Affiliation:
1Diagnostic Medical Imaging, Diagnostic Centre, Unimed Porto Alegre, Porto Alegre 90.510-002, Brazil
Email: mtorres.mt10@gmail.com
ORCID: https://orcid.org/0000-0002-5357-9138
Affiliation:
2Hospital de Clínicas de Porto Alegre, Porto Alegre 90.035-903, Brazil
ORCID: https://orcid.org/0000-0001-6516-7926
Explor Cardiol. 2024;2:265–279 DOI: https://doi.org/10.37349/ec.2024.00039
Received: July 19, 2024 Accepted: September 26, 2024 Published: November 14, 2024
Academic Editor: Andrea Borghini, Institute of Clinical Physiology - National Research Council (IFC-CNR), Italy
The article belongs to the special issue Multimodality Imaging in Ischemic Heart Disease
Pulmonary congestion is a key determinant of heart failure, but for a long time it has been an elusive target for the clinical cardiologist in the pre-B-line era, despite research efforts of Carlo Giuntini, a pneumologist who attempted the quantification of lung water in the seventies with too insensitive chest X-ray lung water score, too cumbersome nuclear medicine, and too complex invasive thermodilution techniques. Daniel Lichtenstein, is a French intensivist who first discovered lung ultrasound as a sign of extravascular lung water in 1997. B-lines (also known as ultrasound lung comets) detectable by lung ultrasound arise from the pleural line, extend towards the edge of the screen, and move synchronously with respiration. In cardiology, B-lines were introduced in 2004 and are now the dominant technique for research applications and clinical purposes. B-lines showed a prognostic value in several clinical scenarios, largely independent and additive over echocardiographic predictors such as ejection fraction. The methodology became user-friendly in the last years, with a reduction of the scanning sites from the original 28 to a simplified 4-site scan now extracting information on lung water in < 1 minute. More recently, B-lines were also studied during physical and pharmacological stress. Signs of pulmonary congestion are found during stress in 1 out of 3 all-comers with normal findings at rest. Artificial intelligence applied to ultrasound and clinical data allows for the detection of B lines, their quantification, and the assessment of their nature. The B-lines phenotype can cluster around different endotypes: dry (in systemic sclerosis and lung interstitial fibrosis); wet (water); sterile (as in cardiogenic edema); infective (as in COVID-19 and interstitial pneumonia); right heart-sided (as in pulmonary arterial hypertension); left-heart sided (as in heart failure or valvular heart disease). Artificial intelligence B-lines and pocket-size insonation of the B-lines-driven decongestion therapy are now on the horizon.
Alveolar gas and blood are separated by an extremely thin membrane, and their anatomic and functional integrity is instrumental for gas exchange between air and blood [1]. An excess rise in pulmonary capillary pressure provokes failure of the alveolar-capillary barrier, with disruption of the capillary endothelium and alveolar epithelium and impairment of gas exchange with an increase in pulmonary congestion and extravascular lung water [2]. The increase in lung water has a detrimental effect on gas exchange, worsens prognosis, and represents an actionable therapeutic target. The identification of extravascular lung water is an old dream of the clinical cardiologist and yet it remained an elusive clinical target for decades. The first systematic attempts to detect and quantify pulmonary congestion used chest X-rays, still employed today for the evaluation of pulmonary congestion in a qualitative or semiquantitative way [3]. A radiologic lung water score includes Kerley A, B, and C lines, hila dimension, haziness and density, subpleural effusion, perihilar haze, micronodules, and widening of interlobar fissures (Table 1). The score assignment of a given sign depends on the entity, stratified into 3 (mild, moderate, or severe) or 2 (mild or severe) degrees as originally described by Giuntini et al. [3]. The cumulative score is obtained by adding the maximum severity scores for each variable. When all signs are absent, the score is 0 (normal dry lung). When all signs are present and severe, the cumulative score is 111 (wet lung with full-blown pulmonary edema).
