Affiliation:
1Department of Cardiopneumology, Instituto do Coração, Hospital das Clínicas, Faculty of Medicine, University of São Paulo (InCor-HCFMUSP), São Paulo 05403-900, Brazil
Email: cristhian.espinoza@hc.fm.usp.br
ORCID: https://orcid.org/0000-0003-0191-7358
Affiliation:
1Department of Cardiopneumology, Instituto do Coração, Hospital das Clínicas, Faculty of Medicine, University of São Paulo (InCor-HCFMUSP), São Paulo 05403-900, Brazil
ORCID: https://orcid.org/0009-0003-7829-4408
Affiliation:
2Faculty of Medicine, Central University of Ecuador, Quito 170521, Ecuador
ORCID: https://orcid.org/0000-0002-1026-8954
Affiliation:
1Department of Cardiopneumology, Instituto do Coração, Hospital das Clínicas, Faculty of Medicine, University of São Paulo (InCor-HCFMUSP), São Paulo 05403-900, Brazil
ORCID: https://orcid.org/0000-0001-5849-5817
Affiliation:
2Faculty of Medicine, Central University of Ecuador, Quito 170521, Ecuador
ORCID: https://orcid.org/0009-0009-8547-2915
Affiliation:
1Department of Cardiopneumology, Instituto do Coração, Hospital das Clínicas, Faculty of Medicine, University of São Paulo (InCor-HCFMUSP), São Paulo 05403-900, Brazil
ORCID: https://orcid.org/0009-0007-9204-9221
Affiliation:
1Department of Cardiopneumology, Instituto do Coração, Hospital das Clínicas, Faculty of Medicine, University of São Paulo (InCor-HCFMUSP), São Paulo 05403-900, Brazil
ORCID: https://orcid.org/0000-0002-7410-3147
Explor Cardiol. 2025;3:101245 DOI: https://doi.org/10.37349/ec.2025.101245
Received: November 01, 2024 Accepted: December 11, 2024 Published: January 14, 2025
Academic Editor: Miodrag Ostojic, University of Belgrade, Serbia
The article belongs to the special issue Multimodality Imaging in Ischemic Heart Disease
We present the case of a woman admitted for an acute ST-segment elevation myocardial infarction. Emergency catheterization was conducted, revealing diffuse lesions affecting 70% of the anterior descending artery from the mid to the distal third but with ventriculography compatible with Takotsubo syndrome. Two magnetic resonances were performed 90 days apart, confirming the unusual coexistence of Takotsubo syndrome and transmural infarction in the same event.
Takotsubo syndrome (TTS) and its variants represent a form of acute heart failure (HF), typically reversible, secondary to a catecholaminergic myocardial storm, and without evidence of obstructive coronary atherosclerotic disease (CAD) to explain the left ventricle (LV) dysfunction. Diagnostic criteria have been established, reviewed by various societies, and mentioned in several reviews on the subject [1]. In the acute phase, the symptoms and signs of TTS are indistinguishable from those of type 1 ST-segment elevation myocardial infarction (STEMI) or atherosclerotic events, as 76% of TTS patients experience chest pain and 44% present with ST-segment elevation [1]. Initially, it was suggested that these two entities were mutually exclusive; however, it is now recognized that stable or unstable CAD may coexist with TTS, either concomitantly or as a trigger [2]. In cohorts evaluating TTS patients, 41.2% were found to have non-obstructive CAD, while 23% had obstructive CAD [2–5].
Differentiating these two conditions can be particularly challenging. In such cases, cardiac magnetic resonance (CMR) imaging plays a pivotal role, being especially useful in the acute phase. Several findings have been documented in the literature, including a combination of typical contractility abnormalities, myocardial edema, and the absence of late gadolinium enhancement (LGE) [6, 7]. It remains unclear whether the presence of TTS alongside a significant but non-occlusive coronary lesion would necessitate primary intervention [2]. An acute coronary syndrome (ACS) may be the initial event, subsequently triggering TTS, but alternatively, TTS could precipitate a type 1 STEMI through a sympathetic discharge that causes vasoconstriction and plaque rupture [2].
