 0.8 (0.6–1.0) | < 0.0011 |
| MV A-wave peak velocity (m/s) | 465 | 0.9 (0.7–1.1) | 0.7 (0.6–0.9) | < 0.0011 |
| MV deceleration time (ms) | 486 | 210.5 (177.5–265.5) | 195.5 (167.8–227.0) | < 0.0011 |
| MV Annulus e’ velocity (m/s) | 523 | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.1951 |
| MV E:e’ | 512 | 13.8 (10.0–18.0) | 11.1 (8.2–16.0) | < 0.0011 |
| Aortic stenosis | 565 | - | - | 0.6702 |
| None | - | 132 (90.4%) | 391 (93.3%) | - |
| Trivial | - | 2 (1.4%) | 4 (1.0%) | - |
| Mild | - | 7 (4.8%) | 7 (1.7%) | - |
| Moderate | - | 4 (2.7%) | 7 (1.7%) | - |
| Severe | - | 1 (0.7%) | 10 (2.4%) | - |
| Aortic regurgitation | 565 | - | - | 0.5522 |
| None | - | 85 (58.2%) | 258 (61.6%) | - |
| Trivial | - | 39 (26.7%) | 93 (22.2%) | - |
| Mild | - | 14 (9.6%) | 52 (12.4%) | - |
| Moderate | - | 6 (4.1%) | 15 (3.6%) | - |
| Severe | - | 2 (1.4%) | 1 (0.2%) | - |
| Mitral stenosis | 570 | - | - | 0.0322 |
| None | - | 141 (95.9%) | 420 (99.3%) | - |
| Trivial | - | 1 (0.7%) | 0 (0.0%) | - |
| Mild | - | 5 (3.4%) | 1 (0.2%) | - |
| Moderate | - | 0 (0.0%) | 2 (0.5%) | - |
| Mitral regurgitation | 570 | - | - | 0.5472 |
| None | - | 19 (12.9%) | 38 (9.0%) | - |
| Trivial | - | 78 (53.1%) | 229 (54.1%) | - |
| Mild | - | 32 (21.8%) | 102 (24.1%) | - |
| Moderate | - | 12 (8.2%) | 46 (10.9%) | - |
| Severe | - | 6 (4.1%) | 8 (1.9%) | - |
| AVA by velocity (cm2) | 469 | 2.8 (2.2–3.5) | 3.0 (2.3–3.5) | 0.1701 |
| AVSP velocity (m/s) | 484 | 1.5 (1.4–2.0) | 1.4 (1.2–1.7) | < 0.0011 |
| AV systolic TVI (cm) | 447 | 33.4 (27.0–43.0) | 28.0 (23.1–34.0) | < 0.0011 |
| LVOT systolic diameter (cm) | 525 | 2.2 (2.1–2.4) | 2.2 (2.1–2.4) | 0.9131 |
| LVEF (%) | 549 | 62.0 (58.0–67.0) | 61.0 (56.0–65.0) | 0.0241 |
Values represent the median (quartile 1 to quartile 3) for continuous variables and frequency (percentage) for ordinal variables. N is the number of non-missing values. P values are calculated by 1 Wilcoxon rank sum or 2 proportion trend tests. -: no data. LS-NVE: left-sided native valve endocarditis; IVSDT: interventricular septum diastolic thickness; LVOT: left ventricular outflow tract; TVI: time velocity integral; LV: left ventricular; LVEDD: left ventricular end-diastolic dimension; LVESD: left ventricular end-systolic dimension; LVPWDT: left ventricular posterior wall diastolic thickness; MV: mitral valve; AV: aortic valve; AVA: aortic valve area; AVSP: aortic valve systolic peak; LVEF: left ventricular ejection fraction
A multivariable conditional logistic regression model was developed to determine the association of anatomical and hemodynamic measurements with the risk of LS-NVE. The selection of variables to be included in the model was based on a priori knowledge and data reduction methods blinded to the case-control group, resulting in 10 variables selected for inclusion (Table 3). Ranked by importance according to their adjusted χ² values from the model (Figure 2), LVOT velocity (P = 0.017), LVEF (P = 0.018), and E:e’ (P = 0.050) were significantly associated with LS-NVE. When re-expressed in terms of grouped variables, the hemodynamic-related variables had a stronger contribution to the model (corrected χ² = 31.7; P < 0.001) than the anatomical landmark-related variables (corrected χ² = 11.1; P = 0.086). A partial effects plot for the continuous variables in the final model is presented in Figure 3.