Lung water in clinical practice: chest X-ray score
| Variable | Mild | Moderate | Severe |
|---|---|---|---|
| Diffuse increase in density | 5 | 10 | 15 |
| Extensive peri-hilar haze | 4 | 8 | 12 |
| Peribronchial cuffs | 4 | 8 | 12 |
| Widening interlobar fissures | 4 | 8 | 12 |
| Subpleural effusion | 5 | 10 | |
| Blurred hilar vessels | 3 | 6 | 9 |
| Increased density of hilar vessels | 2 | 4 | 6 |
| Enlarged hilar vessels | 1 | 2 | 3 |
| Micronodules | 4 | 8 | |
| Kerley A lines | 4 | 8 | |
| Kerley B lines | 4 | 8 | |
| Kerley C lines | 4 | 8 |
Blank cells indicate not applicable
The cumulative radiologic score served clinical cardiologists for a long time, although structurally limited by poor sensitivity, operator dependence, and subjectivity [4].
Although chest X-ray was the only practical noninvasive method available to the clinical cardiologist, there were also other methods of measuring lung water. Their use is more limited to patients admitted to the intensive care unit since they often require a central venous and arterial catheter to obtain the different hemodynamic variables. Still, they have the advantage of being portable methods that can be used repeatedly. The PiCCO™ device (Pulsion, Medical Systems) already allowed the quantification of the extravascular lung water index in the pre-ultrasonography era, using the transpulmonary thermodilution technique, thus providing information on the magnitude of the edema and allowing its evolution to be monitored [3, 4].
Lung ultrasound (LU) was first proposed for the diagnosis of interstitial pulmonary syndrome and pulmonary congestion by the French intensivist Daniel Lichtenstein in 1997 [5]. B-lines (also known as ultrasound lung comets) were introduced for the detection of extravascular lung water in heart failure patients in 2004 [6]. In the hands of cardiologists, LU rapidly evolved in a decade from the status of promising research innovation [7] to the rank of established clinical tool primarily used for the detection and quantification of pulmonary congestion [8, 9].
With LU, 2 signs indicate lung water accumulation: B-lines and pleural effusion. B-lines are usually observed in the third intercostal space with the 4-site simplified scan, symmetrically on the left and right hemithorax, from mid-axillary to anterior axillary, and from anterior axillary mid-clavicular lines, bilaterally and symmetrically. These regions are the wet spots where water preferentially accumulates in cardiac patients [10]. Quantification is also simple, since with the 4-site simplified scan the normal finding (adding up the B-lines of all 4 sites) is 0 to 1 line, with mild, moderate, and severe accumulation of lung water corresponding to 2–4, 5–9, and ≥ 10 B-lines [10] (Table 2). These criteria can also be applied during stress when B-lines, compared to rest, are more frequent and more severe in patients with overt or latent heart failure [11]. For each of the 4 sites, a six-second clip is recorded and analyzed online or offline. The highest number of B-lines in a single intercostal space is counted and then summed across all four spaces. Each site has a possible score from 0 (black lung) to 10 (white lung), generating a total score of all 4 chest zones from 0 (all 4 sites with 0 individual site scores) to 40 (all 4 sites with individual site scores of 10) [10]. The B-line score integrates both the horizontal extent and vertical depth of pulmonary congestion. The horizontal extent is represented by the number of sites with B-lines, while the vertical depth is indicated by the number of B-lines at each site.
Lung water by lung ultrasound
| Lung water | Trivial | Mild | Moderate | Severe |
|---|---|---|---|---|
| B-lines (4-site) | 0–1 | 2–4 | 5–9 | ≥ 10 |
| Pleural effusion (mm) | < 2 | 2–15 | 15–25 | > 25 |
There are other classifications to determine pulmonary edema in this methodologically deregulated field with 1, 2, 4, 8, 28, or 72 regions [6–9], but all share the concept of building an integrated severity score by combining the number of B-lines per region with the number of scanned sites (more frequently 4 or 8). In cardiology, the explored regions are only 4 for time-sparing needs at rest and peak stress imaging; the 4 selected regions are those more likely to show cardiogenic, watery B-lines; only the third intercostal space is scanned, so that the appearance of normal B-lines in volunteers without disease due to lung base atelectasis or horizontal lung fissures is less likely. The 4-site scan method is the only one adopted in multicenter trials and prognostically validated versus outcome. B-lines can indeed be a normal variant found in volunteers without baseline respiratory/cardiology disease, but these findings are rare in the third intercostal space now preferred for a cardiological simplified scan. In some locations, 1 or 2 B-lines are physiological, for instance, anteriorly corresponding to lung fissures or at the bases (likely natural gravity) [6–9].
