Abstract
Tuberculosis (TB) continues to pose a significant public health burden in endemic regions, remaining a leading cause of morbidity and mortality. Although TB is associated with a range of complications, the occurrence of intracardiac thrombus is an exceptionally rare manifestation. We report the case of a 62-year-old male with active pulmonary TB, who had been on anti-tubercular therapy for one month and presented with recurrent syncope and exertional dyspnea. Transthoracic 2D echocardiography revealed a large, mobile thrombus in the left ventricular apex. Evaluation for underlying hypercoagulable states was unremarkable. The patient was managed with anticoagulation therapy in conjunction with ongoing anti-tubercular treatment. This case underscores the importance of clinical vigilance for rare thrombotic complications in TB to facilitate timely diagnosis and appropriate management.
Keywords
Thromboembolism, tuberculosis, anticoagulantsIntroduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is a chronic infectious disease that primarily affects the lungs but can also result in a range of systemic complications. Among these, thromboembolism is an underrecognized yet clinically significant condition that may contribute to increased morbidity and mortality in TB patients. The hypercoagulable state associated with TB arises from chronic inflammation, endothelial dysfunction, increased procoagulant activity, and reduced anticoagulant factors [1]. Elevated levels of inflammatory cytokines, including interleukin-6 (IL-6), activate the coagulation cascade and facilitate clot formation. Activated monocytes and macrophages also play a pivotal role in coagulation by producing tissue factor, which initiates the extrinsic coagulation pathway, resulting in thrombin generation and fibrin clot formation [2]. All these factors predispose patients to thrombotic events, particularly in severe or disseminated TB cases. Recognizing the risk of thromboembolism in TB is essential for timely diagnosis and appropriate management, as delays can lead to life-threatening complications. While thromboembolic events such as deep venous thrombosis (DVT) and pulmonary embolism (PE) have been well documented in association with TB, the development of an intracardiac thrombus remains an exceptionally rare but potentially fatal phenomenon, with risks of systemic embolization or impaired cardiac function. This report describes a unique case of a left ventricular thrombus in a patient with active pulmonary TB, emphasizing the need for increased awareness of such rare complications. Early recognition and prompt management are critical to improving long-term outcomes.
Case report
A 62-year-old male, recently diagnosed with pulmonary TB (microbiologically confirmed) and on anti-tubercular treatment (ATT) for one month (isoniazid, rifampicin, ethambutol, and pyrazinamide), presented with recurrent episodes of syncope and exertional shortness of breath. There was no history of chest pain, orthopnea, palpitations, or swelling. The patient had a history of TB treated 10 years prior and no other known comorbidities. He was a non-smoker and non-alcoholic. On examination, the patient was conscious and oriented. Vitals were as follows: blood pressure 94/50 mmHg, heart rate 102/min, and SpO2 97% on room air. Chest auscultation revealed coarse crepitations and rhonchi in the upper and mid-zones bilaterally. Other systemic examinations were unremarkable.
Routine laboratory investigations were within normal limits except for a raised ESR of 42 mm/h. The chest X-ray (Figure 1) revealed fibrotic changes in the bilateral upper and mid-zones accompanied by a tractional mediastinal shift toward the right. Additionally, infiltrations were observed in the left upper and mid-zones. These radiological findings were consistent with active TB and sequelae of prior infection. A 12-lead ECG revealed sinus tachycardia with no significant ST-segment or T-wave changes. Magnetic resonance imaging (MRI) of the brain was unremarkable. Electroencephalogram (EEG) did not reveal any epileptiform activity or evidence of seizures. Transthoracic 2D echocardiogram was done, which revealed a large, mobile left ventricular apical thrombus measuring 54 mm × 13 mm (Figure 2). All cardiac chambers were of normal size, with a left ventricular ejection fraction of 50% and without any regional wall motion abnormalities. Further tests were conducted to evaluate for hypercoagulable states. The plasma D-dimer level was 596 ng/mL. Levels of protein C, protein S, antithrombin III, and homocysteine were within normal limits. Genetic testing for factor V Leiden, methylene tetrahydrofolate reductase (MTHFR) mutation, and prothrombin gene mutation yielded negative results. Additionally, antiphospholipid antibody testing, including anticardiolipin and anti-beta2-glycoprotein antibodies, was negative, and lupus anticoagulant was not detected. Doppler ultrasonography of the lower extremities showed no evidence of DVT. The patient was initiated on anticoagulation therapy with subcutaneous low molecular weight heparin while ATT was continued. He showed gradual clinical improvement and was discharged after two weeks of hospitalization on ongoing ATT and oral anticoagulation with warfarin.
