Abstract
Age-related atrial fibrillation (AF) is a common condition that has yet to be fully understood, with mechanisms to explain its development under investigation. Notably, cellular senescence, cardiac fibrosis, coronary ischemia, cardiac valvular disease, autonomic dysfunction, channelopathies, and immune system remodeling are processes that have been seen to occur with aging and ample evidence has shown their association with the development of AF. Despite robust therapeutic approaches, the incidence of AF continues to rise, suggesting that the dynamic, multi-faceted interactions leading to AF are incompletely understood. One of the newer mechanisms currently being investigated is the gut microbiome. Although more research is needed to understand its impact on the development of age-related AF and targets for therapies, the gut microbiome is a promising new avenue of research that may provide future benefits in AF prophylaxis or enhanced management. As the field works towards developing this knowledge, there are important questions to answer as to the optimal role of potential gut microbiome targeting therapies and their potential risks versus the benefits they provide. This commentary first summarizes the currently understood mechanisms contributing to age-related AF, which is then followed by an analysis of the current work investigating the role of the gut microbiome in the development of age-related AF, and concludes by highlighting notable questions to consider in future work on the role of the gut microbiome and its relationship to age-related AF.
Keywords
Microbiome, arrhythmia, atrial fibrillation, aging, inflammation, gut dysbiosis, immune, autonomicIntroduction
Atrial fibrillation (AF) is a condition that has been rising in prevalence over the years, increasing the risk of mortality in the general population [1]. While many conditions increase the risk of developing AF, one of the best-established and prominent risk factors is age [2]. However, the molecular mechanisms behind age-related AF are not well-established and are currently under investigation. There are many different proposed, interconnected mechanisms of how cardiac aging will predispose someone to AF (Figure 1). Aging includes a component of spontaneous changes in cell function, often referred to as cellular senescence, as well as an age-associated acquired disease burden which can interact with the cellular senescence to increase the risk of AF. The challenge with any age-related disease is understanding the relative contributions of spontaneous senescence and the accumulation of age-associated disease burden.
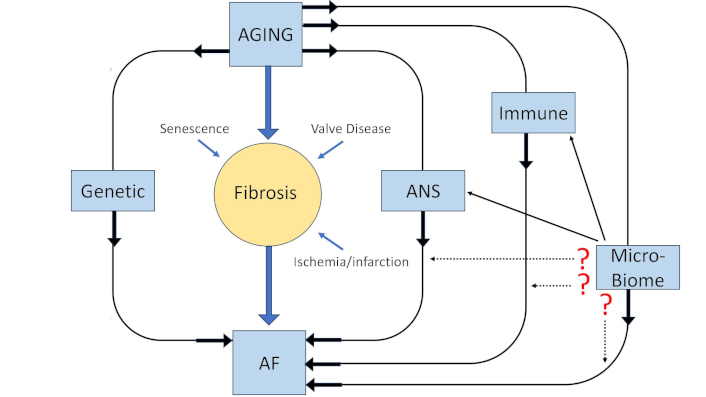
Schematic representation of the factors that could be contributing to age-related AF. The potential role of the microbiome, as well as the potential impact of other systems on the microbiome’s impact, are indicated. ANS: autonomic nervous system; AF: atrial fibrillation
Microbiome and aging
The gut serves as a host to a wide variety of microorganisms. The role of the gut microflora in aging, and the role of aging in the composition of the gut microflora are the topics of widespread interest. In parallel, the role of the gut microflora in the emergence of aging-related co-morbidities has been gaining increasing interest, as have associated strategies to manage the intestinal flora to therapeutic benefit. Dysfunction of the gut microbial ecosystem, or gut dysbiosis, has increased and is associated with many disease processes. For example, a decrease in the proportion of “good bacteria” has been associated with increased inflammation via increased permeability of the gut, and other capillary systems, including the blood-brain barrier, leading to the microbiome-gut-brain (MGB) axis postulate linking gut dysbiosis to increased incidence and accelerated progression of neurological diseases such as Parkinson’s and Alzheimer’s [3]. The authors also suggested that strategically targeting the gut microbiome could have therapeutic benefits.