Pleural effusions are another sign of pulmonary congestion, even simpler to detect than B-lines. Pleural effusion is searched with the sitting patient at the base, along the scapular or posterior axillary line. With pleural effusion, the normal finding is the absence of an echo-free pleural space, and the progressive increase of the echo-free space is directly related to the amount of pleural effusion [12]. A pleural effusion < 15 mm is too small to tap, and a pleural effusion > 25 mm corresponds to at least 500 mL. LU is recommended for the guidance of therapeutic thoracentesis, safer and simpler with ultrasound. Cardiogenic pleural effusions are typically bilateral or right-sided, though left-sided effusions are not uncommon. These effusions occur due to increased pulmonary capillary wedge and/or central venous pressure. The resulting rise in interstitial fluid heightens the lymphatic system’s safety factor, normally draining 20 mL of fluid daily under physiological conditions. This capacity can increase up to 20-fold until the compensatory fluid reabsorption is exceeded, leading to pleural effusion. This condition exacerbates and progresses to heart failure. Therapeutic thoracentesis can enhance ventilatory exchanges and partially correct hypoxemia, providing immediate symptomatic relief [13]. Additionally, it alleviates the restrictive physiology of heart filling and low cardiac output often associated with significant pleural effusion. Consequently, therapeutic thoracentesis can promptly improve symptoms, lung function, and myocardial performance, breaking the cycle of increased pleural pressures, reduced lymphatic drainage, restrictive cardiac physiology, decreased lung ventilation, and impaired tissue oxygenation that leads to worsening pleural effusion and heart failure. It is a straightforward and frequently effective procedure to offer immediate relief from dyspnea and partially restore diuretic response in cases of refractory congestive heart failure. A randomized multicenter, open-label, controlled trial is currently underway to demonstrate the benefits of therapeutic thoracentesis in acute heart failure [14].
B-lines do not identify a disease, but a condition associated with multiple causes such as cardiogenic acute pulmonary edema, acute respiratory distress syndrome, pneumonia, or any chronic interstitial disease. Despite these caveats, the technique is now the best one available for bedside detection of pulmonary congestion (Table 3).
Pulmonary congestion hemodynamic, radiologic, and LU correlates
| Pulmonary congestion | PCWP (mmHg) | Chest X-ray | LU | Lung water (mL) |
|---|---|---|---|---|
| Normal | < 12 | Score 0 | A-lines | < 500 |
| Initial congestion | 12–17 | Score 1–20 | 2–4 B-lines | 500–1,000 |
| Interstitial edema | 17–25 | Score 21–50 | 5–9 B-lines | 1,000–1,500 |
| Alveolar edema | > 25 | Score > 50 | ≥ 10 B-lines | > 2,000 |
LU: lung ultrasound; PCWP: pulmonary capillary wedge pressure
For any given capillary wedge pressure, the permeability of the alveolar-capillary barrier may change acutely (over minutes) or chronically (over days or months) for several extra-hemodynamic factors. These factors may ‘pull’ water within the vessel such as oncotic and osmotic forces (albumin or sodium), or ‘push’ water extraluminally for enhanced permeability due to inflammation [15] or reducing lymphatic drainage (chest radiotherapy) (Figure 1). In heart failure, the amount of lung water for a given pulmonary capillary wedge pressure can vary due to hemodynamic and extra-hemodynamic factors that disrupt the Starling equilibrium in the alveolar-capillary barrier. The leakage of lung water into the interstitium increases with a sudden elevation in pulmonary capillary wedge pressure, particularly without adaptive responses seen in chronic conditions such as increased lymphatic drainage. Additionally, lung water accumulation is exacerbated by elevated central venous pressure, which impairs lymphatic drainage efficiency from the lung interstitium to the central venous system. Furthermore, the development of pulmonary edema is promoted by low plasma albumin levels, reducing oncotic forces, and low plasma sodium levels, reducing osmotic forces, both of which cause water to leave the vessels. The increased ambient air pollution may increase the oxidative stress and the permeability of the alveolar-capillary barrier to edema [16].
