Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical University of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
Email: durajiwona@gmail.com
ORCID: https://orcid.org/0009-0003-5113-2214
Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical University of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
ORCID: https://orcid.org/0009-0003-7335-116X
Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical University of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
ORCID: https://orcid.org/0000-0003-1058-0590
Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical University of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
ORCID: https://orcid.org/0000-0002-7623-5389
Explor Cardiol. 2023;1:59–71 DOI: https://doi.org/10.37349/ec.2023.00008
Received: April 21, 2023 Accepted: June 13, 2023 Published: October 30, 2023
Academic Editor: Andrea Borghini, Institute of Clinical Physiology-National Research Council (IFC-CNR), Italy
The clinical manifestations of COVID-19 which mainly involve the respiratory system may however affect also cardiovascular system. There are a lot and still increasing numbers of reports revealing cardiovascular complications of COVID-19, which may occur in the acute phase as well as during longer follow-up period. The most clinically important diseases include: pulmonary embolism (PE), myocarditis, and acute coronary syndromes (ACS) as well as arrhythmias with the very common atrial fibrillation (AF) and pericarditis. In this review, cardiac imaging options in patients with and after coronavirus infection are presented, showing potential utility for expanding and improving the full and accurate diagnosis of potential complications. Echocardiography, magnetic resonance imaging, and computed tomography (CT) are considered in turn, highlighting their best advantages in patients affected by COVID.
Coronavirus disease 2019 (COVID-19), caused by a new single-stranded RNA virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in December 2019 and quickly became a pandemic leading to a global public health crisis with extremely high mortality and morbidity [1, 2]. According to the World Health Organization (WHO) data until March 2023, more than 750 million people had the confirmed infection, and more than 6 million deaths were reported worldwide [3]. In Poland, more than 6 million infections were reported, and nearly 120,000 people died from COVID-19. During infection, the viral proteins bind to the human angiotensin-converting enzyme 2 (ACE2) receptors in alveolar cells which are the main gateway for the virus, but are present also in vascular endothelium, fibroblasts, and smooth muscle cells [4]. Fortunately, the introduction of global vaccination against COVID-19 and the successful accomplishment of the whole series of injections in a large part of the population reduced significantly the infection rate as well as the severity and mortality of the disease [5].
The most commonly listed symptoms of SARS-CoV-2 infection include fever (83–98%), cough (50–82%), fatigue (25–44%), shortness of breath (19–55%) and muscle soreness (11–44%) [6, 7]. While most SARS-CoV-2 cases in outpatients manifest themselves as relatively mild respiratory illness, about 15% of patients require hospital care for pneumonia [8], see examples in Figure 1. Moreover, some patients may develop acute respiratory distress syndrome (ARDS), blood clotting disorders, shock, and multi-organ failure even within 1 week after admission [9, 10]. The mortality rate varies depending on age, comorbidities, and many other factors. Since the beginning of the pandemic, attention has been drawn to the more severe course of infection in patients with cardiovascular disease (CVD). Specifically, patients with COVID-19 and hypertension were reported to have a higher risk of mortality, and those with hypertension, diabetes, and obesity more often required hospitalization.
