Affiliation:
CNR Institute of Clinical Physiology, 56124 Pisa, Italy
Email: andrea.borghini@cnr.it
ORCID: https://orcid.org/0000-0003-1061-8309
Explor Cardiol. 2024;2:224–240 DOI: https://doi.org/10.37349/ec.2024.00036
Received: August 22, 2024 Accepted: October 11, 2024 Published: November 05, 2024
Academic Editor: Quirino Ciampi, Fatebenefratelli Hospital of Benevento, Italy.
Exposure to ionizing radiation has recognized detrimental cancer and non-cancer health effects. These effects are now well-proven not only for high doses > 1,000 millisieverts (mSv) associated with head radiotherapy but also for moderate (100–1,000 mSv) and even low (< 100 mSv) doses, of interest for professionally exposed cardiologists. The head of interventional cardiologists is highly exposed to ionizing radiation, with possible damage to the eye and brain. Unprotected interventional cardiologists experience head radiation doses up to ten times greater than chest doses below lead aprons, with marked exposure to the left hemisphere of the brain reaching up to 2 Sv—equivalent to 10,000 chest X-rays over a professional lifetime. This narrative review aims to provide an overview of the background of radioprotection, the biological mechanisms involved, and the epidemiological evidence regarding the health effects of head exposure to ionizing radiation in invasive cardiologists. These health effects include cataracts, brain cancer, cerebrovascular diseases, neurodegeneration, and mood disorders. The evidence gathered from other exposed populations, which experienced similar eye and brain doses, has also been reviewed. This is important because the doses, risks, and effects are consistent in cases of repeated exposures, which occur more frequently for patients, and in situations involving chronic low doses, as seen with interventional cardiologists. Despite these risks, effective protective measures—such as suspended lead ceilings, curtains, and specialized eyewear—can reduce radiation exposure to near-zero levels. In some fields, like interventional cardiac electrophysiology, a groundbreaking near-zero radiation approach using non-fluoroscopic methods has been created, eliminating radiation exposure and alleviating orthopedic stress and operational discomfort. The race to zero radiation in interventional cardiology is ongoing.
Ionizing radiation from medical applications is a very significant source of exposure for patients and healthcare professionals working in the cardiac catheterization laboratory. For patients, the exposure per capita per year totaled the effective dose of 3.0 millisievert (mSv) in the USA in the radiological year 2006, corresponding to the biologic risk of 150 chest X-rays. The exposure declined to 2.29 mSv (120 chest X-rays) in the radiologic year 2016, thanks to technological upgrades, greater awareness of radiologic risk, and the development of non-ionizing alternatives such as stress echocardiography and magnetic resonance imaging for the diagnosis of cardiovascular disease [1]. Cardiovascular testing accounts for about 50% of all radiological exposures for patients, and invasive cardiologists are the most exposed health professionals, 3 times more exposed than diagnostic radiologists or nuclear physicians [2]. Ionizing radiation has a wide range of health effects, mostly cancer [3]. However, other non-cancer effects such as cardiovascular and cerebrovascular diseases are also important at the individual and population levels [4]. The head is a highly exposed target for invasive cardiologists and also the potential victim of a variety of damaging clinical effects mediated by different, disease-specific cell populations (Table 1). Damage of cell and mitochondrial DNA is a common initiating molecular step, and low-grade inflammation is a secondary cellular mechanism of progression of cancer, atherosclerotic, and neurodegenerative disease [5–7]. Chronic low-grade neuroinflammation may eventually lead to irreversible damage of neuronal structure and function, hitting cholinergic neurons in Alzheimer’s or dopaminergic neurons in Parkinson’s disease [8]. Ionizing radiation acts not directly on neurons but on microglial cells, key regulators of neuroinflammation and oxidative stress in both these neurodegenerative diseases [9].