Increasingly, focusing on the composition of the gut microbiome has been seen as critical to whether individuals age in a healthy or unhealthy manner, with healthy aging indicated primarily by longevity, and unhealthy aging characterized as early susceptibility to diseases and premature mortality. The associations are highly suggestive, but confounded by wide variations in diet, race, and lifestyle, which can substantially impact the profile of gut microflora. A heavily cited, recent review summarizes many of the working ideas related to the gut microbiota in aging, particularly with respect to the functional role of the microbiota in regulating the overall systemic inflammatory state as a common theme connecting gut dysbiosis to a wide variety of aging-related co-morbidities [4].
Actinobacter, Bacteroidetes, Verrucomicrobia, and Firmicutes dominate the gut microflora in adults. While the gut microbiota is relatively stable over a long period of time during adulthood, aging (> 65 years) impacts the composition and functionality of the microbiota. An increased number of opportunistic pathogens and an overall decrease in microbiota diversity is seen, especially in older individuals consuming a Western diet [5].
A relative loss of commensal bacterial species decreases the availability of beneficial short chain fatty acids (SCFAs), which in turn increases vulnerability to opportunistic infections, translocation of inflammatory mediators from the gut to the bloodstream, and diminished capacity to appropriately regulate systemic immune responses [4]. In 2018, the concept of “inflamm-aging”, the “long-term result of the chronic physiological stimulation of the innate immune system, which can become damaging during aging” was brought forth as a mechanism associating inflammation with several aging related morbidities, including heart failure and frailty [6]. It is reasonable to at least associate gut dysbiosis with chronic inflammation.
To the extent that AF also shows several etiologies with inflammatory components, it is reasonable to consider that age-related gut dysbiosis could directly contribute to the incidence of AF. Gut dysbiosis has been reported to increase both heart failure and AF via the nod-like receptor protein 3 (NLRP3) inflammasome pathway [7, 8]. Gut dysbiosis may also indirectly contribute to age-related AF through modulation of other factors such as ion-channel conductance/expression, and autonomic nervous system (ANS) outflow. An overview of the postulated relationship between gut dysbiosis and AF and its mediators is summarized in Figure 2. The multiple factors that could contribute to AF, along with a focused consideration of therapeutic opportunities in managing AF by targeting the gut microbiome are summarized in the following sections.
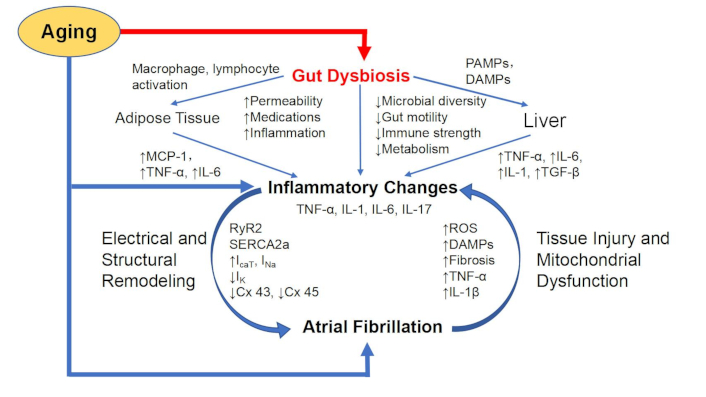
Gut dysbiosis, aging, and atrial fibrillation. Immune remodeling in aging, how it might contribute to atrial fibrillation, and serve as a nexus for potential interaction with the gut microbiome. The immune system becomes less diverse, and proinflammatory with aging. In the heart, increased cytokine levels can lead to both structural and electrical remodeling through effects on calcium storage vesicles, gap junctions, and ion channels predisposing to atrial fibrillations. Arrhythmias can lead to further remodeling, and mitochondrial dysfunction, further exacerbating the inflammatory remodeling. Aging-related gut dysbiosis can serve as an amplifier for any of the aging-associated influences on atrial fibrillation. TNF-α: tumor necrosis factor-alpha; MCP-1: monocyte chemoattractant protein-1; RyR2: ryanodine receptor 2; IL-6: interleukin-6; PAMPs: pathogen-associated molecular patterns; DAMPs: damage-associated molecular patterns; SERCA2a: sarco/endoplasmic reticulum Ca2+-ATPase 2a; Cx 43/45: connexin 43/45; ROS: reactive oxygen species; TGF-β: transforming growth factor-beta; ICaT: calcium current; INa: sodium current; IK: potassium current
Cellular senescence and AF