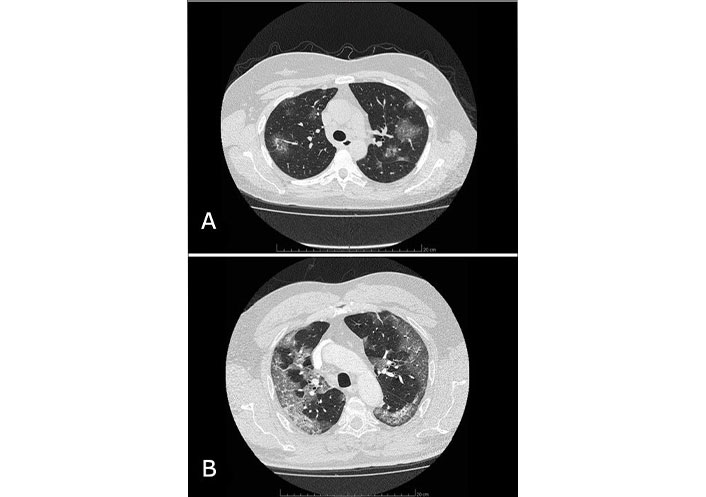
Two examples of CT images of lung involvement in COVID-19 pneumonia. A: Chest computed tomography (CT) image of a 47-year-old female patient admitted to our Clinic in 2020 with confirmed COVID-19 infection by real-time polymerase chain reaction (PCR) test with reverse transcriptase for COVID-19, who required, beyond medical treatment, passive oxygen therapy. Visible glass ground opacities lesions involve 25–30% of the lungs. Patient recovered and stayed alive during long-term follow-up after 2 weeks of hospitalization; B: contrast-enhanced CT image of the chest in a 66-year-old male patient with PCR-confirmed COVID-19 infection. Areas of ground glass opacities with consolidation and air bronchogram involve 40% of the lungs. Despite intranasal high-flow oxygen therapy, followed by mechanical ventilation in an intensive care setting the patient died
A number of scientific studies have emerged to understand the complex pathophysiological mechanisms responsible for the course of infection and its complications caused by coronavirus. A large role has been attributed to ACE2 receptor interaction with virus, cytokine storm with especial function of monocyte chemotactic protein 1 (MCP-1), interleukin (IL)-1β, and tumor necrosis factor (TNF) α with endothelial inflammation and coagulopathy, but still, further studies are needed to fully understand the mechanisms of organ damage, and translate this knowledge into optimal treatment [11]. Additionally, the harmful effects of COVID-19 infection on the cardiovascular system were enhanced by the stress of the pandemic outbreak, as well as the impact of loneliness, lack of positive relationships, and forced isolation required during lockdown.
For example, in patients hospitalized for COVID-19 in Qatar who were assessed for chest pain, heart failure symptoms, elevated troponin T, electrocardiogram (ECG) changes and arrhythmias C-reactive protein (CRP), troponin T, urea, creatinine, and leukocyte count were higher in those with fatal outcome, while heart rate, diastolic and systolic blood pressure, and O2 saturation showed lower value [12]. The most common manifestation of cardiovascular complications in COVID-19 was an increase in troponin, ECG abnormalities: ST-segment and T-wave (ST-T) changes, T-waves inversion, QT interval prolongation or arrhythmias, and chest pain.
The present review is aimed at the presentation of cardiovascular complications in the course of COVID-19 and at the discussion of most utile imaging modalities in such patients.
Although the clinical manifestations of COVID-19 involve mainly the respiratory system, cardiovascular damage is also possible. The prevalence of cardiovascular involvement in the course of SARS-CoV-2 infection is reported to range from 12% [13] to 20% [14], and is significantly higher compared to the approximately 1% incidence of circulatory complications in acute viral infections other than COVID-19. The mechanisms of cardiovascular damage in COVID-19 are multifactorial and are still not fully understood. SARS-CoV2 virus can lead to immediate cardiac damage via ACE2 receptors located in myocytes [15] as well as to damage to the cardiovascular system by interfering with the immune response, systemic inflammatory mechanisms with increased cytokines activity, and a subsequent increase in the cardiac oxygen demand, and increased prothrombotic activity. COVID-19 infection can also induce systemic complications such as sepsis and DIC, which in turn can cause various cardiac aftermaths. There is data that patients with COVID-19 are at an increased risk of CVD including pulmonary embolism (PE) [16].
Although the frequency of cardiovascular consequences in patients with COVID-19 is not known in a detailed manner, it was observed that a history of prior CVD may be associated with a more severe course of infection [17]. As an example, Wu et al. [8] recorded a fivefold increase in COVID-19 mortality in patients with pre-existing CVD, which amounted to 10.5% versus 2.3%.