Different cell subtypes are involved in main cancer and non-cancer effects on the brain
| Disease | LEC | EC and SMC | Arachnoid cells | Astrocytes | Schwann cells | Microglial cells |
|---|---|---|---|---|---|---|
| Cataract | √ | |||||
| Stroke | √ | |||||
| Meningioma | √ | |||||
| Glioblastoma | √ | |||||
| Neuroma | √ | |||||
| Alzheimer | √ | |||||
| Parkinson | √ |
LEC: lens epithelial cells; EC: endothelial cells; SMC: smooth muscle cells; √: involved
These effects were first demonstrated after high dose (> 1,000 mSv) of head radiotherapy in cancer, but in the last decade increasingly recognized after moderate (100–1,000 mSv) and even low dose (< 100 mSv) exposures. Consequences of radiation with moderate or low dose exposures appear after a long latency time, and can be less easy to recognize, but are present and linearly related to the dose exposure, probably without a threshold effect. Every dose counts, there is no safe dose, and the low doses at low dose rates typically met in medically exposed patients and professionals, tend to be more toxic than acute high-dose exposures [10]. Tissue reactions (such as skin effects with erythema and ulcers from high radiation exposure in patients during intensive cardiac interventions) require medium to high doses of radiation on the target tissue, recognizing a threshold dose, and a latency period of days or weeks [4]. Tissue reactions were traditionally considered tissue reactions (previously named deterministic effects), conceptually different from stochastic effects, such as cancer and hereditary effects. However, in the last 15 years evidence has accumulated that eye, cardiovascular, cerebrovascular, and neurologic effects of radiation exposure tend to deviate from the definition of tissue reactions, show significant risks also at low dose and for low-dose rates, and their effects are apparent at doses and dose rates substantially lower than previously thought, sometimes many years after exposure [10].
This narrative review will briefly describe the radioprotection background, the biological basis, and the epidemiological evidence supporting the health effects of head exposure to ionizing radiation in invasive cardiologists. The evidence obtained in patients, for similar eye and brain doses, has also been reviewed since the doses, the risks, and the effects are very much the same in the case of repeated exposures as more frequently happens for patients and in the case of chronic, low-dose, low-dose-rate refracted doses as happens with interventional cardiologists. Doctors may become patients, and the exposures as patients add up to professional exposures.
For patients, head computed tomography or diagnostic cerebral angiography with whole-body exposures of 5 mSv to 10 mSv are associated with brain organ dose exposure in the range of 50 mSv to 100 mSv per scan [11]. Interventional neuroradiology intervention with endovascular procedures such as closure of cerebral aneurysm involves a brain dose exposure between 200 mSv and 1,000 mSv [12–15].
Brain organ doses are derived from recent literature dating back to the 1990s, but it is important to apply correction factors for earlier exposures. Specifically, a correction factor of 1.5 is necessary for radiographic procedures conducted in the 1980s, and a factor of 2.0 applies to nuclear medicine scans performed prior to 1980 [16].
Regarding interventional cardiologists, the cumulative lifetime professional exposure ranges from 100 mSv to 200 mSv [17], with 1 Sv associated with right-sided cranial radiation and up to 2 Sv for left-sided cranial radiation [18, 19]. In fact, in a typical cardiac catheterization room, the cardiologist stands on the right side of the patient, and the left side of the head of the operator is closer to the scatter radiation coming from the patient [20]. The organ dose is far in excess to the low dose range (below 100 mSv or 5,000 chest X-rays). In addition, the reassuring concept that refracted low-dose exposures can be less damaging because there is time to repair damaged DNA has been disproved and the low, low-dose-rate exposures are at least equally, and probably more damaging than acute high-dose exposures, possibly because low-dose it is more likely that the cells suffer non-lethal DNA damage that can be passed to next cell generations [21–23].
The doses of medical or professional exposures are lower than the doses employed for head and neck cancer radiotherapy (Table 2). In the case of radiotherapy, high doses (> 1,000 mSv) are involved, with brain organ doses in the range of hundreds of thousands of chest X-rays. Following head radiotherapy, various essential neurological structures, including the brain, brainstem, spinal cord, cranial nerves, nerve plexuses, autonomic pathways, brain vasculature, and neurosensory organs, are exposed to varying degrees of radiation. This exposure can lead to a wide array of long-term neurological side effects in survivors. These complications often cause permanent symptoms, negatively impact patients’ quality of life, and account for a significant number of non-cancer-related deaths [24].
| Subjects | Whole body dose (mSv) | Brain organ dose (mSv) |
|---|---|---|
| Patients | ||
| Skull X-ray | 1.8 | 0.9 |
| Thyroid isotope scan | 3.2 | 1 |
| Cerebral angiography | 8 | 5 |
| Head CT | 1.6 | 20 |
| Interventional Neuroradiology | 20–100 | 200–1,000 |
| Doctors | ||
| Per procedure | 0.03 | 0.3 |
| Lifetime (40 years) | 200 | 2,000 |
| Radiotherapy | ||
| Per cycle | 1,000–40,000 | 10,000–400,000 |
Ionizing radiation exposure can lead to significant cerebrovascular and neuronal damage in the brain, primarily through a cascade of molecular and cellular mechanisms. These effects are often seen in both therapeutic settings, such as radiation therapy for brain tumors, and in accidental or occupational exposure to radiation.