The exact pathophysiology of TTS remains unclear, though significant advances have been made in understanding the syndrome. One central hypothesis is that intense sympathetic activation and elevated circulating catecholamine levels play a critical role in TTS. Acute episodes are often triggered by stress or physical illness, with patients showing signs of intense sympathetic activation. Elevated plasma catecholamine levels, especially in patients with pheochromocytoma or those receiving catecholamine-based treatments, support this hypothesis [1, 5, 8]. These findings suggest that excessive sympathetic stimulation contributes to the pathophysiology of TTS, leading to reversible myocardial dysfunction [5, 8].
The diagnostic criteria for TTS, according to the InterTAK registry, include: 1) clinical evidence of transient LV dysfunction, with apical or mid-ventricular hypokinesia or akinesia on echocardiography; 2) the absence of significant coronary artery obstruction, confirmed by coronary angiography; 3) the absence of pre-existing conditions that could explain the ventricular dysfunction, such as myocardial infarction or myocarditis; 4) the presence of an emotional or physical trigger, often with initial chest pain or angina, particularly in postmenopausal women [5, 7]. Spontaneous recovery of ventricular function is common within weeks to months. The InterTAK score, used to assess the risk and severity of the syndrome, assigns points for factors such as age, sex, presence of coronary artery disease, type of trigger (emotional or physical), and the extent of ventricular dysfunction, with a score greater than 70 points indicating a high probability of TTS [7, 8].
We present an unusual case of TTS with concomitant ACS, confirmed by transthoracic echocardiogram (TE) and CMR during the acute episode. The edema resolved within three months, but transmural LGE persisted, as evidenced in the initial event.
A 42-year-old woman was admitted to the external emergency room complaining of typical angina associated with dyspnea, diagnosed with STEMI on the lateral wall, not undergoing thrombolysis, having received a double anti-aggregation with acetylsalicylic acid and clopidogrel. On physical examination, the patient had a heart rate of 116 beats per minute, blood pressure of 156/96 mmHg, respiratory rate of 18 breaths per minute, temperature of 36.0°C, good peripheral perfusion, and rhythmic and normophonetic sounds without murmurs and vesicular murmurs present without adventitious noises. She reported a history of right nephrectomy and many episodes of stress in the last few months at the office. The patient does not have other comorbidities such as diabetes, hypertension, or a family history of cardiovascular disease. She denies smoking or alcoholism.
The electrocardiogram (ECG) on admission to emergency department showed ST-segment elevation on the inferior wall (Figure 1). Laboratory tests are shown in Table 1. Emergency coronary catheterization was performed, which found left anterior descending artery (LAD) involving the inferior wall, with diffuse lesions of 70% since the middle to distal-third, suggesting type 2b coronary dissection. Ventriculography showing marked systolic dysfunction, with akinesia in the anterior, anteroapical, anterolateral, apical, and inferoapical walls. The echocardiogram revealed LV with apical dyskinesia, anterior and inferior septal akinesia, ejection fraction (EF) estimated at 40%, in addition to basal hyperkinesia suggestive of TTS (Figure 2). Due to the suspicion of type 2b coronary dissection, a coronary computed tomography angiogram was performed, but without meeting the criteria for this suspicion. The exam also showed middle calcified plaque with a mild luminal reduction in LAD.