In the course of SARS-CoV2 infection, myocardial damage, myocarditis including those with a fulminant course, acute coronary syndrome (ACS), acute myocardial infarction (AMI), Takotsubo cardiomyopathy, various arrhythmias up to cardiac arrest, venous thromboembolism with PE as well as heart failure are possible [18]. The prothrombotic activity in COVID-19 may increase the risk of venous as well as arterial thrombosis [19]. It is important to note that myocardial involvement can occur even in the absence of symptoms of upper respiratory tract infection [20], which underscores the importance of cardiovascular monitoring in such patients. Moreover, elevated levels of cardiac injury biomarkers such as N-terminal pro-B-type natriuretic peptide (NT-proBNP), cTn, or D-dimer were associated with a worse prognosis in the course of SARS-CoV2 infection [21]. There are findings of elevated creatine kinase MB fraction (CK-MB) as well as IL-6 levels in COVID-19 patients with acute myocardial injury [22]. Similarly, elevated fibrinogen and D-dimer have been shown predictors of poor prognostic outcome in hospitalized patients with COVID-19 [23].
Motloch et al. [23] retrospective study involving 1,746 patients hospitalized for COVID-19 pneumonia evaluated the effect of two anticoagulant treatment regimens used after discharge with an oral anticoagulant (rivaroxaban, dabigatran, or apixaban) or dipyridamole. The smaller control group consisted of patients without anticoagulant treatment. Both therapies oral anticoagulant and dipyridamole were associated with a decrease in overall mortality and cardiovascular mortality. Moreover, the treatment with an oral anticoagulant led to a reduction of stroke and PE, while dipyridamole resulted in a diminution in the incidence of PE without affecting the stroke and myocardial infarction prevalence.
Zagidullin et al. [24] in a retrospective analysis showed a higher frequency of J-wave in ECG (late positive wave following the QRS complex), similar to those observed in hypothermia, in patients hospitalized because of COVID-19 pneumonia compared to the general population (12% versus 5–6%). Factors associated with its presence were older age, female sex, stroke, heart failure, and high CRP levels. The presence of J-wave was confirmed to be an independent predictor of mortality.
Cardiac arrhythmias may occur during COVID-19 infection although their frequency has not been well described yet. It is also possible that some arrhythmias may be related to electrolyte disturbances in infected patients. According to current knowledge, the most common arrhythmia in COVID-19 is atrial fibrillation (AF), and COVID-19 itself is increasingly seen as an independent risk factor for stroke in patients with AF [25]. Other arrhythmias in patients with COVID-19 include sinus tachycardia, atrial flutter, atrial tachycardia, monomorphic or polymorphic ventricular tachycardia, bradycardia, and atrioventricular conduction block [26]. It is worth noting that while COVID-19 infection itself is a factor that increases the risk of arrhythmias, also pharmacologic treatment is problematic due to the risk of bronchospasm when using beta-blockers. Moreover, some medicines used especially in the early stages of the pandemic, e.g., macrolide antibiotics, revealed proarrhythmic properties by prolonging the QTc interval [27]. Myocardial damage can lead to atrial or ventricular fibrosis, which can be a substrate for subsequent arrhythmias, and may be assessed with cardiovascular magnetic resonance (CMR); in such a way CMR can be useful for better assessment of the arrhythmic risk in patients recovered from COVID-19.
There have been a lot of recent studies on PE in patients with COVID-19 infection. In a meta-analysis by Suh et al. [28] including 3,342 patients with COVID-19, it was shown that the incidence of PE was 24.7% in patients admitted to the ICU and 10.5% in non-acute patients. The researchers noted that studies in which pulmonary angiography was performed in all patients showed a higher incidence of PE compared to those in whom it was used based on clinical presentation and additional tests, raising the idea that the incidence of PE in COVID-19 patients may be underestimated. PE was more likely to involve peripheral segments of the pulmonary arteries, and D-dimer levels > 1,000 ug/L showed high sensitivity but low specificity in the diagnosis of PE in COVID-19.
In a multicenter observational study including patients with COVID-19 admitted to 7 Italian hospitals, the incidence of PE was 14% [29]. Serum D-dimer and high-sensitivity troponin levels were significantly higher in PE compared with non-embolic patients. Patients with PE had also lower tricuspid annular plane systolic excursion (TAPSE) (18 mm versus 21 mm) and higher sPAP values on transthoracic echocardiography (TTE). In a single-center, prospective study, PE was detected in 23% of patients with new or persistent dyspnea after COVID-19 infection [30]. In this study, patients in New York Heart Association (NYHA) class III and IV showed a higher rate of PE compared to patients in class I and II.