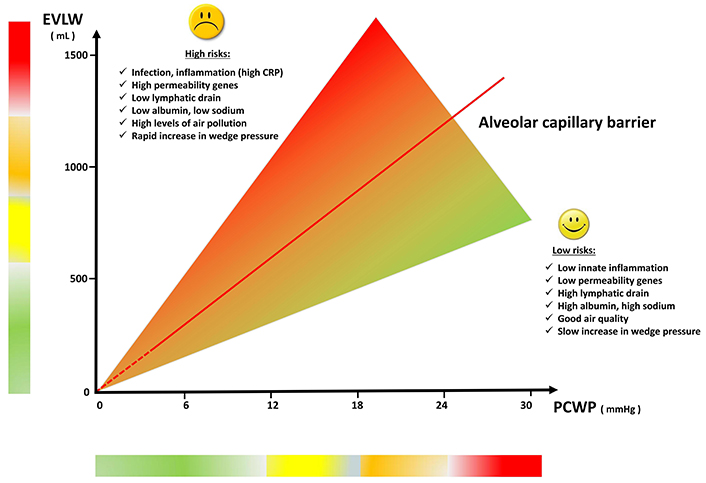
Modulators of lung water accumulation for any given capillary wedge pressure. EVLW: extravascular lung water; PCWP: pulmonary capillary wedge pressure
Even in the absence of left heart failure, pulmonary congestion may occur through an entirely venous, right-sided mechanism. The increase in systemic venous pressure is determined by post-capillary but also by purely pre-capillary pulmonary hypertension, with normal left heart function. The dominant mechanism of lung water accumulation in left heart failure is increased fluid filtration. The prevailing mechanism for right heart failure is decreased fluid efflux due to impaired lymphatic drainage caused by high resistances due to the increased systemic venous pressure in the inferior vena cava accepting lymphatic flow from the lung [17]. B-lines are also detectable with a normal left heart, in the presence of systemic venous hypertension [18].
A very good correlation is shown between B-lines and extravascular lung water evaluated experimentally by gravimetry in pigs with acute lung injury induced by oleic acid and clinically by thermodilution in patients with acute lung injury-acute respiratory distress syndrome, showing that in a controlled setting even mild degrees of congestion (2–4 B-lines) corresponds to initial pulmonary congestion (for a review of individual studies, see [8]). This level of mild congestion also corresponds to a significant impact on outcome. The 2-year hard event rate in a population of all comers submitted to stress echo is 3% for patients without B-lines, 4% in those with mild (2–4) B-lines, 8% with moderate (5–9) B-lines, and 20% in those with severe peak stress (≥ 10) B-lines [11].
Quantification of pulmonary congestion is indispensable for diagnosing heart failure, conducting risk stratification, and guiding therapy guidance. However, it remains unattainable through physical examinations based solely on lung auscultation or body weight. Historically, chest X-ray served as the primary screening tool for detecting pulmonary congestion before the advent of LU. Nonetheless, the interpretation proves challenging and imprecise, exhibiting high interobserver variability. It is the simplest of all methods, although less accurate than other more advanced and complex imaging methods such as chest CT (computed tomography), magnetic resonance imaging, nuclear medicine, or invasive methods based on the double-indicator thermodilution method.