Cellular senescence is a prominent age-associated mechanism that is defined as a state of cell growth arrest where the cell is unable to proliferate, and it can be triggered by various age-related cellular stressors such as DNA damage or telomere attrition. It is a key marker of aging and many studies have shown higher levels of senescent cells in older patients [9]. This has been an area of interest for many researchers as mouse models have shown evidence that cellular senescence contributes to age-related diseases such as Parkinson’s disease, atherosclerosis, and chronic obstructive pulmonary disease (COPD). Much research has been done on senolytic therapies aimed at clearing senescent cells by targeting specific up-regulated apoptotic modulators or enhancing immune clearance in animal models. While results from those studies are encouraging, it remains to be seen if senolytic therapy is safe in human patients. Further study is also needed to better characterize the diverse senescent phenotypes [10].
Cellular senescence appears to correlate with the development and progression of AF. Studies have found increased senescent cell concentrations in the atria of human and rat models with AF [11]. It has been proposed that senescent cells secrete bioactive molecules, named the senescence-associated secretory phenotype, which is implicated in the formation of atrial fibrosis, increasing AF susceptibility. Many of the same proteins involved in atrial fibrosis have shown a relation to the development of cellular senescence [12]. Interestingly, senolytic therapies have shown some promise in the possibility of AF prophylaxis, at least in animal studies. One study demonstrated that senolytic therapy reduced atrial fibrosis and the development of an AF substrate in rats [13]. However, further work needs to be done before this can become a clinical reality, and perhaps future senolytic therapy will include a microbiome-targeted component.
Cardiac fibrosis and AF
With aging, senescence in cardiac cells can lead to structural changes in the heart. In particular, cardiac fibrosis has been well-studied to contribute to this structural reorganization. Cardiac fibrosis typically is defined by an increased imbalance of collagen synthesis and arrangement in the extracellular matrix put in cardiac tissue by fibroblasts, usually in response to inflammation or mechanical stress. Regulation of fibrotic formation is thought to be mediated by various neurohormonal pathways, most prominently by transforming growth factor beta-1 (TGF-β1) and angiotensin II (ANG II) [14]. These signaling pathways stimulate cardiac fibroblasts and encourage the formation of extracellular matrix and collagen around cardiomyocytes, forming fibrosis. In turn, fibrosis will often lead to non-homogeneity and disorder of electrical conduction and excitability throughout cardiomyocytes leading to conduction abnormalities and arrhythmias such as AF. In particular, fibrosis in the left atrium is noted to have close ties to the development of AF and related adverse effects [14]. Major contributors to cardiac fibrosis, in addition to aging, are coronary ischemia (CI) and cardiac valve disease, both of which require substantial structural remodeling, of which fibrosis is an inherent component. While vascular aging also occurs and contributes to hypertension and cardiac remodeling, that is beyond the scope of this review.
One of the leading future directions in advancing our understanding of the relationship between fibrosis and AF is enhancement of cardiac fibrosis evaluation. The two current primary methods are imaging and serological. However, imaging has its limitations. While cardiac MRI is perhaps the best current modality, it is expensive, time and labor intensive, making it less practical for widespread use. Serology is perhaps the ideal modality, but more work is needed to study the accuracy of various forms of those assays. Thus, one of the leading directions in this field is the discovery of biomarkers to track the extent and severity of cardiac fibrosis. Another direction is identifying potential targets in the fibrotic pathway to focus on for anti-fibrotic therapies in animal models. Future studies will focus on finding more biomarkers and targets to optimize anti-fibrotic therapy for possible AF prophylaxis [15]. Some of those may include applications of gut microbiome profiling as an additional strategy to consider (Figure 2).