Echocardiography played the role of an important bedside test in suspected cardiac damage in the course of COVID-19, especially in hemodynamically unstable patients, see example in Figure 2. It allows us to visualize features of myocarditis, myocardial damage in the course of myocardial infarction and its complications, Takotsubo syndrome, right ventricular (RV) overload from PE as well as the presence of pericardial effusion. In the course of myocarditis, TTE examination reveals thickening of the heart walls, ventricular dilatation, pericardial effusion, and left ventricular (LV) systolic dysfunction. In patients with Takotsubo cardiomyopathy, the typical echocardiographic picture of transient hypokinesis, akinesis, or dyskinesis of the middle segments with or without involvement of the LV apical segments is one of the primary criteria for diagnosis.
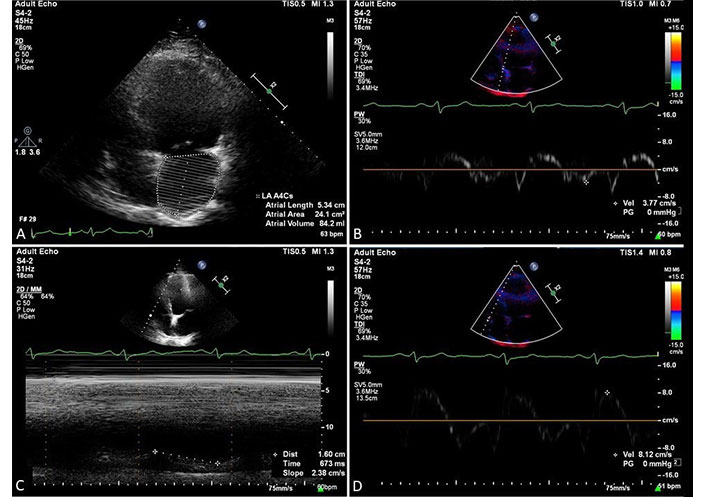
The examples of cardiac involvement were found in echocardiography in a 74-year-old male patient with COVID-19 pneumonia and clinical suspicion of PE. A: moderate dilatation of the left atrium as expressed by left atrial volume of 84 mL; B: spectral display of septal mitral annulus velocity with decreased peak early wave velocity, E’ = 3.77 cm/s; C: slightly decreased global RV systolic function parameter, TAPSE = 16 cm; D: mild decrease of tricuspid annulus systolic velocity obtained by tissue Doppler method. S’ = 8.12 cm/s
Giustino et al. [31] in a multicenter cohort study conducted in New York and Milan, showed that myocardial damage, defined as elevated cardiac troponin levels, affected 67% of hospitalized patients, with in-hospital mortality rates of 18.6% and 31.7% in patients with myocardial damage without TTE abnormalities and with myocardial damage and echocardiographic abnormalities, respectively. TTE abnormalities that affected almost two-thirds of the patients with myocardial damage included segmental LV wall motion abnormalities, global LV dysfunction, grade II or III diastolic dysfunction as well as RV dysfunction and pericardial effusion.
In a retrospective cohort study, TTE was performed in patients with COVID-19 lung inflammatory infiltrates described on X-ray or CT and elevated troponin I levels or in emergency situations requiring TTE [32]. In the patients studied, LV systolic function was hyperdynamic or normal in most cases (89%), while RV dilatation was present in 41%, and RV function impairment affected 27% of patients. RV systolic dysfunction was associated with PE in 20% and elevated D-dimer and CRP levels but no association with increased hsTnI was proven. In the cited study, RV size and function were not associated with the use of vasopressors or mechanical ventilation.
Dweck et al. [33] in a prospective, international study involving about 1,200 patients with COVID-19 showed that echocardiographic abnormalities occurred in more than half of the group. In most, the heart damage involved the left ventricle and was nonspecific, although some patients had myocardial infarction, myocarditis, and Takotsubo cardiomyopathy. RV abnormalities were more common in severe COVID-19 and were probably associated with severe pneumonia and PE. In this study, 60% of the examinations were performed in the intensive care setting, and the imaging result changed the management in as many as 30% of patients, often leading to the initiation of disease-specific treatment, hemodynamic support, or a change in the level of patient care. On the basis of TTE, treatment was initiated for heart failure, ACS, tamponade, PE, and infective endocarditis. Changes in the management were more common in patients with prior heart disease and elevated levels of cardiac biomarkers.
