Affiliation:
1Department of Obstetrics and Fetal Medicine, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
2Department of Cardiology, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0002-9569-6551
Affiliation:
1Department of Obstetrics and Fetal Medicine, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
†These authors contributed equally to this work.
Affiliation:
2Department of Cardiology, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
ORCID: https://orcid.org/0000-0002-8654-7634
Affiliation:
3Department of Neonatal and Pediatric Intensive Care, Division of Neonatalogy, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
ORCID: https://orcid.org/0000-0002-7300-4577
Affiliation:
2Department of Cardiology, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
4Department of Intensive Care Medicine, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
ORCID: https://orcid.org/0000-0002-7897-4628
Affiliation:
1Department of Obstetrics and Fetal Medicine, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
ORCID: https://orcid.org/0000-0002-9206-5279
Affiliation:
4Department of Intensive Care Medicine, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
ORCID: https://orcid.org/0000-0002-2011-5169
Affiliation:
1Department of Obstetrics and Fetal Medicine, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
ORCID: https://orcid.org/0000-0001-8801-5546
Affiliation:
1Department of Obstetrics and Fetal Medicine, Erasmus MC, University Medical Center Rotterdam, 3000 CA Rotterdam, The Netherlands
Email: j.cornette@erasmusmc.nl
ORCID: https://orcid.org/0000-0001-8482-5138
Explor Cardiol. 2025;3:101244 DOI: https://doi.org/10.37349/ec.2025.101244
Received: August 26, 2024 Accepted: December 05, 2024 Published: January 13, 2025
Academic Editor: Quirino Ciampi, Fatebenefratelli Hospital of Benevento, Italy
The article belongs to the special issue Cardiovascular Risk for Mothers and Offspring Resulting from Complicated Pregnancy
Data on the use of mechanical circulatory support devices in pregnant women are limited. A 27-year-old woman at 27 weeks and 6 days of gestation was supported by three different mechanical circulatory support devices due to cardiogenic shock. She came into spontaneous labor, which was complicated by major hemorrhage at the cannulation site, fetal distress, and transverse position, requiring emergency cesarean section. The postpartum period was complicated by intra-abdominal bleeding and arterial occlusion of lower extremity. When using mechanical circulatory support devices in pregnant women, a multidisciplinary approach is recommended.
Cardiogenic shock in pregnancy is a life-threatening condition, characterized by inadequate cardiac output leading to organ hypoperfusion. It is associated with a high risk of maternal and fetal morbidity and mortality [1]. When pharmacological therapy is insufficient, mechanical circulatory support devices should be considered to support hemodynamics. Short-term mechanical circulatory support devices, including veno-arterial extracorporeal membrane oxygenation (VA-ECMO), intra-aortic balloon pump (IABP), and Impella improve organ perfusion. They are used as a “bridge to recovery” [2] (Figure 1). The last two decades these devices are used more commonly, but experience in pregnancy remains limited [3]. Our case report describes the use of VA-ECMO, IABP, and an Impella device in a woman with peripartum cardiogenic shock. The challenges associated with the use of these devices in pregnant women are highlighted.
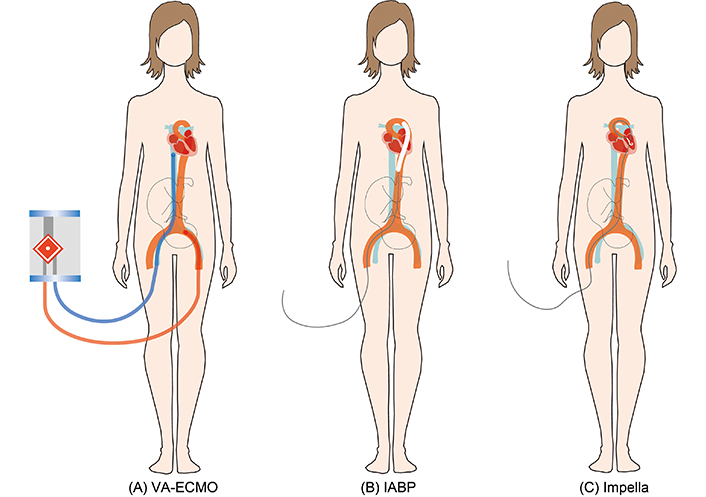
Mechanical circulatory support devices in cardiogenic shock. (A) VA-ECMO. The blue line represents the cannula inserted in the femoral vein, and the red line represents the cannula inserted in the femoral artery. (B) IABP. (C) Impella. VA-ECMO: veno-arterial extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump
A 27-year-old gravida 3 para 2 with uncomplicated history was admitted at the intensive care unit (ICU) with dyspnea and fever at 27 weeks and 6 days of gestation after COVID-19 infection. Workup showed a dilated left ventricle (LV) and reduced left ventricular ejection fraction (LVEF) of 26%. Troponin was not increased. Peripartum cardiomyopathy or dilated cardiomyopathy was the most likely diagnosis, with an unknown role of the previous COVID infection. She was treated for the COVID infection and received bromocriptine, and it was decided not to perform cardiac biopsy. The woman was anticoagulated with a prophylactic dosage (5,700 IU) of nadroparin. Rapid hemodynamic deterioration and progression to cardiogenic shock necessitated mechanical ventilation and inotropes (enoximone 2 µg/kg/min). Due to rapidly progressive refractory shock evolving over the following hours with norepinephrine as high as 0.75 µg/kg/min, the patient was put on VA-ECMO with cannulas introduced in the right femoral vein [25 French (Fr)] and artery (19 Fr). At the same time, an IABP was implanted through the left femoral artery (14 Fr) for left ventricular unloading. The fetal condition was assessed by ultrasound and cardiotocogram and remained reassuring despite maternal condition and presence of large bore cannulas filling both common iliac arteries and vein. As there were signs of blood stasis in the dilated LV, the IABP was replaced by an Impella device (14 Fr, catheter shaft 9 Fr), which unloads the LV independent of the phase of the cardiac cycle, thereby reducing the cardiac work load and improving myocardial perfusion [4]. Back in the ICU, the woman came into spontaneous labor. Considering the compromised maternal condition, anticoagulation, cephalic position and reassuring fetal condition, progression of cervical dilatation, previous vaginal births, and completed steroid course, a multidisciplinary consultation decided to await vaginal delivery in ICU with an obstetric and neonatologic team on standby. At full dilatation, the fetus was in cephalic position with reassuring heart tracing. Suddenly, a bleeding occurred at the right femoral artery cannulation site along with persistent fetal bradycardia. It was controlled with a hemostatic suture, but the fetus turned into transverse position. Groin cannulation limited pelvic access for fetal manipulation. An attempt of internal conversion of the fetal body and breech extraction was unsuccessful and an emergency cesarean section was performed at the ICU. A baby girl of 1,030 g was born with Apgar 1/3 and an umbilical cord pH of 6.99, base excess (BE) –10 mEq/L. After successful cardiopulmonary resuscitation (CPR), she was breathing spontaneously and transferred to the neonatal ICU. Immediately after the infant was delivered, it was necessary to adjust vasopressors due to the increase in preload. Unfractionated heparin was started at a low dose one day after the cesarean section, and gradually increased over the following days targeting an activated partial thromboplastin time (APTT) of 40–55 s. During the first days, the woman received multiple blood transfusions for persisting hemorrhage at the right groin cannulation site. Five days postpartum, a laparotomy was required for a new active bleeding at the hysterotomy scar. Heparin was lowered peri-procedurally to 5,000 EI/day. The Impella was replaced on day seven due to visible, mobile thrombus formation at the tip, probably due to a lack of systemic anticoagulation in the days before. Removal of the VA-ECMO at day 9 was complicated by an acute arterial occlusion of the leg and a successful fasciotomy was performed. Two weeks after the caesarean section, the Impella was successfully weaned. After 12 weeks the infant was discharged in good condition and is doing well at 1 year of age corrected for premature birth. The mother was discharged from the hospital after 5 weeks with an implantable cardioverter defibrillator. Additional genetic research showed a mutation in filamin C gene, associated with dilated cardiomyopathy. As the LV function did not recover and the patient remained New York Heart Association III despite optimal guideline-directed therapy, the patient is listed for heart transplant 1.5 years after delivery.
Our case report describes the need of three different mechanical circulatory support devices in a pregnant woman with cardiogenic shock. Overall, these devices can be used as temporary additional support when inotrope drugs are insufficient. While there is substantial experience with veno-venous ECMO (VV-ECMO) in pregnant women, experience with VA-ECMO in pregnancy is rare [5]. The maternal survival after VA-ECMO in pregnancy is around 75–80%, and the fetal survival rates are relatively low (25–50%) [3, 6]. As VA-ECMO often increases afterload, additional cardiac support with inotropes, or mechanical unloading by IABP or Impella can be necessary to prevent stasis, LV overdistension and/or pulmonary edema. The use of an IABP seems safe for the mother and fetus [3]. An Impella device can provide more hemodynamic support than IABP and progressively unloads the LV [7]. Experience with the Impella in the peripartum period is limited with only two antepartum cases described [8, 9].