The emergence of LU in cardiology in 2004 has overshadowed the clinical and research utility of other methods [19]. In 2023, the number of papers with LU exceeded the number of publications with all the other techniques combined: positron emission tomography, single-photon emission tomography, CT, and magnetic resonance imaging. In addition, these techniques require transfer to the radiology department, and only LU and chest X-rays are applicable at the bedside. Although the radiation exposure of a single chest X-ray is modest compared with a chest CT or a nuclear medicine test equivalent to 400 chest X-rays, the assessment of pulmonary congestion must be repeated sometimes day by day, or even within hours in critically ill patients. As shown in Table 4, LU is always useful to minimize radiation exposure in all ages of life, especially when the damaging effects of radiation are highest, such as children, and pregnant women (for the potential damage to children) and women [9]. Compared to physical examination based on pulmonary crackles at the lung bases and peripheral edema, LU is more sensitive and accurate and less dependent on the operator’s experience [15]. LU is characterized by much less environmental impact, with carbon dioxide emissions of 1 kg per scan compared to 20-fold higher emissions of nuclear medicine and CT and 50-fold higher emissions of magnetic resonance. It is a bedside technique, with a cost of 10 to 1,000-fold lower than positron emission tomography [19].
The main non-invasive imaging techniques for extravascular lung water
| Imaging techniques | Chest X-ray | Chest CT | CMR | PET-SPECT | LU |
|---|---|---|---|---|---|
| Relative cost | 1 | 10 | 20 | 100 | 1 |
| Radiation | 1 | 200 | 0 | 250–500 | 0 |
| CO2 (kg) | 1 | 20 | 50 | 20–30 | 1 |
| Bedside | Yes | No | No | No | Yes |
CMR: cardiovascular magnetic resonance; CT: computed tomography; LU: lung ultrasound; PET: positron emission tomography; SPECT: single-photon emission tomography. Radiation is expressed in multiples of chest X-ray (postero-anterior projection)
In a 2019 statement of the European Respiratory Society, it is concluded that LU will become more frequently used in patients with acute respiratory failure, and future studies will assess if the information provided will improve clinical management and outcomes [20].
The use of LU and B-line quantification directly impacts clinical outcomes, patient management, and decision-making in heart failure and other conditions such as respiratory tract infections or neonatal dyspnea.
For a cardiologist, LU is an add-on to transthoracic echocardiography (TTE), just as lung auscultation is part of cardiac physical examination. A cardiac 2.5–5.0 MHz transducer is generally suitable since the small footprint makes it ideal for scanning intercostal spaces. Patients are examined in the supine or semi-recumbent position with the cardiac transducer at an imaging depth of 16 or 18 cm in sagittal orientation (perpendicular to the ribs).
Over the years, LU scanning for cardiologists underwent a marked simplification, with fewer regions to scan meaning shorter imaging time and simpler analysis. In 10 years, the initial 28-site scan at rest and peak stress [6, 21–23], and even 72-site including posterior chest for interstitial lung fibrosis [24], has become the 4-site simplified scan, starting from the observation that some wet sites are the first to be involved by pulmonary congestion and those with highest B-lines. In practical terms, this means that if you scan only 4 regions instead of 28 or 72 you do not miss any significant information. The additional scan time for a TTE to add a LU is < 1 min, and in this way, LU becomes a clinically practical tool rather than a research toy, passed through the multicenter study validation as a feasible and simple tool easily performed by all laboratories at rest and during stress [11, 25].
A 4-site simplified scan is adopted, including only the “wet spots” with most B-lines, in the third intercostal space, symmetrically in the right and left hemithorax: from mid-axillary to the anterior axillary line, and from anterior axillary to the midclavicular line (‘Ma’am’ as a memory trick) [26].
B-lines are a truly universal sign of pulmonary congestion, applicable to all experimental models and to different specialties when pulmonary congestion is an issue. B-lines have been found in rats, cows, dogs, rabbits, pigs, and horses, in experimental models of acute pulmonary distress syndrome and cardiogenic pulmonary edema [27–33]. B-lines are now extensively used not only in the original fields of intensive care [34] and pneumology [35, 36]. They are especially useful in pediatrics to avoid the radiation burden inherent to chest X-rays and chest CT for the diagnosis of interstitial pneumonia (including COVID-19) and lung disease in children [37, 38]. LU is always useful to minimize radiation exposure in all ages of life, especially when the damaging effects of radiation are highest, such as children, and pregnant women (for the potential damage to the fetus) and women. The risk of an adult is 4 times higher in a child, 38% higher in women, and highest in the embryo or fetus.