CI, fibrosis, and AF
CI is the leading cause of mortality worldwide. The connection between CI and AF lies in the pathophysiology of CI. Fundamentally, there is an inflammatory reaction following ischemia and associated infarction that includes disparate, but related processes. The injured tissue undergoes a remodeling process in which the infarction is ultimately replaced by scar tissue. At the same time, the non-injured tissue undergoes a hypertrophic response as the remaining healthy tissue adapts to the changing mechanical load dynamics within the heart. ANG II inhibitors are routinely prescribed as part of the post-infarction medical management to limit “adverse remodeling”.
CI, even without overt infarction, also can predispose someone to AF. The development of atherosclerotic plugs in arterial endothelium is partly due to the oxidation of foam cells by various chemokine and growth factor signals. Endothelial changes lead to a decrease in arterial diameter with a 70% reduction in luminal diameter noted to significantly restrict blood flow. It is these structural changes in arterial diameter and subsequent ischemia that may lead to changes in gap junction proteins in cardiomyocytes causing AF [16]. AF may in turn cause endothelial dysfunction due to high levels of proinflammatory mediators, which in turn promotes atherosclerosis and the generation of plaques in patients with CI, creating a positive-feedback loop [16]. Separate animal models have shown that dysfunctional gap junctions, as seen in the development of AF, also occur in the development of atrial ischemia (AI). Preclinical models from a matched case control study found that AI is a cause and a trigger for AF [17].
Cardiac valvular disease and AF
Cardiac valvular disease increases in incidence with age and has an association with increased susceptibility to cardiac arrhythmias and associated thrombotic events. In particular, patients with rheumatic heart disease (RHD) have been noted to have an increased risk of developing AF with age [18]. Atrial enlargement, fibrosis, and cardiac remodeling have been noted to increase in RHD patients, which may predispose these patients to arrhythmias. Early intervention of the valvular condition has been shown to relieve atrial pressures and reverse cardiac remodeling. Post-operative AF complicating valvular surgery, while attenuated with co-surgical therapies such as ablation, has remained frequent [19]. Questions still remain on how best to manage and prevent it, and further research focuses on optimizing ablative therapies to prevent post-operative AF.
ANS and aging
The role of the ANS changes with age, although the mechanisms by which it occurs are not as well understood. The parallel between rising systolic blood pressure and increasing age has been appreciated for quite some time, but the mechanisms responsible for it are less clear. Understanding the role of the ANS with age, especially in conjunction with the cardiovascular system, often is confounded by the simultaneous increases in age-related pathology such as atherosclerosis, myocardial infarction, and heart failure. Histologically, aging is associated with a decrease in arterial elastin content along with an increase in collagen content in the vascular wall. The loss of distensibility contributes to a decrease in diastolic pressure, with mean arterial perfusion pressure maintained by a simultaneous increase in systolic blood pressure, supported by increased sympathetic outflow from the ANS. A great deal of investigation into the ANS either as a cause, or as a consequence of aging alone, or in conjunction with associated pathophysiology (such as hypertension) has been done in both human and animal systems. However, much of that research was done 30–40 years ago [20] and has waned in more recent years as interest has shifted towards the management of thrombosis, ischemia/revascularization, and heart failure. The heart is heavily influenced by the ANS, and therapies for managing cardiovascular pathophysiology usually include a significant role for modifiers of autonomic function. Yet, relatively little is understood regarding the actual contributions of aging to the ANS.
Sometimes confusion is introduced inadvertently by how the material is discussed. For example, it has been reported that the baroreceptor reflex is not affected by aging in rats or humans when evaluated by efferent sympathetic nerve activity [21, 22]. Unfortunately, those studies exclude the end effector response. Studies consistently show that orthostatic hypertension or postprandial hypotension, both reflective of inadequate baroreceptor reflex responses, is common in elderly patients [23, 24]. Increasingly, what is becoming more evident is that the aging effect might be end-organ specific, tied to changes in some aspect of receptor expression, sensitivity, or affinity, including nicotinic receptors in the brain regulating regional blood flow responses impacting autonomic outflow [25–32]. It is imprecise and can be misleading to suggest the baroreceptor reflex is intact without better clarification of what exactly was measured and where.