In a prospective study involving 100 patients hospitalized for COVID-19 who had TTE, the most common abnormality was RV dilatation and/or dysfunction, followed by LV diastolic dysfunction [34]. LV contractility [ejection fraction (EF)] remained normal in 90% of examined. In patients in severe conditions, further deterioration of RV parameters was visualized, which could be related to increased pulmonary resistance. The cited study showed that the most common cause of B-lines in patients with COVID-19 infection was non-cardiogenic pulmonary edema due to infection typically not related to elevated LV filling pressures.
It has been increasingly emphasized that 2D-STE may have a potentially beneficial role in the evaluation of the cardiovascular system in patients with COVID-19 [35] with LV global longitudinal strain (LV-GLS) and RV free wall strain (RV-GLS) offering the prognostic value as independent predictors of mortality. It is noteworthy that patients with COVID-19 who described LV and RV dysfunction on TTE showed improvement after recovery from the acute phase of the disease. In a multicenter, observational cohort study, the frequency of adverse ventricular remodeling of the heart was evaluated in patients who survived COVID-19 pneumonia requiring hospitalization [36]. Parameters of TTE performed during hospitalization were normal in 46% of patients, abnormalities such as RV dilatation were visualized in 39% of patients, RV dysfunction affected 27% and LV dilatation and dysfunction were described in 4% and 13% of patients, respectively. In a 3-month follow-up examination, normal TTE was noted in 71% of patients and 20% had unfavorable RV remodeling. In the last group of patients, almost half of them were diagnosed with PE during hospitalization and, additionally, 6% presented unfavorable LV remodeling.
The COVID-19 pandemic has become the largest healthcare challenge in recent years. Echocardiography, including bedside echocardiography, played an important role in identifying patients at high risk of death and cardiovascular complications. The easy availability of the test, its lack of harmful effects for patients, and its ability to quickly assess the anatomy and function of the cardiovascular system and evaluate hemodynamics are well-known great advantages. However, the protection of personnel performing the test has to be observed in a detailed manner and appropriate clinical indications should be applied due to possible transmission of infection [37].
CMR imaging is a test that can indicate myocardial damage associated with both ischemic and non-ischemic diseases, and assesses myocardial function and tissue characteristics. This test is a valuable source of information on pathological processes, including myocardial edema, congestion, necrosis, and fibrosis by using changes in the basic magnetic properties of the tissue (T1 and T2 relaxation). CMR can confirm or exclude active myocarditis using the Lake Louise criteria [38] with secondary criteria being pericardial effusion and LV systolic dysfunction, although these criteria need validation for patients with COVID-19. In the study evaluating the results of cardiac CMR imaging in 199 patients with COVID-19 and elevated troponin levels, myocarditis was the most common diagnosis and affected 40% of patients screened [39]. Abnormalities included T1 and T2 mapping, myocardial edema at T2, and late gadolinium enhancement (LGE) most often observed in the subepicardial location, however, the systolic function of both ventricles remained normal in most patients. CMR may also improve diagnostic specificity being a method of identifying features of myocarditis which has implications for treatment and prognosis [40].
Interesting conclusions were provided by Putmann et al. [41] based on a cohort study involving 100 patients recently treated for COVID-19. Patients with confirmed COVID-19 infection by RT-PCR in an upper respiratory tract swab test underwent magnetic resonance imaging 2 weeks after the initial diagnosis, which revealed cardiac involvement in 78% of them, defined as increased native T1 and T2 myocardial mapping, LGE or pericardial enhancement. Ongoing myocarditis defined as abnormal native T1 and T2 measurements was diagnosed in 60% of patients. It is noteworthy that the results were independent of the severity and overall course of the acute illness. The results of this study indicate the need to follow the long-term cardiovascular consequences of COVID-19.