At the molecular level, ionizing radiation primarily causes damage by generating reactive oxygen species [25]. These highly reactive molecules can damage cellular components, including lipids, proteins, and DNA. The endothelial cells lining the blood-brain barrier are particularly vulnerable. DNA damage in these cells can lead to apoptosis (programmed cell death) or senescence (a state of permanent cell cycle arrest), disrupting the integrity of the blood-brain barrier. This disruption allows for the extravasation of blood components into the brain parenchyma, leading to inflammation and further tissue damage.
One critical pathway activated by ionizing radiation is the DNA damage response (DDR) pathway, which includes the activation of ataxia-telangiectasia mutated and p53 proteins. These proteins initiate cell cycle arrest, DNA repair, or apoptosis depending on the extent of damage. Persistent activation of the DDR pathway can result in endothelial cell dysfunction, contributing to chronic cerebrovascular damage [6, 26–28].
At the cellular level, ionizing radiation induces changes in the microenvironment of the brain’s vasculature. This includes endothelial cell activation, which promotes the expression of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). These molecules facilitate the adhesion and infiltration of inflammatory cells like macrophages and lymphocytes into the brain tissue, exacerbating inflammation and vascular damage [20, 26].
Radiation also impacts astrocytes, the star-shaped glial cells in the brain that play a key role in maintaining and supporting neuronal function. Astrocytes exposed to ionizing radiation can undergo reactive gliosis, a process characterized by hypertrophy and proliferation. Reactive astrocytes secrete cytokines and chemokines that further promote inflammation and can alter the function of the blood-brain barrier [26].
Microglia, the resident immune cells of the brain, also respond to ionizing radiation. Activated microglia release pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which contribute to a pro-inflammatory milieu. This chronic inflammatory state can perpetuate vascular damage and promote neurodegenerative processes [26–28].
The central nervous system is exceptionally radiosensitive and thus more liable to neurological insult even at low doses [26]. Neurogenesis occurs not only in the developing brain but also in the adult brain in specific regions such as the hippocampus and the impairment of adult neurogenesis has been implicated in depressive disorders such as anxiety and depression [29]. Fractionated low-dose irradiation has been shown to impair adult hippocampal neurogenesis and cognitive function in experimental animals [30].
In summary, ionizing radiation exposure leads to cerebrovascular damage through a complex interplay of molecular and cellular mechanisms. DNA damage and reactive oxygen species generation trigger endothelial dysfunction, inflammation, and disruption of the blood-brain barrier. The involvement of various cell types, including endothelial cells, astrocytes, pericytes, and microglia, underscores the multifaceted nature of radiation-induced injury (Figure 1).
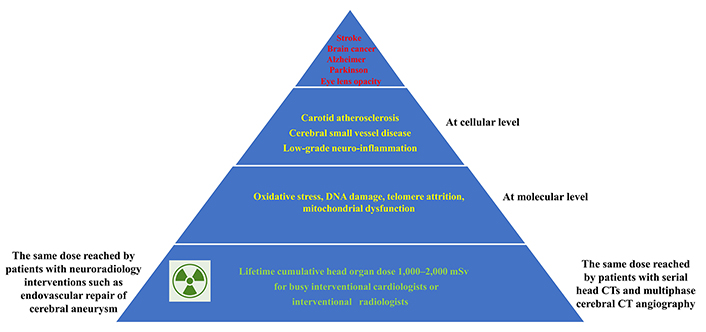
From radiation exposure to the head through molecular and cellular effects to the clinical manifestations of radiation-induced disease
A deeper understanding of the biological mechanisms involved is essential for developing protective strategies and treatments for individuals exposed to low-dose ionizing radiation. In this context, the use of three-dimensional (3D) brain culture models to investigate oxidative stress induced-DNA damage, telomere attrition, mitochondrial dysfunction, and neuroinflammation can offer valuable insights into the intricate cellular responses to ionizing radiation, serving as simplified representations of the structures and functions of in vivo organs. This is highlighted by recent results from Oyefeso et al. [31] that demonstrated the potential of using brain organoids to characterize cell-specific radiosensitivity and early radiation-induced gene expression changes within the human brain at low-to-moderate doses [31].
