Affiliation:
1Department Rheumatology, Centro Hospitalar Universitário de São João, 4200-319 Porto, Portugal
ORCID: https://orcid.org/0000-0003-3911-5959
Affiliation:
1Department Rheumatology, Centro Hospitalar Universitário de São João, 4200-319 Porto, Portugal
ORCID: https://orcid.org/0000-0001-7581-4074
Affiliation:
2Department of Cardiology, Centro Hospitalar Universitário de São João, 4200-319 Porto, Portugal
ORCID: https://orcid.org/0000-0003-0671-009X
Affiliation:
3MEDCIDS - Departamento de Medicina da Comunidade, Informação e Decisão em Saúde, Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal
ORCID: https://orcid.org/0000-0002-1277-3401
Affiliation:
4Nuclear Medicine, Lenitudes-Medical Center & Research, 4520-103 Santa Maria da Feira, Portugal
Affiliation:
5Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal
ORCID: https://orcid.org/0000-0002-8388-1848
Affiliation:
5Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal
Email: inesfortuna@gmail.com
ORCID: https://orcid.org/0000-0002-7010-2104
Affiliation:
1Department Rheumatology, Centro Hospitalar Universitário de São João, 4200-319 Porto, Portugal
ORCID: https://orcid.org/0000-0003-0518-776X
Affiliation:
6Department of Orthopaedics, Centro Hospitalar Universitário de São João, 4200-319 Porto, Portugal
ORCID: https://orcid.org/0000-0002-4962-0302
Affiliation:
7Nuclear Medicine Department, Centro Hospitalar Universitário de São João, 4200-319 Porto, Portugal
Affiliation:
1Department Rheumatology, Centro Hospitalar Universitário de São João, 4200-319 Porto, Portugal
Affiliation:
2Department of Cardiology, Centro Hospitalar Universitário de São João, 4200-319 Porto, Portugal
8Department of Medicine, Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal
ORCID: https://orcid.org/0000-0002-6944-2953
Explor Cardiol. 2024;2:217–223 DOI: https://doi.org/10.37349/ec.2024.00035
Received: April 12, 2024 Accepted: August 15, 2024 Published: October 24, 2024
Academic Editor: Erkin Mirrakhimov, Kyrgyz State Medical Academy, Kyrgyzstan
Carpal tunnel syndrome (CTS) is the most common type of entrapment neuropathy and affects approximately 1% to 5% of the general population, mostly patients older than 50 years. CTS is present in various conditions and diseases and could also be an early sign of systemic transthyretin amyloidosis (ATTR), associated with amyloid cardiomyopathy and subsequent heart failure. With advances in the treatment of cardiac amyloidosis, patient prognosis could be significantly improved with an early diagnosis. Amyloidosis represents a group of disorders characterized by the extracellular deposition of destabilized protein fragments, aggregating as amyloid fibrils, thereby leading to organ dysfunction. Among these, ATTR is a significant subgroup. This study protocol aims to explore the potential association between CTS and systemic and cardiac ATTR. The study design involves a case-control approach, with assessments including physical examination, laboratory tests, electromyography, electrocardiogram, wrist ultrasound, and scintigraphy with 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD). Histopathological analysis and genetic testing will be performed when appropriate. Statistical analysis will be conducted to evaluate the relationship between CTS and ATTR. The study seeks to contribute to a better understanding of the diagnostic and therapeutic implications of identifying ATTR in patients with idiopathic CTS (ClinicalTrials.gov identifier: NCT05409833).
Amyloidosis are diseases caused by the extracellular deposition of amyloid in organs and tissues. Amyloid is a proteinaceous substance composed of an insoluble fibrillar matrix, which leads to progressive damage and failure of the affected organs. There are several types of amyloidosis, determined by different configurations of the amyloid protein. Distinct amyloidosis syndromes imply different diagnostic and therapeutic approaches [1, 2].
Amyloidosis transthyretin-related (ATTR) is an important group of these disorders. Transthyretin (TTR) is a tetrameric protein primarily produced by the liver and present in human serum [3] and has the ability to aggregate into insoluble amyloid fibers. There are two types of ATTR, the hereditary and the wild type, which can sometimes be distinguished depending on the main symptoms and organs they affect.