A prospective, multicenter study conducted in the UK in patients hospitalized with COVID-19 and elevated troponin levels who had CMR performed, showed that they were twice as likely to have cardiac abnormalities as in the COVID-19 population without troponin elevation as well as in those without infection [42]. Left and RV wall motion abnormalities, pericardial effusion, and myocardial scarring were described more frequently in these patients, which were mainly acquired due to the myocardial ischemia, including microinfarcts, highlighting the prothrombotic consequences of COVID-19. In the cited study, myocardial scarring was an independent predictor of adverse cardiovascular events. A retrospective study including 64 outpatients with COVID-19 infection showed cardiac involvement in 71% defined as the presence of LGE or pericardial effusion on CMR [43]. Patients with cardiac involvement showed predominantly focal myocardial edema and often normal left ventricular function and volume, which may indicate common cardiac involvement in non-severe COVID-19 patients without high TnT values since in the cited study 14% of patients had significantly elevated TnT levels.
According to current studies, in patients with COVID-19 infection, CMR can observe the following: left and RV dilatation and dysfunction, myocardial infarction, although it is emphasized that micro-infarcts may occur, myocarditis: non-ischemic CMR patterns of the right ventricle involvement have been described, but are not specific for COVID-19, pericardial effusion as well as a thrombus in both the left and right ventricles [32]. Although there are currently no pathognomonic CMR patterns specific to COVID-19 myocarditis, more studies are needed to understand this issue in more detail. To sum up, CMR is a test of high diagnostic value, has many imaging benefits, and the number of reports of myocardial involvement in COVID-19 using this method is rapidly increasing [44], nevertheless, its availability, the need for patient cooperation during long examination time and the requirement for post-examination disinfection of the machine in patients with an infectious disease remain main problems.
In most patients with suspected COVID-19 inflammatory pneumonia, a CT scan of the chest is performed to detect the disease, assess its severity, monitor progression and potential complications. CT lesions in patients with COVID-19 lung involvement are usually bilateral, with peripheral, subpleural distribution. Typical are interstitial ground glass opacities with or without consolidation, reticular pattern with air bronchogram, then fibrosis, pleural thickening and effusion as well as nodules with halo sign, lymphadenopathy, and pericardial effusion [45]. Perincek et al. [46] in their study showed that a cutoff value of 5.5 for global CT score (depending on the degree of lung lobe involvement, in the possible range of 0–25) predicted in-hospital mortality with 74% sensitivity and 73% specificity, and global CT score was correlated with levels of CRP, D-dimer, natriuretic peptide and procalcitonin [47]. In Table 1 commonly used CT score in patients with COVID-19 pneumonia is presented.
Semi-quantitative scoring system to quantify COVID-19 lung involvement [41]
| Area involved | Scores |
|---|---|
| No involvement | 0 |
| < 5% involvement | 1 |
| 25% involvement | 2 |
| 26–49% involvement | 3 |
| 50–75% involvement | 4 |
| > 75% involvement | 5 |
The total CT score is the sum of the scores of each of the five lung lobes and ranges from 0 (no involvement) to 25 (maximum involvement), which approximates the degree of lung involvement and has prognostic significance for subsequent disease progression [41]
There are reports on the prognostic value of epicardial adipose burden on CT, which acts as a reservoir for coronavirus and may contribute to myocarditis perseveration and enhancement [12]. Özer et al. [48] assessed epicardial adipose thickness on chest CT in patients with COVID-19, who were divided into two groups with and without myocardial damage defined as elevated troponin levels. The thickness of the epicardial adipose layer ≥ 4.85 mm was associated with myocardial damage with a sensitivity of 65% and specificity of 39%. Moreover, the amount of epicardial adipose was correlated with BMI.