Adjusted odds ratios and 95% confidence intervals
| Variable | Contrast | Odds ratio (95% confidence interval) | Test statistic |
|---|---|---|---|
| IVSDT (mm) | 12:10 | 1.13 (0.82–1.56) | χ22 = 3.48, P = 0.176 |
| LVESD (mm) | 35:28 | 1.38 (0.95–2.01) | χ12 = 2.81, P = 0.094 |
| LVEF (%) | - | - | χ22 = 8.02, P = 0.018 |
| - | 46:61 | 0.34 (0.16–0.73) | - |
| - | 69:61 | 1.07 (0.67–1.71) | - |
| LVPWDT (mm) | 12:9 | 1.35 (0.83–2.20) | χ12 = 1.43, P = 0.232 |
| Aortic regurgitation | Mild to severe: none or trivial | 0.71 (0.39–1.28) | χ12 = 1.32, P = 0.250 |
| Mitral regurgitation | Mild to severe: none or trivial | 0.96 (0.58–1.58) | χ12 = 0.03, P = 0.868 |
| LVOT velocity (m/s) | - | - | χ22 = 8.20, P = 0.017 |
| - | 0.9:1.1 | 0.51 (0.32–0.82) | - |
| - | 1.4:1.1 | 1.20 (0.75–1.91) | - |
| LV cardiac index [L/(min·m2)] | 3.6:2.6 | 1.05 (0.78–1.43) | χ12 = 0.11, P = 0.742 |
| MV E:e’ | 16.7:8.6 | 1.42 (1.00–2.02) | χ12 = 3.85, P = 0.050 |
| AVSP velocity (m/s) | 1.7:1.2 | 1.08 (0.84–1.40) | χ12 = 0.37, P = 0.543 |
For all continuous variables except LVOT and LVEF, the odds ratios represent an interquartile increase in that variable (i.e., an increase from the 25th to the 75th percentile). For LVOT and LVEF, which appear to be non-monotonically related to the outcome (see Figure 3), odds ratios are calculated at 2 data points compared with the median (i.e., 50th percentile) to represent the effects of having an extremely low value (10th percentile) and an extremely high value (90th percentile). As IVSDT, LVOT, and LVEF each showed evidence of nonlinearity, these variables were modeled with regression splines, and their overall tests of the association are based on 2 degrees of freedom. -: no data. IVSDT: interventricular septum diastolic thickness; LVOT: left ventricular outflow tract; LV: left ventricular; LVESD: left ventricular end-systolic dimension; LVPWDT: left ventricular posterior wall diastolic thickness; MV: mitral valve; AVSP: aortic valve systolic peak; LVEF: left ventricular ejection fraction