Importantly, human induced pluripotent stem cells (hiPSCs) can serve as a powerful modeling system to elucidate the cellular and biological effects of radiation exposures on the central nervous system using conventional 2D arrays and more advanced tissue engineering approaches with organoids and other 3D models. The health effects of ionizing radiation may be influenced by genetic polymorphisms, which can make certain individuals more susceptible to its effects. Thus, since hiPSC lines reprogrammed from selected donors reflect their individual genetic backgrounds, iPSC disease modeling may reveal important gene-environment interactions that exacerbate cerebrovascular disease and predispose certain individuals to adverse outcomes.
Cataract, or opacification of the lens, is often associated with visual impairment and may be classified into three main categories: nuclear, cortical, and posterior subcapsular, according to their anatomic location. Among the three major areas of age-related cataracts, posterior subcapsular is the least common but it is the one most frequently associated with ionizing radiation exposure. New exposure limits set by the International Commission on Radiological Protection (ICRP) in 2012 markedly reduced the allowed annual exposure of the eye from 150 mSv (ante-2012) to 20 mSv/year (since 2012). The eye is a very radiosensitive organ. Interventional cardiologists and staff have a 4-fold higher risk of posterior subcapsular opacity and cataracts compared to non-exposed controls [32–37]. Although traditionally considered a deterministic effect with threshold dose, it is not clear if the cataract is explained by a stochastic effect (without threshold, like cancer) or a tissue reaction (with threshold, a deterministic effect, like skin ulcer after high dose exposure). Ionizing radiation can induce cataract formation through complex molecular and cellular mechanisms that disrupt lens transparency. The lens, composed of epithelial and fiber cells, is particularly vulnerable due to its high metabolic activity and lifelong accumulation of damage. Ionizing radiation causes direct DNA breaks and generates reactive oxygen species, leading to oxidative stress. This oxidative stress can result in DNA mutations, apoptosis, and cellular senescence. Reactive oxygen species can oxidize lens lipids and proteins, particularly crystallins, leading to protein aggregation and insolubilization. These aggregated proteins scatter light, impairing lens transparency [38, 39]. At the cellular level, ionizing radiation can cause epithelial cell death, DNA damage, and dysfunction, disrupting cell proliferation and differentiation necessary for lens fiber cell formation. This complex interplay of molecular and cellular damage disrupts lens transparency, resulting in cataract formation [38, 39]. There is emerging evidence that the risk of normal-tension glaucoma, diabetic retinopathy, and macular degeneration, may be increased after ionizing radiation exposure [40].
In theory, the brain is a relatively radioresistant organ. A tissue weighting factor of 0.01 was applied to the brain by the ICRP in Document 103 released in 2007. The tissue weighting factor is 12 times lower for the brain compared to highly radiosensitive organs such as the lung, bone marrow, colon, and stomach [41]. To classify radiation-induced tumors, Cahan et al. [42] proposed back in 1948 certain criteria for radiation-induced carcinogenesis as follows: the tumor should arise in the field of irradiation, it must be histologically different from the disease that occasioned the irradiation; a suitable duration must elapse between the radiation and clinical onset of tumor; no other obvious predisposing conditions for oncogenesis be present [42].
Brain cancer is a recognized complication of brain radiotherapy and has been increasingly recognized also after low to moderate doses of radiation in patients and occupationally exposed workers [32–48]. The 3 types of brain cancer associated with chronic low-dose radiation exposure are acoustic neuroma, meningioma, and glioblastoma. Vestibular schwannomas (also known as acoustic neuromas) are benign, slow-growing tumors of the eighth cranial nerve, that arise from Schwann cells ensheathing the vestibular portion of the vestibulocochlear nerve. Ionizing radiation exposure is a recognized risk factor for schwannomas [43–46].