A single mutation can increase the probability of TTR misfolding, leading to amyloid deposits in the peripheral nerves, heart, aorta, lungs, gastrointestinal tract, liver, kidney, and connective tissue [3]. TTR mutations may occur again or be inherited. There are more than 120 known mutations of the protein, some of them are associated with familial forms of the disease [3, 4].
The most common ATTR mutation in the world is Val30Met considered endemic in Portugal, Sweden, and Japan. ATTR mutation Val30Met is the main responsible for familial amyloid polyneuropathy (TTR-FAP) [4]. This is a rare autosomal dominant hereditary condition with adult-onset [4], typically leading to progressive neurodegenerative systemic disease in the majority of cases. If left untreated, it often results in the patient’s death within the first decade after diagnosis [3, 4]. Patients with Val30Met mutation may also present cardiomyopathy (43%) and carpal tunnel syndrome (CTS) [3].
The largest population with TTR-FAP is in Portugal, with nearly 20% of worldwide patients. In 2016, in Portugal, were identified 1,865 living people with this disease, with an estimated prevalence of 22.93 per 100,000 adult inhabitants [4].
Wild-type ATTR (ATTRwt) results from misfolding of non-mutated TTR into amyloid configurations [3]. Systemic ATTRwt is an aging-related disorder, usually associated with cardiac disease, but extends also to other organs. This condition is rarely recognized before death, and at diagnosis, most patients have advanced stage disease, with few therapeutic options. Postmortem studies have found ATTRwt deposition in 12% to 25% of people older than 80 years and in 37% of people older than 95 years. Concerning people older than 80 years, 25% have TTR deposits in the heart [5, 6].
Recent studies have shown that CTS could be an initial symptom of systemic ATTRwt [5, 7] and hypothesized it as an early symptom of ATTRwt cardiomyopathy [8].
Histopathology has been necessary for the accurate diagnosis of ATTR, with tissue samples obtained from the myocardium, gastroduodenal mucosa, subcutaneous fat pad, or tenosynovial tissue. Recently, cardiac scintigraphy has provided an alternative minimally invasive procedure noninvasive technique, with high specificity and sensibility for the diagnosis of cardiac ATTR [2].
Despite the described associations, there is a lack of evidence on how we should approach patients with idiopathic CTS, considering the fact that they could be at risk for systemic amyloidosis, namely cardiac disease.
The protocol was designed to explore the association between idiopathic CTS and systemic and cardiac ATTR. We propose a case-control study with the following objectives:
To determine whether the presence of TTR deposition is associated with idiopathic CTS;
To assess whether or not idiopathic CTS is associated with systemic ATTR;
To assess whether or not idiopathic CTS is associated with cardiac ATTR.
This study adopts a case-control design to investigate the relationship between idiopathic CTS and ATTR.
As we lack epidemiological studies, we will test the protocol through a pilot study in our center for 3 months to reconsider the sample size. Consecutive patients from the orthopaedics consultation of a university tertiary hospital (Centro Hospitalar Universitário de São João) will be selected according to the inclusion and exclusion criteria and referred to the rheumatology consultation. They will be prospectively enrolled in this case-control study.
The study population will consist of 100 patients aged above 60 years. The cohorts will include 50 cases with symptomatic CTS and 50 controls without CTS symptoms. Controls will comprise patients without symptomatic CTS but with hand surgery indication for other causes. Patients with known secondary causes of CTS, such as diabetes mellitus, hypothyroidism, chronic renal failure under haemodialysis, rheumatoid arthritis and other inflammatory arthropathies, multiple myeloma, gout, chondrocalcinosis, Colles’s fracture, space-occupying lesions, and infectious synovitis will be excluded. The main study features and patient characterization are presented in Table 1.