The age-related changes in autonomic outflow (ARCAF) and receptor mediated changes also may be sex-dependent [33]. For example, in a well-controlled study of 42 women spanning in age 16–76, Baker et al. [33] identified a strongly positive correlation between increasing age and the dose of an autonomic ganglionic blocker (Trimethapan) needed to block changes in heart rate or muscle sympathetic nerve activity in response to a Valsalva maneuver [33]. Measures of heart rate variability (HRV), sympathetic nerve activity on the peroneal nerve, and blood pressure responses led them to conclude that there was both a loss of parasympathetic tone and an increase in sympathetic tone with increasing age. The findings were especially remarkable because all subjects in the study were healthy, non-smoking, non-obese (BMI < 28), non-diabetic (fasting glucose < 126), normotensive, and taking no systemic medications for cardiovascular dysfunction [33].
ANS, aging, and AF
While general interest in autonomic function impacted by aging had waned for some time, in favor of directed therapy for specific conditions, there has been a renewed interest lately, in part because non-invasive tools for evaluating autonomic function are increasingly accessible and user-friendly. Most are predicated on more or less sophisticated measures of HRV, obtained from analysis of continuous recordings of the electrocardiogram. Different components of the HRV analysis can provide specific information about relative changes in the sympathetic and parasympathetic arms of the ANS [34]. Increased parasympathetic activity is associated with longevity [35] and increased, as well as decreased, sympathetic activity has been associated with a higher risk of sudden cardiac death, specifically in AF patients [36, 37]. To the extent that AF is a known risk factor for stroke, and to the extent that ARCAF is a risk factor for AF, Barthelemy et al. [34] have suggested that HRV should be added to account for age and autonomic influences to improve the risk predictions for stroke [34]. Given that age is associated with increased risk for AF, that age is associated with increased sympathetic and decreased parasympathetic outflow, and that increased sympathetic outflow is a risk for arrhythmia, it is reasonable to associate aging and the ANS, perhaps augmented by changes in the microbiome (Figure 2), as contributing mechanisms to the prevalence of AF with aging.
Channelopathies and aging
Channelopathy refers to changes in the function of ion channels, often to the detriment of tissue function, but in cardiac tissues specifically, also often undetected until some adverse event occurs. Channelopathies exist in two forms: genetic/inherited, and acquired, secondary to drugs, toxins, or pathological changes in this leading to channels in expression of channel proteins. A significant number of inherited channelopathies are transmitted in autosomal dominant fashion [38–41] and yet arrhythmias in general, and AF specifically are uncommon in children. Many inherited abnormalities in cardiac channel function are not identified until other events occur later in life, such as myocardial ischemia, or the onset of the arrhythmia.
Channelopathies and arrhythmia
Cardiac muscle displays two distinct action potentials, one associated with pacemaker events, and one associated with the muscle cell response [42]. All the currents in all the channels are the result of ion flux through ion-specific channels. The pacemaker current is dominated by a sodium leak channel, while depolarization of pacemaker cells is attributed to an inward calcium current, and repolarization to an outward potassium current. Depolarization of cardiac muscle cells is the result of an inward sodium current, with inward calcium currents primarily responsible for the plateau phase, and outward potassium currents responsible for repolarization [42]. Changing the dynamics of the channels opening or closing channels changes the balance of ion fluxes/currents and contributes to the potential risk of arrhythmia. For example, defects in the sodium channel that delay depolarization, or that prolong repolarization are considered pro-arrhythmic [43]. Parallel observations can be identified with abnormalities in the potassium channels [41]. The most common clinical hallmark of a potential channelopathy is prolongation of the QT interval of the electrocardiogram. At least nine different channel mutations have been associated with long QT syndromes, and another six with familial AF [40].