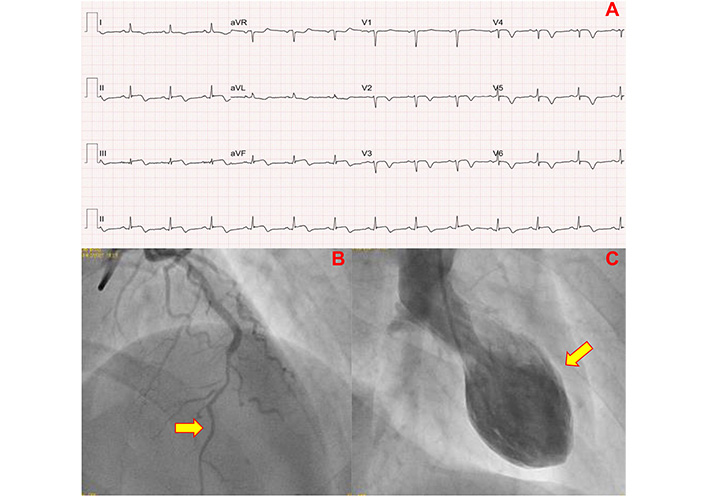
Admission exams of the patient. (A) Electrocardiogram after 12 hours with ST-segment elevation of the inferior wall. (B) Coronary angiography in the anterior descending artery with lesions of 70% from middle to distal third, image suggestive of type 2b coronary dissection (arrow). (C) Ventriculography showing ballooning of the middle and apical segments (arrow)
Laboratory values during hospitalization
| Laboratory tests | Laboratory values | |
|---|---|---|
| Hemoglobin | 151 g/L | |
| Leukocytes | 6,770/mm3 | |
| Platelets | 264,000/mm3 | |
| INR | 1.1 | |
| LDL cholesterol | 142 mg/dL | |
| CRP | 8.2 mg/dL | |
| Triglycerides | 124 mg/dL | |
| Creatinine | 0.8 mg/dL | |
| Urea | 20 mg/dL | |
| Potassium | 3.7 mEq/L | |
| Troponin I hs (NV up to 40 ng/L) | External | 1,642 |
| Admission | 13,872 | |
| Peak | 18,455 | |
| CK-MB (NV up to 3.8 ng/L) | External | 11.6 |
| Admission | 20 | |
| Peak | 32 | |
INR: international normalized ratio; NV: normal value; CK-MB: creatine kinase-MB; CRP: c-reactive protein
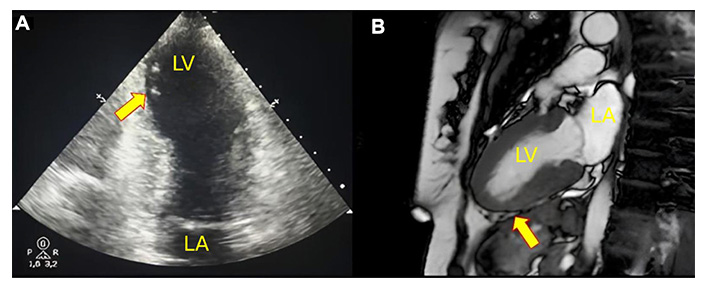
Multimodality imaging of the patient with takotsubo syndrome. (A) Two-dimensional 2-chamber echocardiogram shows mid-apical ballooning due to segmental change during systole (arrow). (B) Cardiac magnetic resonance (CMR) in systole with “steady-state free precession” pulse sequence showing segmental changes in the apical segments (arrow). LA: left atrium; LV: left ventricle
CMR imaging was performed at 5 days after STEMI with findings of left atrium and LV of preserved dimensions, but with EF at the lower limit of normality (55%), anterior and inferoseptal hypokinesia of the segments middle and inferior, septal, and anterior of the apical segment. Signs of myocardial edema in the inferior, anteroseptal, and anteroseptal walls of the midsegment and apical circumferential. Presence of transmural LGE in the inferior apical segment compatible with acute myocardial infarction (AMI) and absence of viability. Low-signal foci were compatible with associated microvascular obstruction, with altered strain in the apical segments and preservation of the basal segments (Figures 2 and 3).