In cardiology, B-lines have been observed in virtually all diseases, from heart failure across all values of resting ejection fraction [39–42] to valvular heart disease [43–45] and hypertrophic cardiomyopathy [46]. B-lines are currently used in nephrology to titrate dialytic therapy [47–50]. B-lines are frequently observed in sports medicine to identify pulmonary congestion during extreme physiology settings such as apnea diving or strenuous efforts in marathon runners or triathletes, or altitude trekkers to detect high altitude pulmonary edema at a preclinical stage [51–66]. Dry, fibrotic B-lines characteristically located in the lung bases in the posterior lung are very frequent and prognostically unfavorable findings in interstitial lung fibrosis, systemic sclerosis, and other connective tissue diseases [67, 68].
B-lines are not specific to a single disease, and they represent rather a peculiar feature (the B-lines phenotype) shared by different conditions (Figure 2). B-lines can be wet (composed of water) or dry (composed of connective tissue as in connective tissue disease associated with systemic sclerosis and interstitial lung fibrosis). Wet B-lines can be categorized as sterile (hydrostatic cardiogenic pulmonary edema or acute respiratory distress syndrome) or infective (inflammatory exudates in viral or bacterial pneumonia). Cardiogenic pulmonary edema can have a left-sided origin (in valvular heart disease or left-heart disease with heart failure with either reduced or preserved ejection fraction) or a purely right-sided origin, for instance in pulmonary arterial hypertension with right ventricular-pulmonary artery uncoupling. All these conditions can usually be easily differentiated based on the clinical presentation, the physical examination, and the peculiar TTE or LU findings. With TTE, the heart is normal for morphology and function in acute respiratory distress syndrome, infective disease, or systemic sclerosis. The ejection fraction is reduced in heart failure with reduced ejection fraction, with normal ejection fraction but altered indices of diastolic function in heart failure with preserved ejection fraction: E/e’ > 15, dilated left atrium volume index, increased estimated systolic pulmonary systolic pressure, and possibly reduced left ventricular global longitudinal strain. In patients with acute and chronic heart failure, B-lines are correlated with cardiac natriuretic peptide levels, and can usefully replace or complement them for the diagnosis of cardiogenic origin of acute dyspnea, since LU does not suffer from biological variability, slow time course, poor specificity, cost, and venipuncture of cardiac natriuretic peptides.
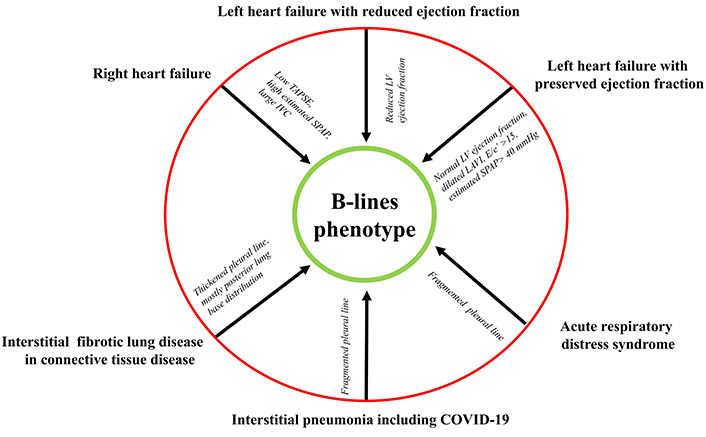
The B-lines phenotype shared by different conditions. IVC: inferior vena cava; LAVI: left atrium volume indexed; LV: left ventricle; SPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion
The pleural line is regular and thin in heart failure, irregular and fragmented in acute respiratory distress syndrome, irregular and thickened in interstitial lung fibrosis, and irregular in interstitial pneumonia. The use of linear probes with higher frequencies can help to study the pleural line more accurately [9].