When confronted with a pregnant woman with cardiogenic shock, a multidisciplinary approach and discussion on the use of circulatory support and timing, place, and mode of delivery are essential, taking both mother and child into account. Although vaginal delivery is usually preferred in women with heart failure, an emergency cesarean section may be required in unstable patients or in case of fetal distress [10]. Everything for sudden obstetric interventions, emergency surgery, and neonatal resuscitation needs to be prepared in an unfamiliar, suboptimally prepared ICU setting to optimize the chance for a favorable outcome.
The large bore cannulas of the circulatory support devices have obstetric implications. The gravid uterus can make femoral cannulation more difficult. Supine position with left lateral uterine displacement is important and can permit uncomplicated vaginal delivery. Still, the cannulas partly obliterate the common iliac arteries and veins and may further reduce downstream perfusion, including utero-placental circulation and reserve. Severe fetal distress can then rapidly develop on top of other complications like hemorrhage or hypotension. We assume that our premature fetus suddenly turned into a transverse position in the last stage of delivery due to hypoxia-induced hypotonia. Furthermore, it is important to take into account that the femoral catheters impede lithotomy position which is necessary for certain vaginal obstetric interventions. Implantation of an Impella 5.5 device by axillary approach could be an alternative strategy in severe isolated LV failure, avoiding cannulas in the groin.
In addition to the obstetric implications of the large cannulas of mechanical circulatory support devices, another challenge is the need of anticoagulation to reduce thrombotic complications. Delivery, either by vaginal route or caesarean section, induces substantial wound beds at the placental bed, hysterotomy site, abdominal wall and/or vaginal. Therapeutic anticoagulation should be stopped in time and haemostatic factors supplied if necessary. Bleeding is initially controlled by haemostatic sutures, electrocautery, strong uterine contraction and/or compression immediately after delivery permitting cloth formation. As the postpartum period is a prothrombotic state, anticoagulation is essential when indicated to prevent thrombotic complications and initiated within hours to days after delivery when postpartum blood loss seems controlled. The anticoagulative drugs can however dissolve these newly formed organised cloths [11]. With the use of therapeutic anticoagulation, intra-abdominal or vaginal bleeding complications a few days postpartum, after initial blood loss control, is therefore not uncommon. Our case illustrates both the need and danger for therapeutic anticoagulation by the occurrence of Impella thrombosis and vascular occlusion as well as bleeding complications at the insertion site and hysterotomy a few days postpartum. The fine balance between thrombosis and haemorrhage is challenging. We recommend very strict surgical haemostasis and prolonged use of uterotonics. Heparins have a shorter half-life permitting easier control in case of haemorrhage in the days postpartum and therefore, we suggest to delay the conversion of heparin to oral anticoagulants to 14 days postpartum.
In short, the use of short-term mechanical circulatory support devices in pregnant women with cardiogenic shock can be life-saving. A multidisciplinary approach is crucial in order to obtain the best possible outcome for mother and child.
IABP: intra-aortic balloon pump
ICU: intensive care unit
LV: left ventricle
VA-ECMO: veno-arterial extracorporeal membrane oxygenation
The case has also been filmed and broadcasted in a national Dutch TV-program on prematurity with consent of the parents and can be viewed on the website of the broadcasting company: https://npo.nl/start/serie/handen-aan-de-couveuse/seizoen-1/doodziek-maar-zwanger/afspelen.
JAvdZ: Writing—original draft, Writing—review & editing. DB: Conceptualization, Writing—original draft, Writing—review & editing. RMK, HRT, JJHB, AG, HE, and AF: Writing—review & editing. JMJC: Conceptualization, Writing—original draft, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
In accordance with the regulations of the Ethics Committee, “Institutional Review Board and Ethics Committee of Erasmus University Medical Center” ethical approval is not required for case report publications.
Informed consent to participate in the study was obtained from the participant.
Informed consent to publication was obtained from the relevant participant.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1965
Download: 59
Times Cited: 0
Kazim Raza Talpur ... Muhammad Waleed Abdullah
Jingwen Guo, Xueying Wang