Meningioma is the most frequent type of brain cancer and is highly radiosensitive. Meninges are made of highly proliferating tissue, and host neural precursor cells with high mitotic activity. Meningiomas are mostly benign tumors originating from arachnoid cap cells [47–56]. Glioblastoma originates from somatic mutations in neural stem cells and glial precursor cells. Repeated head CT with whole-body exposures of 5 mSv to 10 mSv are associated with brain organ dose exposure in the range of 50 mSv to 100 mSv per scan [57]. A large-scale study of close to 1 million patients with CT at a pediatric or young age shows a significant linear dose—response relationship for all brain cancers and gliomas individually [58]. Clusters of mostly left-sided meningioma and glioblastoma have been described by interventional radiologists [59–61]. The famous cardiac surgeon and interventional cardiologist who pioneered hybrid coronary revascularization techniques Edward B. Diethrich developed a brain tumor (oligodendroglioma) in 2012, as well as cataracts in both eyes and dense, calcified plaque in his carotid artery [62]. He then made it his mission to educate other healthcare workers about the dangerous effects of radiation, joining the Organization for Occupational Radiation Safety in Interventional Fluoroscopy to release a documentary describing the effects that radiation had upon him [63].
Interventional neuroradiology with endovascular procedures such as the closure of cerebral aneurysms involves a brain dose exposure between 500 mSv and 1,000 mSv and therefore also patients may develop brain cancer after diagnostic and therapeutic exposure [64].
A 2019 meta-analysis of 26 studies supports a significant association between dental X-rays and thyroid cancer and meningioma [56], but recent large-scale studies and meta-analysis failed to find an association between diagnostic X-rays and brain cancer [65].
Retinoblastoma is a malignant tumor of the developing retina that occurs in children, usually before age five years. Retinoblastoma may be unifocal or multifocal. About 60% of affected individuals have unilateral retinoblastoma with a mean age of diagnosis of 24 months; about 40% have bilateral retinoblastoma with a mean age of diagnosis of 15 months [66]. Maternal or paternal gonadal exposure (with radiologic or nuclear medicine diagnostic examinations) in the pre-conception time (9 weeks before conception in males) increases 3-fold to 6-fold the risk of retinoblastoma resulting from new germline mutation in offspring [67, 68]. This finding is of potential concern for interventional cardiologists since their cumulative gonadal exposure in a professional lifetime can range between 0.5 Sv and 1 Sv [69, 70].
Radiation exposure increases cardiovascular disease and particularly cerebrovascular disease risk. This has long been known for radiotherapy doses. Still, now evidence has also accumulated with moderate (100–500 mSv) and low (< 100 mSv), and the atherosclerotic effect is considered proven for the dose ranges common for interventional cardiologists and for patients exposed to repeated head and neck radiology examinations.
From the epidemiologic viewpoint, large-scale meta-analyses have conclusively proven on millions of patients that the risk of cardiovascular disease is of the same order of magnitude as radiation-induced cancer risk [71–74]. This risk is detectable at low doses, and probably low dose rates are even more dangerous for their pro-atherosclerotic effects than high-dose acute administration. Radiation is a risk factor for atherosclerosis and cardiovascular disease, especially cerebrovascular disease [75]. The level of risk is less uncertain at lower doses and lower dose rates, with the possibility that risk per unit dose is greater at lower doses and dose rates [76]. Recently, Cha et al. [77] found limited evidence in support of a positive association between occupational radiation exposure and the overall risk of circulatory disease over a short follow-up period among diagnostic medical radiation workers in South Korea. However, the excess relative risk of morbidity from circulatory diseases was significantly higher in female workers compared to male workers, and for cerebrovascular diseases compared to other subtypes of circulatory diseases [77]. In another study using data from the UK National Registry for Radiation Workers cohort, Hinksman et al. [78] identified an increased risk of mortality from cerebrovascular disease related to low-doses of ionizing radiation. This finding supports the notion that radiation exposure may significantly affect cerebrovascular health at doses lower than those currently recommended by ICRP protection guidelines [78].
The epidemiological findings are supported by molecular epidemiology and imaging studies that reveal alterations in intermediate and preclinical markers of vascular aging and early atherosclerosis. These include telomere shortening in circulating leukocytes and early thickening of carotid intima-media thickness observed in exposed interventional cardiologists [79]. Telomere shortening is a well-known hallmark of both cellular senescence and organismal aging. Current research consistently indicates that telomere dysfunction plays a key role in the progression of age-related vascular diseases, particularly coronary atherosclerosis, myocardial infarction, ischemic heart disease, and stroke [80, 81]. DNA damage and critically shortened telomeres can induce senescence in both vascular endothelial and smooth muscle cells resulting in an enhanced pro-inflammatory response and endothelial activation [81].