Channelopathies, arrhythmia, autonomics, and aging
Normal regulation of cardiac rate and rhythm is modulated by the ANS. Normal depolarizing events occur via sodium channels and typically are mediated primarily through actions of the sympathetic nervous system, and repolarizing events to the parasympathetic nervous system. To the extent that the autonomic nervous outflow tends to become more sympathetically driven with aging (see previous section), increased stimulation of dysfunctional sodium channels, or decreased modulation of dysfunctional potassium channels both are likely to be increased with aging. Channelopathies are not limited to cardiac cells. Mitochondria express and are profoundly regulated by ion channels, impacting apoptotic responses, bioenergetics, and reactive oxygen species, all of which can impact the risk of AF [43]. Similarly, cellular responses of the immune system involve ion channel signaling [39, 40, 43]. Aging-associated changes in the immune system are known, and a strong relationship between the immune system and the gut microbiome is becoming more consistently appreciated. It seems at least plausible that there is a dynamic relationship between channelopathies, the autonomic and immune systems, the gut microbiome (Figure 2), and the emergence of age-related AF.
Immune system and AF
The immune system has been suggested to have some part in modulating homeostasis in cardiac tissue. Studies have suggested that immune cells may have a role in maintaining electrical conductance between cardiomyocytes [44]. With aging, the immune system loses its cellular repertoire and ability to fight off infections, undergoing remodeling. Activation of immune cells leads to the secretion of many pro-inflammatory mediators which over time, can induce electrical remodeling in the atria. Some of these pathways have been suggested to downregulate connexin expression, creating changes in conduction pathways between cardiomyocytes [45]. The pro-inflammatory mediators can also lead to the upregulation of NLRP3 inflammasome expression which has been shown to be elevated in elderly patients with AF, and correlated with AF induction in rat models [46, 47]. Giving fecal transplants and lowering the levels of NLRP3 inflammasome were even shown to lower the risk of developing AF in rat models [47]. Speculatively, there is some evidence that suggests that the NLRP3 inflammasome may even play a role in modulating the ANS’s cardiac regulation [48].
Therapies targeting the inflammatory pathway for AF prophylaxis, however, have been unreliable. Colchicine, for example, had previously been used to prevent post-operative AF, but later research showed mixed results. Further in-depth analysis and delineation of targets within the immune pathway are needed to find a more effective treatment [47].
Gut microbiome and AF
While many of the pathways discussed above have been established as having a role in the development of age-related AF, one of the newer mechanisms brought to light recently is the impact of the gut microbiota. While this is a promising new avenue of study, more studies are needed to further define the mechanisms behind how the gut microbiome impacts or regulates AF [12] (Figure 2).
Presently, there is an understanding of a correlation between metabolites produced by the gut microbiome and age-related AF in rat models and human subjects. Many of these metabolites such as lipopolysaccharide (LPS), trimethylamine-N oxide (TMAO), and SCFAs have reportedly been raised in elderly patients and have been found to be associated with AF and cardiac fibrosis [12]. As noted earlier, a recent study showed that fecal matter transplantation could reverse AF and development of cardiac fibrosis in rat models through modification of metabolite levels produced by the gut microbiome [46]. Several other studies focused on sequencing the gut microbiome saw notable changes in the composition of the microbiome in AF patients as compared to non-AF patients [48, 49].
Current therapeutic ideas have included identifying targets in pathways in the aging gut microbiome to prevent AF or cardiac fibrosis. This can be through specific targets or through broad modification via probiotics and prebiotics or long-term dietary changes [50]. Gut microbiome-related therapies have promising uses, as recent research has shown. The benefits of microbial supplements in patients with diabetes and allergies have been studied in studies with encouraging results, although much further trials are needed. Fecal matter transplant therapy has also been observed to offer potential benefits in patients with intestinal inflammation, metabolic syndromes, infection, etc. While more research is needed to further solidify the role and clinical benefits of this therapy, this is a developing area that may offer benefits in the future [51].