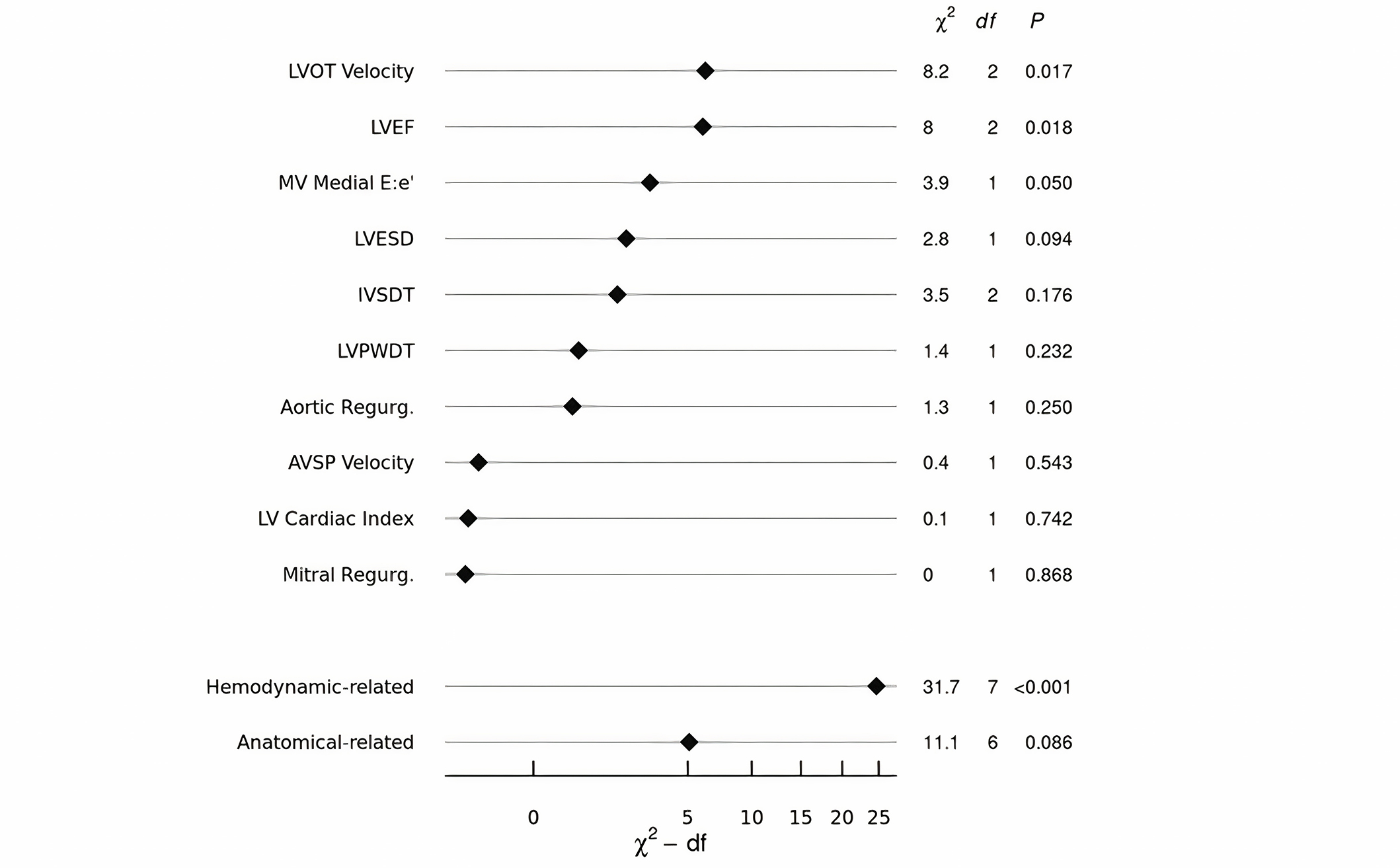
The relative importance of individual and grouped echocardiographic variables. LVOT: left ventricular outflow tract; LVEF: left ventricular ejection fraction; MV: mitral valve; LVESD: left ventricular end-systolic dimension; IVSDT: interventricular septum diastolic thickness; LVPWDT: left ventricular posterior wall diastolic thickness; AVSP: aortic valve systolic peak; LV: left ventricular; df: degrees of freedom
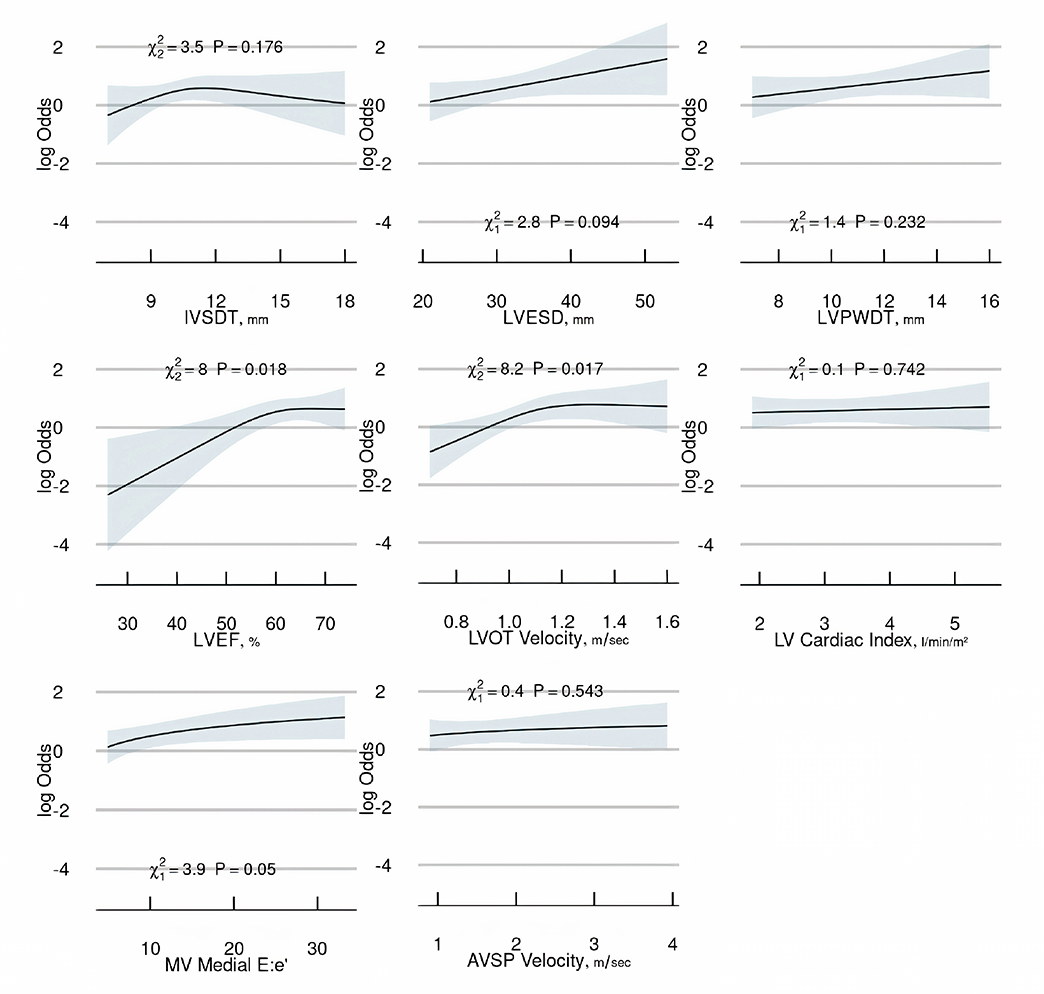
Partial effects plot for continuous variables. IVSDT: interventricular septum diastolic thickness; LVESD: left ventricular end-systolic dimension; LVPWDT: left ventricular posterior wall diastolic thickness; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; LV: left ventricular; MV: mitral valve; AVSP: aortic valve systolic peak
The bias-corrected C statistic of the model was 0.67, indicating modest discrimination in predicting LS-NVE for an individual patient. Using the bootstrap method to internally validate and calibrate the model, the estimated slope shrinkage factor of 0.79 suggested minor overfitting. The bias-corrected calibration curve presented in Figure 4 demonstrates that the model is generally well calibrated across the risk range, except for higher risk profiles where the model tends to underestimate the probability of IE. Model-based predictions of LS-NVE (in the matched sample), as estimated by the mathematical formula in Figure S1, are illustrated for ten hypothetical patients in Table S1.
A secondary analysis in which terms for diabetes mellitus and chronic kidney disease were added to the original model yielded similar findings concerning echocardiographic variables, except for E:e’ which was no longer significant (Figures S2 and S3).