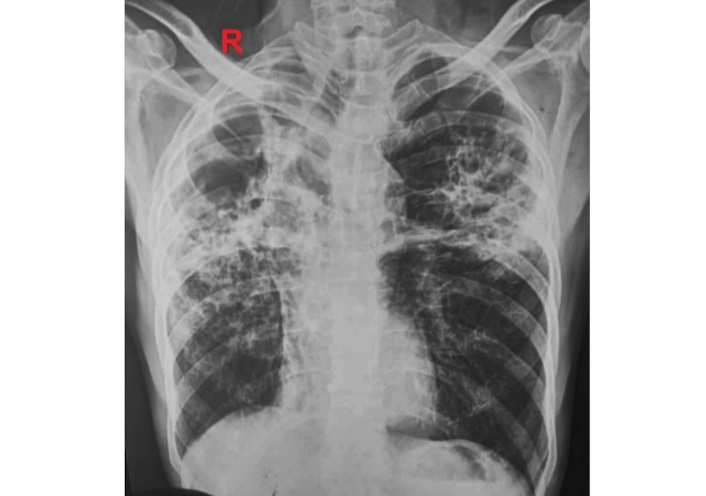
Chest X-ray showing features suggestive of active tuberculosis and sequelae of prior infection
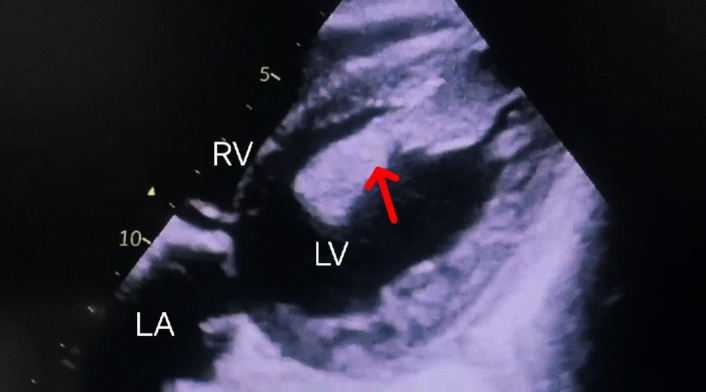
Bidimensional transthoracic echocardiogram (subcostal view). Showing a large thrombus (red arrow) in the left ventricular cavity, measuring 54 mm × 13 mm. The thrombus is partially adhered to the apex and partially mobile. LV: left ventricle; LA: left atrium; RV: right ventricle
Discussion
The hypercoagulable state associated with TB can lead to thromboembolic events in approximately 0.6–1.0% of patients, with venous thromboembolism (VTE) being the most frequently observed manifestation [3]. Several case reports have demonstrated a direct association between TB and VTE, independent of other established risk factors [4, 5]. Thrombotic events may occur within days of diagnosis or later in the course of the disease, even in patients undergoing antitubercular treatment. Robson et al. [6] observed 35 patients with pulmonary TB and DVT, finding that in 33 cases, DVT developed within seven days of the TB diagnosis, while it was the presenting feature in two cases. Similarly, Ambrosetti et al. [7] reported a 0.6% incidence of DVT (five cases) and PE (two cases) among 1,237 TB patients in Italy. Cases of thrombosis in unusual sites, such as the cortical venous sinus, hepatic vein, and retinal vein, have also been documented in patients with TB [8–10]. Additionally, there have been reports of arterial thrombosis associated with TB. Bansal et al. [11] reported a case of pulmonary TB complicated by multiple venous and arterial thrombi involving the pulmonary artery, pulmonary vein, splenic vein, common hepatic artery, and gastroduodenal artery. Similarly, Sharma A and Sharma V [12] documented a case of abdominal aortic thrombosis in a patient with abdominal TB. Nicholson et al. [13] described axillary artery thrombosis in a patient diagnosed with pulmonary TB. However, the development of a left ventricular thrombus, as seen in this case, is an exceptionally rare complication of pulmonary TB. Intracardiac thrombi are more commonly associated with conditions like myocardial infarction, cardiomyopathy, and atrial fibrillation. Razafimanjato et al. [14] have described a case of tubercular pleural effusion syndrome associated with left ventricular thrombus.