Study design of protocol
| Items | Parameters |
|---|---|
| Type of study | Case-control |
| Location | Centro Hospitalar Universitário de São João |
| Patients | Both genders and ≥ 60 years old |
| Cohorts | Cases: symptomatic CTS and surgical treatment indication |
| Controls: absence of symptomatic CTS and indication for hand surgical treatment | |
| Exclusion criteria | Patients with diabetes mellitus, hypothyroidism, chronic renal failure under haemodialysis, rheumatoid arthritis and other inflammatory arthropathies, multiple myeloma, gout, chondrocalcinosis, Colles’s fracture, space-occupying lesions, or infectious synovitis |
CTS: carpal tunnel syndrome
Patients will undergo comprehensive clinical evaluations, including physical examination, laboratory tests, electromyography, electrocardiogram, wrist ultrasound (only in symptomatic CTS), scintigraphy with 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) and synovial biopsy (Congo red). Mass spectrometry analysis or genetics tests will be applicable if it is applicable. To better understand and specify the procedures, we present a schema and table with detailed information (Table 2 and Figure 1).
Description of study procedures
| Procedure | Description |
|---|---|
| Physical examination | Includes the Tinel sign at the wrist, Phalen test, and sensory disturbance detected over the median nerve-innervated area. |
| Laboratory tests | Includes blood tests of antinuclear antibodies, rheumatoid factor and troponin I to exclude rheumatic disease. |
| Electromyography | The electrical testing of median nerve function can detect the presence, location, and extent of CTS, and help to determine the most effective treatment. |
| Electrocardiogram | The ECG will be analyzed for rhythm, presence of previous pseudoinfarction, conduction abnormalities and QRS amplitudes, including left ventricular hypertrophy and low voltage. |
| Wrist ultrasound (only in symptomatic CTS) | The ultrasound of the wrist will be done by the rheumatologist and will be used to measure the transversal area of the median nerve and confirm the absence of lesion space occupying or anatomical abnormalities. |
| Whole-body, cardiac and wrists/hands scintigraphy with 99mTc-DPD | It will be performed to detect amyloid deposits. Before surgery, patients (cases and controls) will undergo a scintigraphy with 99mTc-DPD, obtaining whole-body scans; acquisition of images of vascular, blood-pool and late phases of the radiopharmaceutical’s distribution in the hands and wrists; cardiac images synchronized with cardiac rhythm and acquired with low-dose CT scan (total amount of radiation of 6.3 mSv); cardiac disease will be characterized according to the semiquantitative Perugini categories: grade 0: no cardiac uptake and normal rib uptake; grade 1: cardiac uptake which is less than rib uptake; grade 2: cardiac uptake with intensity similar to rib uptake; grade 3: cardiac uptake greater than rib uptake with mild or absent rib uptake.Cardiac SPECT/CT imaging acquired at 2 hours and whole-body at 2h30 after injection. |
| Synovial biopsy (Congo red) | Tenosynovial tissues obtained during surgery (cases and controls) will be analyzed using Congo red. Histopathological analysis will be conducted when necessary. The degree of amyloidosis will be divided into 3 categories and evaluated semiquantitatively: grade I, II, III representing mild, moderate and severe amyloid deposition, respectively. |
| Mass spectrometry analysis | If the Congo red stain is positive, mass spectrometry will be performed to differentiate and identify the type of amyloid protein. |
| Genetics tests | If the Congo red stain is positive, genetic test will be carried out to detect TTR gene mutations. |
CT: computed tomography; CTS: carpal tunnel syndrome; ECG: electrocardiogram; TTR: transthyretin; 99mTc-DPD: 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid
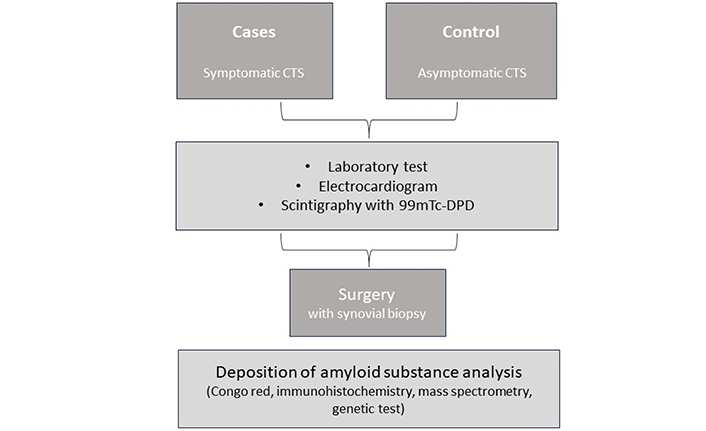
Sequence of study procedures. CTS: carpal tunnel syndrome; 99mTc-DPD: 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid
Statistical analysis will be done to characterize the cohorts and explore the association between CTS and ATTR. Descriptive statistics will be used to summarize patient features and logistic regression models will be employed to assess the associations mentioned. Statistical significance will be defined at p < 0.05.