This is the first study to evaluate multiple specific TTE measurements and determine whether they were associated with the subsequent development of LS-NVE in patients with no known cardiac predisposing conditions. Our primary finding was that increased peak LVOT velocity and LVEF were hemodynamic echocardiographic measurements associated with the subsequent development of LS-NVE. It is conceivable that increased LV pressures and velocities could be operative in IE predisposition by causing substantial turbulence that could create a substrate for endocardial injury as highlighted in an experimental model highlighting a “damage-induced” pathway [12]. Nevertheless, it is important to mention that there is limited clinical application for these results, future larger-scale studies should be performed to assess the relevance of our findings.
It has been estimated that more than 800,000 individuals are at a high risk of IE in the United States alone [21]. Nevertheless, there is not a clear NVE diagnostic scheme for patients with ‘formally’ structural normal heart valve conditions. Thus, implementing our analysis, which included an in-depth characterization of cardiac disease with the use of quantitative echocardiographic measurements, but also recognizing clinical parameters, may provide the most useful foundation for a future strategy in identifying patients who may have an increased risk of IE.
We did not detect an association between the degree of mitral and aortic regurgitation and subsequent development of IE in our multivariable model. Previous work has shown that valvular dysfunction is associated with IE, however, in contrast to our study, they included patients with predisposing cardiac conditions. Namely, the degree of mitral regurgitation could be a risk factor associated with the development of IE in patients with mitral valve prolapse [22]. Of note, the inclusion of stenotic lesions of the mitral and aortic valves in our multivariable model was not possible due to their limited prevalence in our study cohort.