In vascular cells, ionizing radiation can initially induce irreparable DNA damage and telomere erosion, which activate permanent DDR-inducing vascular cellular senescence [82]. This, in turn, increases senescence-associated secretory phenotype signals, promoting a heightened pro-inflammatory response within the vessel wall and accelerating the progression of atherosclerosis, ultimately resulting in cardio-cerebrovascular complications [82].
Systematic reviews and meta-analyses concordantly show an association between ionizing radiation exposure and increased risk for neurodegenerative disease for both patients and doctors. Srivastava et al. [83] identified five types of exposed populations: 1. survivors of atomic bombings in Japan; 2. patients treated with radiation therapy for cancer or other diseases; 3. occupationally exposed workers; 4. those exposed to environmental radiation; 5. patients exposed to radiation from diagnostic radiation imaging procedures. The meta-analysis of over 18 studies shows an increased risk (of 10–17% for every 100 mSv exposure) for Alzheimer’s disease incidence and mortality [83]. The pooled analysis included the One Million Worker study including nuclear power plant workers, industrial radiography workers, exposed military personnel, and 109,019 medical radiation workers with 2,779,838 person-years of follow-up, with a median exposure dose of 9.8 mSv and only 1% with an exposure > 100 mSv. The association remained significant when considering only Parkinson’s disease mortality as a distinct outcome [84]. For cognitive effects, the detrimental effects of low-dose exposure are even more marked than acute high-dose exposures [85, 86]. The epidemiological data are also corroborated by molecular epidemiology and functional testing data showing changes in intermediate proximal, preclinical biomarkers of neurodegeneration such as brain-specific microRNA expression and early neurocognitive changes with reduction of olfactory acuity in exposed interventional cardiologists [87–89]. The brain-specific microRNA-134 is involved in synapse development and is directly connected to learning and memory. It has been shown to be dysregulated in conditions such as Alzheimer’s disease, bipolar disorder, oligodendrogliomas, and glioblastomas [90]. Notably, a recent study provided the first evidence that the microRNA-134-mediated post-transcriptional regulation of cAMP response element-binding protein 1 (CREB-1) and brain-derived neurotrophic factor (BDNF) is an important molecular mechanism underlying the plasticity deficit in Alzheimer’s disease, underscoring the critical role of miR-134-5p as a potential therapeutic target for restoring plasticity in Alzheimer’s disease condition [91]. Given its significant dysregulation in interventional cardiologists, it strongly indicates that brain damage could be a major long-term risk of unprotected head irradiation, potentially leading to enduring cognitive impairments [87].
Long-term, low-dose-rate radiation exposure early in life might cause subsequent psychological stress and an increased risk of depression decades later. The radiation-induced dysfunction of the cortico-limbic system in the left dominant hemisphere of the human brain with specific involvement of the impaired neurogenesis in the hippocampus is considered to be the key cerebral basis of post-radiation organic brain damage [92, 93]. The risk of diseases rises with radiation dose. Radiation risks are revealed for organic psychoses, non-psychotic organic brain damage, and acute and chronic cerebrovascular pathology in exposed populations such as Chernobyl liquidators [94–98]. Although there is a tremendous biological complexity and multifactorial nature of these symptoms, from stress to sleep deprivation to reduced sunlight exposure, the possibility that these mood disorders exist and have a plausible biological mechanism should be considered in planning future studies and looking for the missing convincing evidence. X-ray stimulation induced the generation of reactive oxygen species in the prefrontal cortex in a dose-dependent manner, leading to the occurrence of depression-like behaviors in the mice, resulting in neuron death triggered by pro-inflammatory signals both in vivo and in vitro [99]. In a survey by Andreassi et al. [100] in interventional cardiologists, mood disorders such as anxiety/depression were 6 times more frequent in exposed professionals compared to unexposed controls.