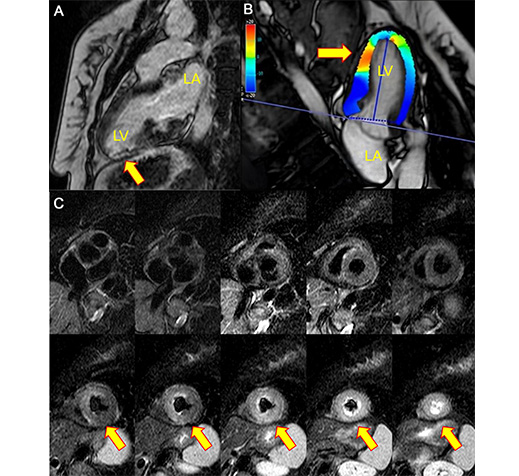
Cardiac magnetic resonance imaging at admission. (A) Late gadolinium enhancement sequence showing focal contrast in the lower apical segment, in addition to a focal hypointense image in the infarcted area compatible with microvascular obstruction (arrow). (B) T2-edema sequence, short axis, an increase in a homogeneous signal in the middle and apical segments, compatible with active inflammation (edema) (arrow). (C) Global longitudinal strain of the left ventricle of –15% and with regional changes (arrows). LA: left atrium; LV: left ventricle
The patient evolved stable, without the need for vasoactive drugs, and therapy for HF was started with enalapril, carvedilol, and spironolactone, in addition to aspirin, clopidogrel, and atorvastatin.
In the clinical follow-up, the patient evolved stable with good response to clinical treatment and underwent a control CMR at 90 days with total resolution of the edema compared to the previous exam, but with persistence of late enhancement in the lower apical segment (Figure 4).
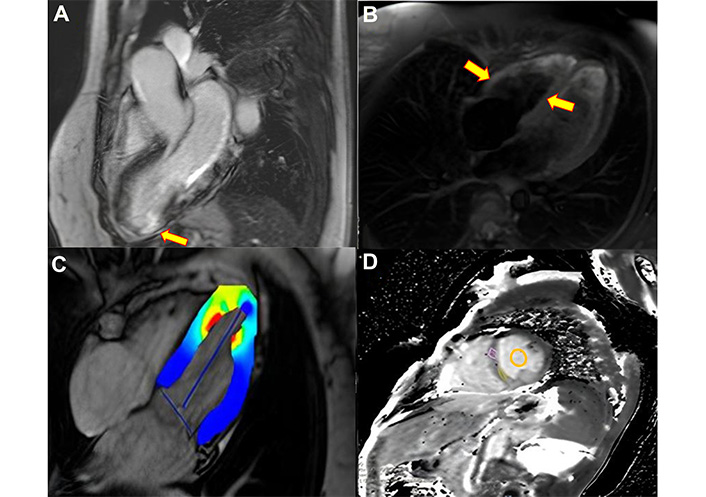
Cardiac magnetic resonance imaging after 90 days. (A) Late gadolinium enhancement sequence, there is an area of transmural hypersignal throughout the lower segment compatible with infarction (arrow). (B) Absolute global longitudinal strain shows improvement compared to the previous study (arrows). (C) T2-edema sequence, long axis, without signs of active inflammation (edema) in the segments affected in the previous study. (D) The tissue characterization of the native T1 map, and pre- and post-contrast extracellular volume shows normal and absence of interstitial fibrosis in the segments without infarction (circle), with a value of 900 msec, lower than the previous study (1,300 msec)
This case highlights a diagnostic challenge in cardiology, where two entities with remarkably similar clinical presentations are difficult to differentiate in practice: TTS and type 1 STEMI. According to the 2004 Mayo Clinic diagnostic criteria, which considered both entities to be mutually exclusive, our patient would have been diagnosed with ACS only [9]. However, these criteria were revised in 2008 to account for the coexistence of the two entities, and later, a cohort study revealed that such an association is not as rare as previously thought [2, 10].