The project has been approved by the Ethics Committee for Health of Centro Hospitalar Universitário de São João (reference No: CE 200/2020), according to the principles of the Helsinki Declaration, the Convention on Human Rights and Biomedicine and the guidelines of the Council for International Organizations of Medical Sciences [1, 9], and written informed consent was signed for each patient. The study protocol was registered in Clinicaltrials.gov with the registration number: NCT05409833.
Our understanding of the pathogenesis of systemic amyloidosis has grown over the last years, particularly regarding ATTR cardiomyopathy, due in part to significant advances in cardiac imaging diagnostic techniques [10]. Furthermore, the importance of an early diagnosis came from the availability of recent specific therapeutic strategies, such as tafamidis, which are capable of changing the natural history of the disease and improving the patient’s prognosis [11].
One of the red flags for ATTR diagnosis is the coexistence of a history of CTS and unexplained cardiomyopathy (particularly hypertrophic cardiomyopathy). Theoretically, as this entrapment neuropathy manifests some years before the onset of cardiac disease, it offers an opportunity for an earlier diagnosis [12] or even for therapeutic interventions [8].
On the other hand, CTS is also considered a red flag of other hypertrophic cardiomyopathy conditions, such as Fabry disease [13], and similar considerations around CTS diagnosis should be taken into account for this disorder.
However, nowadays it remains unclear what should be the ideal clinical approach for a patient with the diagnosis of idiopathic CTS, especially if the patient is male and has bilateral involvement, since these characteristics may indicate a higher probability of ATTR. In this sense, we can question several aspects: if we always obtain tenosynovial tissue for TTR deposits search; if we recommend a long-term follow-up protocol of cardiac assessments, similar to what is indicated for asymptomatic pathogenic carriers of cardiomyopathy mutations. The clarification of this kind of questions is important since CTS is a relatively common diagnosis and usually considered a benign condition.
Establishing the causal link between ATTR deposits and the development of CTS is of paramount importance, and one of the steps is to clarify if tenosynovial ATTR deposits are also found in older patients even without the CTS diagnosis. Although amyloid deposition can also be present in other specific clinical conditions as patients undergoing chronic hemodialysis [14], these patients will not be included. We will apply strict diagnostic criteria, excluding “secondary” causes of CTS to overcome the difficulty of establishing the causal relationship between ATTR deposits and CTS.
In conclusion, it is hoped that the results of this study on CTS patients allow a comprehensive approach to investigate the relationship between idiopathic CTS and ATTR that will provide useful data for fruitful discussions between cardiologists, rheumatologists or orthopedics’ surgeons that could contribute to the implementation of new protocols and stimulate research in this area to optimize patient care and promote further advancements in this field.
The aim of the present study is to obtain clinical, histological, and imaging data from patients with CTS. Based on the results, our goal is to promote multidisciplinary discussion on optimizing the use of tissue biopsies, imaging technics, and clinical follow-up protocols in CTS patients. Our study will be performed in a Portuguese population known to have a particular incidence of a genetic variant of the TTR gene [15].
99mTc-DPD: 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid
ATTR: amyloidosis transthyretin-related
ATTRwt: wild-type ATTR
CTS: carpal tunnel syndrome
TTR: transthyretin
SP and AM: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. MC, BSP and ASP: Conceptualization. JS and IF: Writing—review & editing. PM, PN, JP and LC: Conceptualization. EM: Validation, Writing—original draft, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study protocol was approved by the Ethics Committee for Health of Centro Hospitalar Universitário de São João, reference No: CE 200/2020, and complied with the principles of the Helsinki Declaration.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This study is supported by a research grant from Pfizer Grant [59852013]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2595
Download: 47
Times Cited: 0