Despite the abundant evidence of detrimental long-term health effects of chronic low-dose, low-dose-rate radiation exposure, radiation unawareness, and suboptimal protection have been for a long time the rule rather than the exception in the catheterization laboratory [101–103]. Genuine lack of information, additive work discomfort and orthopedic strain connected to wearing protection, dose limits perceived as a barrier to personal productivity, and underestimation of radiation risk also encouraged by limited direct evidence in medically exposed populations contributed to exposure above and beyond all regulatory limits of a first generation of invasive cardiologists now suffering an epidemic of radiation-induced damage. Now it is mainstream knowledge that a culture of respect for radiation hazards is needed to enter the cardiac catheterization laboratory [104–105] and the primary duty of the invasive cardiologist must protect the operators, the patients, and the staff from the negative effects of chronic occupational exposure to radiation [106]. These effects include significant effects on the eye, brain cancer, cerebrovascular disease, neurodegeneration, and mood disorders.
Known, proven, and uncertain, possible eye and brain effects of radiation exposure have all the same therapy: prevention through protection. Therefore, a legitimate question is how, not if and when, to protect the head of invasive cardiologists from ionizing radiation (Table 3).
Protection of the head of interventional cardiologists
| Types of protection | Logistics | Protection | Comfort |
|---|---|---|---|
| Cap, collar, glasses | Simple | 50% to 90% | Low |
| Suspended ceilings | Complex | > 90% | Intermediate |
| Cabin | Advanced | 99% | High |
| Zero radiation | Very advanced | 100% | Very high |
Once a culture of respect towards radiation hazards has been built, the therapy is simple: to apply the principle of justification, dose optimization, and responsibility embedded in the law and to protect ourselves and the staff most efficiently. There are now many different effective solutions, although probably not all equally effective: the leaded cap, the suspended ceiling, the cabin, and even robotic intervention with remote control from the operator away from the patient and the radiation source [107–118]. There is also the most radical and effective solution to move from ionizing radiation imaging to zero-fluoroscopy and ionizing radiation-free methods, now the standard state-of-the-art in cardiac electrophysiology. Each protection system has different logistics, learning curve, cost, and efficacy, and the best solution may vary according to these variables, but the worst solution is to ignore the radiation issue important for the safety of doctors and patients. The final protection is the progressive shift to zero radiation as it has already been achieved for electrophysiology procedures [119–122]. In the meantime, careful optimization practices can reduce 10-fold the dose to patients and doctors in the catheterization laboratory [123]. The radiation exposure is dramatically reduced with intravascular ultrasound complementing or replacing fluoroscopy and fluorography [124]. Robotic interventional cardiology allows one to operate at a distance with remote control of catheters [125]. The race to near-zero radiation cardiology continues.
In summary, epidemiological evidence suggests a connection between exposure to ionizing radiation and an increased risk of brain cancer, cerebrovascular disease, and neurodegenerative disorders in both patients and doctors. These findings are supported by molecular epidemiology and imaging studies that reveal early changes in intermediate and preclinical disease markers. Despite these risks, effective protective measures such as the use of lead-lined ceilings, curtains, and specialized eyewear can significantly minimize radiation exposure to near-zero levels.
However, large-scale studies that involve individual dose reconstruction and thorough data collection on potential confounding factors are necessary to more accurately assess the risk of disease. Future research should investigate the extent of brain effects resulting from the interplay of various traditional and environmental risk factors, particularly the potential synergistic effects between pollution and medical radiation exposure.
Detailed research into the biological mechanisms by which ionizing radiation impacts brain cells is also essential. For instance, employing brain organoids to study neuroinflammation, DNA damage, telomere attrition, and mitochondrial dysfunction could significantly enhance our understanding of these processes. Since genetic research has provided evidence of variability in individual responses to ionizing radiation—demonstrating that some individuals are more susceptible than others—hiPSC disease modeling derived from selected donors may reveal important gene-environment interactions that exacerbate cerebrovascular and neurodegenerative disorders, predisposing certain individuals to adverse outcomes. This approach will be crucial not only for deepening our knowledge of brain disease risks following low-dose ionizing radiation exposure but also for developing effective protective strategies.
3D: three-dimensional
DDR: DNA damage response
hiPSCs: human induced pluripotent stem cells
ICRP: International Commission on Radiological Protection
mSv: millisieverts
AB: Writing—original draft, Conceptualization, Validation, Supervision. The author read and approved the submitted version.
Andrea Borghini who is the Editorial Board Member of Exploration of Cardiology had no involvement in the decision-making or the review process of this manuscript.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4414
Download: 288
Times Cited: 0