Differentiating the two entities or recognizing their association is not straightforward, as there is considerable overlap in pathophysiological, clinical, and diagnostic characteristics. Emotional and physical triggering factors can occur in both situations, and one may precipitate the other [2]. In patients with ACS, troponin and creatine kinase-MB (CK-MB) levels are elevated [7, 11, 12]. In TTS, CK-MB typically shows mild elevations, while brain natriuretic peptide (BNP) levels increase significantly, which was not observed in this ACS case [11, 12]. Comparing echocardiography with coronary angiography is crucial; in ACS, segmental ventricular dysfunction correlates with the anatomy of the coronary artery responsible for the acute event [7, 11]. In TTS, however, regional ventricular dysfunction typically does not adhere to the coronary anatomy, often extending beyond the boundaries of the affected artery [11]. If TTS is associated with CAD, CMR plays a fundamental role in addition to comparing echocardiography with coronary angiography. The presence of subendocardial or transmural LGE indicates myocardial necrosis, confirming an acute coronary event [7, 11]. Patient follow-up is also critical, as recovery of myocardial function and reduction in myocardial edema across various walls should raise suspicion for TTS [11].
This patient had a non-obstructive coronary lesion in the LAD artery, but the ventricular dysfunction observed on ventriculography and echocardiography extended beyond the LAD territory. CMR showed edema in multiple walls, but LGE was only present in the lower apical segment, consistent with the lesion in the LAD. At the 90-day follow-up, the complete resolution of the edema compared to the previous exam, alongside persistent LGE in the lower apical segment, supports the hypothesis of TTS associated with type 1 STEMI. This is further corroborated by the recovery of ventricular function in regions not compatible with the LAD lesion, while myocardial necrosis persisted in a segment consistent with the LAD lesion.
The treatment of TTS and ACS shares similarities in managing complications such as HF and arrhythmias, utilizing beta-blockers, diuretics, mineralocorticoid antagonists, ACE inhibitors, and, when necessary, advanced mechanical support in cases of cardiogenic shock. However, therapeutic strategies differ in the acute phase. The management of TTS primarily involves supportive care, with an emphasis on preventing recurrence and monitoring for arrhythmias and HF. In the presence of CAD, aspirin and statins are indicated. In contrast, the management of ACS includes dual antiplatelet therapy, anticoagulation, and early revascularization of the target vessel to reduce mortality and prevent HF [7, 13].
In conclusion, TTS is a challenging diagnosis, particularly when associated with CAD. Therefore, the combined evaluation of echocardiography and coronary angiography is crucial, as it allows for comparison between the territory of the affected coronary artery and the dysfunctional myocardial segment. Additionally, CMR evaluation of LGE, myocardial edema, and segmental contractility changes play an important role in aiding the differential diagnosis.
ACS: acute coronary syndrome
CAD: coronary atherosclerotic disease
CMR: cardiac magnetic resonance
EF: ejection fraction
HF: heart failure
LAD: left anterior descending artery
LGE: late gadolinium enhancement
LV: left ventricle
STEMI: ST-segment elevation myocardial infarction
TE: transthoracic echocardiogram
TTS: Takotsubo syndrome
CER, LBV, and GOP: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. VMC: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Validation, Supervision. WL: Validation, Supervision. KDPM: Writing—original draft, Writing—review & editing. TTL: Conceptualization, Writing—original draft, Writing—review & editing, Validation, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Ethical approval was not required for this study as it was routine treatment according to the local committee.
Informed consent to participate was obtained from the participant.
Informed consent to publication was obtained from the participant.
The data for this manuscript are available upon request from the corresponding author.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Marco Fabio Costantino ... Luisiana Stolfi
Marco Antonio Rodrigues Torres, Natália Moraes de Quevedo
Praveen Kumar Chandra Sekar, Ramakrishnan Veerabathiran
Emma Cerracchio ... Quirino Ciampi