One such benefit that gut microbiome-targeted therapies can provide is as a safer adjunct to current ablative or medical therapies for AF, reducing the reliance on current treatments (Figure 3). There has been some evidence that probiotic and prebiotic medications can lower levels of TMAO metabolites, a key player in the development of AF, through modulation of the gut microbiome [52]. One study showed that resveratrol, which has some prebiotic effects, was able to reduce levels of TMAO in mice and attenuate the development of atherosclerosis [53]. While this area of study is still in its infancy, a further study analyzing the efficacy of probiotics/prebiotics in consistently lowering the development of AF could highlight their use as a prophylactic or adjunctive agent. By helping to modulate key metabolites in the gut microbiome that have been found to lead to inflammation and fibrosis that cause AF, probiotics/prebiotics could play a bigger role in lowering the risk or slowing the development of age-related AF. This could reduce the AF prevalence in the elderly and decrease exposure to drugs and procedures later. There may even be a role for prebiotics/probiotic therapies in helping to control AF or reduce AF burden in patients with existing AF alongside existing medications and procedures, should solid data be found. This could allow patients, particularly the elderly, to use safer medication to reduce their reliance on other medical therapies that have a number of adverse effects. Recent studies analyzing the efficacy of various AF management strategies have shown issues of efficacy and post-treatment complications, particularly in the elderly [54, 55]. Anti-arrhythmic medications, for example, are known to have toxicities and invasive procedures such as ablations become riskier with age.
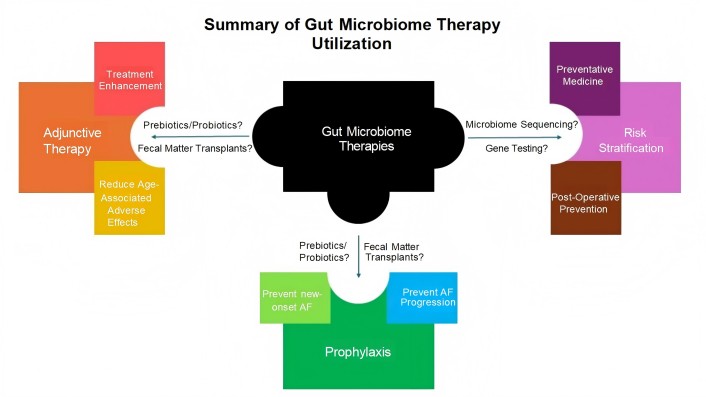
Summary of gut microbiome therapy utilization. The role of gut microbiome directed therapies in three broad categories. One use is as an adjunct to current therapies for atrial fibrillation (AF) (i.e. ablations and medical therapy), mitigating the adverse effects from existing treatments and/or enhancing their effectiveness. Another is the alteration of the microbiome, via medicines or fecal matter transplants, as a low-risk option for AF prophylaxis in the elderly patient population to prevent cardiovascular complications and potentially improve quality of life. Finally, there is the function of gut microbiome sequencing as a method of identifying patients more likely to develop AF, particularly in a post-operative setting. It is important to note that these potential roles for gut microbiome directed therapies are not exclusive and may work in conjunction with each other. Furthermore, it is imperative to analyze the risks versus benefits of adding on any potential therapies for age-related AF
As further research is being done to elucidate this unique pathway, a possibility is to analyze for a protective role of the gut microbiome. Much of the current research seeks to identify gut microbiome profiles in AF patients and potential biomarker targets. However, an interesting avenue of thought is analyzing the gut microbiomes of patients who do not develop AF, but who develop the cardiac pathophysiology thought to increase their susceptibility to AF. There is a growing amount of evidence that the gut microbiome plays a role in maintaining the health of patients with cardiovascular disease [56, 57]. Perhaps there is a protective mechanism offered by the gut microbiome against the development of AF. This could be a mechanism that may unearth a potential therapeutic target used in future AF treatments or for AF prophylaxis.