Diabetes mellitus and chronic kidney disease were identified as predisposing comorbidities for the development of LS-NVE in the current investigation. Diabetes mellitus is well recognized as an independent risk factor associated with IE; these patients can have an impaired immune response, delayed healing, reduced tissue vascularization, and endothelial dysfunction [23]. Chronic kidney disease patients, particularly those with end-stage renal disease receiving hemodialysis, are at a significantly higher risk of developing IE when compared to the general population due to a higher prevalence of bacteremia related to vascular access and because of uremia-related impaired immune response [24, 25]. In addition, both diabetes mellitus and chronic kidney disease have a higher rate of S. aureus colonization than the general population, and hence, an increased risk of S. aureus bacteremia, which consequently increases the risk of IE [26].
Our study is based on a retrospective case-control design and is subject to inherent limitations. First, given our relatively modest sample size, the large number of echocardiographic variables available posed statistical challenges with model development, therefore, we used data reduction based on clinical sensibility, lack of redundancy, and data completeness. Second, the retrospective nature of this study precluded the acquisition of complete echocardiographic measurements in all patients; however, a strength of our modeling approach is that only parameters that were routinely available (i.e., measured in ≥ 80% of the subjects) were included. Third, this study was conducted at a tertiary medical center which may have introduced selection and referral bias. Fourth, our population was composed of adult patients who were predominantly white and of northern European descent; thus, the generalizability of our results to other populations is uncertain. Fifth, interobserver variability is an inherent limitation of echocardiography, as interpretations of imaging data can vary among different echocardiographers. Despite efforts to standardize protocols, discrepancies in findings may arise which could have affected our results. Finally, despite the association of peak LVOT velocity and LVEF measurements with the development of IE, the differences between the compared study groups were small and are not applicable in clinical practice given that they can be confounded by the inherent interobserver variability of TTE. Furthermore, both variables are functional and dynamic echocardiographic measurements, that may change in different clinical and hemodynamic conditions. However, it is crucial to recognize the potential value and opportunity this presents for conducting a larger-scale investigation to assess the importance of these findings. A larger sample size would enhance statistical power, allowing for a more precise estimation of effect sizes and detection of smaller, yet clinically relevant effects.
Specific hemodynamic echocardiographic measurements were independently associated with LS-NVE in patients with no known predisposing cardiac conditions in our preliminary investigation. These measurements underpin the importance of alterations in hemodynamic function in predisposing to NVE. While our results demonstrated an association of echocardiographic measures with the subsequent development of IE, further investigation is needed to validate these preliminary findings.
CCI: Charlson comorbidity index
df: degrees of freedom
IE: infective endocarditis
IQR: interquartile ranges
IVSDT: interventricular septum diastolic thickness
LS-NVE: left-sided native valve endocarditis
LV: left ventricular
LVEF: left ventricular ejection fraction
LVOT: left ventricular outflow tract
LVPWDT: left ventricular posterior wall diastolic thickness
TTE: transthoracic echocardiography
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/101234_sup_1.pdf.
The authors are extremely grateful for the philanthropic support provided by a gift from Eva and Gene Lane (Baddour LM), which was paramount in our work to advance the science of cardiovascular infections and has been an ongoing focus of investigation at Mayo Clinic for over 60 years.
JAQM: Conceptualization, Data curation, Investigation, Methodology, Writing—original draft, Writing—review & editing. JRH: Data curation, Writing—original draft, Writing—review & editing. HRV: Supervision, Writing—review & editing. BDL: Software, Formal analysis, Validation, Methodology, Writing—original draft, Writing—review & editing. MJD, MHT, JCO, HIM, NSA, PS, and DCD: Supervision, Writing—review & editing. LMB: Conceptualization, Supervision, Investigation, Methodology, Writing—original draft, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Larry M. Baddour, M.D. reports over the past 12 months UpToDate, royalty payments (authorship duties). The other authors declare that they have no conflicts of interest.
This study was approved by the Mayo Clinic Institutional Review Board, the reference number is 20-011665.
Informed consent to participate was obtained from all subjects involved in the study.
Not applicable.
The data underlying this article cannot be shared publicly because the privacy of the individuals who participated in the study must be maintained and the data underlying this article were provided by the Mayo Clinic under license and permission. The data will be shared with a reasonable request to the corresponding author (Juan A. Quintero-Martinez) with the permission of the Mayo Clinic.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2236
Download: 86
Times Cited: 0