The pathophysiology of intracardiac thrombus formation in TB likely involves a combination of factors, including myocardial inflammation, impaired cardiac contractility, and a prothrombotic state driven by haemostatic and inflammatory changes. TB has been established as an independent risk factor for thrombosis. It triggers a robust immune response characterized by the release of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), IL-1, IL-6, interferon-gamma (IFN-γ), and IL-12, all of which contribute to a prothrombotic state. Elevated IL-6 levels are associated with increased fibrinogen synthesis, promoting coagulation [15]. TNF-α plays a crucial role in phagocyte activation but also mediates tissue damage, which can lead to the release of tissue factor and activation of the coagulation cascade. In addition, in vitro studies have demonstrated that Mycobacterium tuberculosis can directly induce tissue factor expression in monocytes and macrophages, serving as a key trigger of the coagulation cascade [16]. Endothelial dysfunction, resulting from the systemic inflammatory response, disrupts vascular homeostasis through alterations in key mediators such as vascular endothelial growth factor (VEGF), nitric oxide (NO), endothelin-1 (ET-1), and von Willebrand factor antigen (vWF) [17]. Furthermore, levels of ADAMTS13 (a metalloproteinase that regulates vWF) are significantly reduced in TB, resulting in elevated vWF concentrations and thereby enhanced platelet adhesion and aggregation [18]. Collectively, these changes contribute significantly to a prothrombotic milieu that may complicate the clinical course of TB. In the MEDENOX study, patients with TB demonstrated a significantly increased risk of VTE, with an odds ratio of 1.62—comparable to that observed in malignancy [19]. Turken et al. [20] reported that patients with active pulmonary TB exhibited thrombocytosis, elevated levels of fibrinogen, factor VIII, and plasminogen activator inhibitor-1, along with decreased levels of anticoagulants such as antithrombin III and protein C. In addition, rifampicin—an enzyme-inducing antitubercular drug—may further aggravate hypercoagulability by altering hepatic synthesis of coagulation and anticoagulation factors, increasing the risk of thromboembolic events by up to 4.74 times in patients with pulmonary TB [21].
In our patient, the development of an intracardiac thrombus can be attributed to a multifactorial prothrombotic environment caused by TB. Although the patient had normal left ventricular function and no regional wall motion abnormalities, localized endocardial injury from inflammatory mediators or transient flow disturbances may have contributed to thrombus formation in the left ventricular apex. Thus, the interplay between systemic inflammation, endothelial dysfunction, a hypercoagulable state, and possibly localized cardiac factors likely contributed to the rare occurrence of intracardiac thrombus in this patient with TB. Normal MRI brain and EEG findings suggest that the recurrent syncopal episodes may be attributed to intermittent obstruction of left ventricular outflow or transient reductions in cardiac output caused by the mobile thrombus, leading to cerebral hypoperfusion.
In conclusion, intracardiac thrombus secondary to TB poses significant diagnostic and therapeutic challenges. Echocardiography is critical for timely diagnosis, and anticoagulation therapy, along with antitubercular treatment, remains the cornerstone of management. This case underscores the importance of maintaining a high index of suspicion for thromboembolic complications in TB, as early recognition and prompt management can significantly improve clinical outcomes.
Abbreviations
| ATT: | anti-tubercular treatment |
| DVT: | deep venous thrombosis |
| EEG: | electroencephalogram |
| IL-6: | interleukin-6 |
| MRI: | magnetic resonance imaging |
| PE: | pulmonary embolism |
| TB: | tuberculosis |
| TNF-α: | tumor necrosis factor-alpha |
| VTE: | venous thromboembolism |
| vWF: | von Willebrand factor antigen |
Declarations
Author contributions
AK: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervision. AD: Investigation, Writing—original draft, Writing—review & editing. YS: Investigation, Validation, Writing—review & editing. ST: Validation, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The Declaration of Helsinki was adequately addressed, and the study was approved by the ESIC PGIMSR institutional ethics committee [42/2024].
Consent to participate
Informed consent to participate in the study was obtained from the participant.
Consent to publication
Informed consent to publication was obtained from the participant.
Availability of data and materials
The data of this manuscript could be available from the corresponding authors upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.