The clinical presentation of cough and fever are the presenting symptoms of COVID-19 or interstitial pneumonia, with a typical pleural line fragmentation while the pleural line is smooth in cardiogenic pulmonary edema. The wet right-sided B-lines are characterized by a normal left ventricle but a dilated right ventricle, with dilated inferior vena cava and high estimated systolic pulmonary arterial pressure [18, 69]. It is the accumulation of water in the kitchen sink. This can occur due to an increased flow of water from the faucet caused by higher upstream pressure (left heart failure) with a normal drainage system, or due to a normal flow from the faucet but water accumulation caused by an obstruction in the drain trap (right heart failure with normal left heart function). In both cases, water accumulates in the sink, and the lungs exhibit B-lines.
The effectiveness of diuretic therapy guided by resting B-lines has been validated in three randomized trials, with a number needed to treat between 5 and 7 in heart failure patients [70–73]. In with end-stage renal disease on dialysis, B-lines guided therapy over 24 months of follow-up showed a steady reduction in pulmonary congestion and decreased hypotensive episodes during dialysis [74]. Whether using diuretics or dialysis in heart failure, B-lines guided therapy outperforms conventional therapy in gently reducing lung congestion, resulting in better outcomes. However, the advantage of B-lines guided therapy for stress B-lines, which theoretically reflect pulmonary congestion during daily activities more accurately than rest B-lines, still needs to be demonstrated.
B-lines assessment both at rest and during stress is increasingly recognized as crucial for diagnosing heart failure, gauging its severity, evaluating therapy effects, and refining risk stratification, as acknowledged by recent guidelines and cardiology recommendations [75–79]. According to the universal definition of heart failure by the European Society of Cardiology in 2021, diagnosis relies on the presence of heart failure symptoms and signs confirmed by elevated natriuretic peptides or objective evidence of cardiogenic pulmonary or systemic congestion [80]. Nowadays, TTE is only deemed complete with a concise yet effective assessment of lung B-lines, both at rest and during stress.
The assessment of B-lines and pleural effusion through LU is operator-independent when employing artificial intelligence, thereby enabling the distinction between the nature of B-lines (fibrotic versus congestive) and the determination of their origin (infective versus hemodynamic) [81, 82].
There are still barriers to implementing LU in clinical practice, especially the need to implement universal training across various specialties including ED (emergency department), intensive care providers, cardiologists, and neonatologists, although remote online web-based training modules and artificial intelligence-assisted acquisition and interpretation may enormously simplify the task [83, 84]. Fully automated robotic acquisition of LU images is possible with a remotely controlled, probe with a scanning time for a single person of less than 4 minutes [85]. Among several ongoing projects, in September 2023 the Bill and Melissa Gates Foundation awarded a 44-million-dollar project to create user-friendly, artificial intelligence-assisted ultrasound imaging auto-assessment tools across maternal and fetal care as well as pediatric lung health, intending to expand access to low-and-middle-income countries and across diverse sites of care. Among the commercially available solutions based on artificial intelligence, smart B-line or auto B-lines software allows to perform automatically time-consuming measurements and enables the real-time analysis of the obtained data. The presence of B-lines is also quantified as the number of B-lines per region or the percentage of area filled by B-lines in a given zone [86].
Of special relevance, LU application in pediatric populations for diagnosis of community-acquired pneumonia or neonatal acute respiratory failure will allow sparing radiation exposure due to chest X-ray and chest CT. It will also serve as a guide to surfactant therapy in neonates [87, 88].
B-lines by LU in cardiology are of special importance. Its interest is growing since it is a real-time imaging method, with easy acquisition, low cost, performed with portable equipment, and free of radiation, available in different settings, from the ED and intensive care units to outpatient clinics, for adults and pediatrics.
The existence of barriers to LU implementation in the routine evaluation and quantification of extravascular lung water is gradually being surpassed, and will very probably gain increasing acceptance.
CT: computed tomography
ED: emergency department
LU: lung ultrasound
TTE: transthoracic echocardiography
MART: Writing—original draft, Conceptualization, Validation, Supervision. NMdQ: Investigation, Visualization. Both authors read and approved the submitted version.
Both authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Marco Fabio Costantino ... Luisiana Stolfi
Cristhian Espinoza Romero ... Tatiana Torres Leal
Praveen Kumar Chandra Sekar, Ramakrishnan Veerabathiran
Emma Cerracchio ... Quirino Ciampi