Studies have also shown that increased clinically adverse events occur during the transition period as compared to paroxysmal AF and persistent AF patients [58]. During this transitional period, molecular changes are noted to be more prominent in the heart. A recent study noted that atrial myocyte compositional changes occurred mostly during this transition period [59]. Therapies targeting the gut microbiome could serve as a mechanism to prevent or delay this transition in AF frequency, thereby reducing the risk of adverse effects in the elderly. One recent study analyzed the differences in the gut microbiome compositions between patients with paroxysmal AF, persistent AF, and non-AF patients. While there were many similar changes in the microbiome compositions of patients with AF, there were specific bacterial species that were distinct in levels between patients with paroxysmal AF and persistent AF such as “ethylovulum miyakonense, and certain metabolites like choline” [60]. Future studies could further solidify the species and metabolites that differ between patients with paroxysmal AF and persistent AF, specifying targets for future therapies. The establishment of a threshold level of a trigger intermediate associated with an increased risk of transitioning in AF frequency could also help physicians take earlier, prophylactic steps in their management. Implementing this as a non-invasive stool test could give clinicians more data to better predict the trajectory of their patients’ AF, enhancing their clinical management of the condition.
Research on gut microbiome sequencing and its use in predicting health and occurrence of disease has been a fascinating avenue of study on the rise. A group of researchers created a “Gut Microbiome Health Index” using metagenomic data from the microbiota profiles of “healthy” and “unhealthy” patients, which was able to predict the prevalence of disease in an individual. A near 74% accuracy in this algorithm in distinguishing between “healthy” and “unhealthy” patients was established, showing promise for the use of microbiota analysis as a tool to analyze patient health non-invasively [61]. Gut microbiome sequencing could also play a role in predicting post-procedural AF. A recent study analyzed how the gut microbiome composition could predict AF recurrence in patients who undergo a cardiac ablation. The researchers found a clear increase in gut dysbiosis in patients who transitioned from non-AF to recurrent AF following a radiofrequency ablation. Further analysis allowed them to make a taxonomic scoring system that showed the difference in bacterial species composition in the gut microbiome between patients who had recurrent AF and those who did not post-ablation. The study’s results suggested that gut microbiome compositional profiles could be used to distinguish patients at the highest risk for developing recurrent AF post-ablation [62]. Future work is needed to clarify the reliability and predictive value of microbiome sequencing, but it may open the door for non-invasive stool test analyses to be used for risk stratification, giving clinicians and surgeons better foresight into which patients are more susceptible to developing AF and adjusting their management accordingly (Figure 3). It may also serve as a predictor for the effectiveness of ablative therapies, providing another data point to help manage AF in a complex elderly patient.
Conclusions
In the end, the role of the gut microbiome in helping us better understand age and its role in developing AF is promising and may help clinicians better manage the ever-growing condition. However, as more research is done studying the gut microbiome, its impact on the development of age-related AF, and the role of this knowledge in AF therapies, there needs to be thought about the scope of treatment and its added value (Figure 3). What is the added value of giving fecal transplants or starting an additional medication to elderly patients and how much would that reduce the risk of AF? Treating the elderly is a more delicate process and potential issues such as polypharmacy, added risks from invasive procedures, and bleeding risk come into question. Thus, as treatments are being curated, there should remain a more omniscient view on how much added benefit comes from a gut microbiome related therapy and if it is meant for prophylaxis or to accentuate current treatments.
Abbreviations
| AF: | atrial fibrillation |
| AI: | atrial ischemia |
| ANG II: | angiotensin II |
| ANS: | autonomic nervous system |
| ARCAF: | age-related changes in autonomic outflow |
| CI: | coronary ischemia |
| HRV: | heart rate variability |
| NLRP3: | nod-like receptor protein 3 |
| RHD: | rheumatic heart disease |
| SCFAs: | short chain fatty acids |
| TMAO: | trimethylamine-N oxide |
Declarations
Author contributions
ML: Conceptualization, Visualization, Writing—original draft, Writing—review & editing. RML: Conceptualization, Visualization, Supervision, Writing—original draft, Writing—review & editing. EC: Visualization, Writing—original draft, Writing—review & editing.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.