CT pulmonary angiography (CTPA) is the principal method that confirms PE, which is a severe and frequent complication in patients with COVID-19 pneumonia, who are at higher risk for thromboembolic events. In a prospective study involving 179 patients with confirmed COVID-19 pneumonia and D-dimer > 1 ng/mL, CTPA was performed [49] showing a high prevalence of PE, reaching almost 40%, regardless of clinical suspicion. PE showed a peripheral distribution, most often involving the segmental, lobar, or subsegmental arteries. Another interesting retrospective study using contrast-enhanced CT including pulmonary artery angiography emerged, in which the CT assessment was performed in all patients with severe COVID-19 requiring intensive care in the ICU setting [50]. PE was diagnosed in 47% of patients despite ongoing heparin prophylaxis, and in 35% of cases, the thrombi were located in the main pulmonary artery and/or proximal branches.
A retrospective study involving 180 patients evaluated the association between visual assessment of coronary artery calcification index [coronary artery calcium (CAC)] on non-contrast chest CT and the severity of COVID-19 [51]. The results showed that COVID-19 patients with coronary artery calcification were more likely to need intubation. Moreover, higher CAC was associated with increased mortality. Similar conclusions were reached by Dillinger et al. [52] showing that the presence and degree of CAC was associated with a worse prognosis in patients hospitalized for COVID-19. Subclinical coronary artery atherosclerosis superimposed on an enhanced immune response, endothelial dysfunction, and higher myocardial oxygen demand can significantly worsen the clinical course of COVID-19 in patients without previously diagnosed CAD, which was also confirmed by the results of a French observational study. In the cited study, visual CAC score was an independent predictor of 6-month mortality in patients hospitalized for COVID-19 without known CVD [53]. During the COVID-19 pandemic, chest CT scanning became the primary method for diagnosing viral pneumonia as well as tracking its complications. Additional CAC detection, although not routinely performed, maybe a relatively available marker of worse prognosis in patients with COVID-19. In Table 2 the main features which may be assessed in the respective noninvasive modalities are presented. There are studies aimed at an estimation of the prevalence of main imagined symptoms in patients with COVID-19. For example, Szekely et al. [34] show that the most common echocardiographic sign observed in COVID-19 patients was RV dilation with or without dysfunction, which affected 39 patients out of 100 included in the study, followed by left ventricular diastolic dysfunction observed in 19 patients. However, the data concerning the sensitivity and specificity of imaging signs in COVID-19 patients are so far sparse.
Imaging of cardiovascular complications and features in COVID-19
| TTE | MRI | CT |
|---|---|---|
| RV dilatationa | Nonischemic injury (T1-based) 1. Abnormal T1 mapping 2. Increase extracellular volume quantification 3. Late gadolinium enhancement subepicardial midwall, patchy or scattered distribution of LGE | Pericardial effusion |
| Left ventricular contractile dysfunction | Myocardial edema (T2-based) 1. Abnormal T2 mapping 2. Abnormal T2- weighted imaging | Epicardial fat thickness |
| Left ventricular diastolic dysfunction | Global or regional abnormality systolic function (cine) | CTPAb |
Pericardial effusion Decreased GLS | Pericardial effusion | CAC detectionc |
a RV dilatation and dysfunction as the most common echocardiographic abnormalities with prognostic significance in most patients resolve after a period of acute infection. b CTPA is the principal method that confirms PE. c Additional CAC detection, although not routinely performed, maybe a relatively available marker of worse prognosis in patients with COVID-19
Myocardial perfusion scintigraphy can be helpful in the evaluation of cardiac involvement in patients with COVID-19, including the diagnosis of myocardial ischemia, assessment of myocardial viability and also in choosing the best treatment for patients with heart failure [54].
There are reports on the use of myocardial single photon emission computed tomography (SPECT) in patients with a history of COVID-19. A retrospective analysis of patients who underwent SPECT after COVID-19 infection within the past 6 months compared to age- and sex-matched controls who had the test before the pandemic was conducted, noting an increase in the rate of ischemia compared to the uninfected cohort [55]. The hypothesis has been proposed that COVID-19 may induce myocardial ischemia, although evidence is lacking to establish a direct causal relationship between symptomatic COVID-19 infection and coronary artery disease. Similar conclusions were reached by Çap et al. [56], who demonstrated an increase in myocardial ischemia indices on SPECT in patients with chest pain and/or dyspnea after COVID-19 without a history of prior coronary artery disease [56].
Recent studies show that cardiovascular imaging in COVID-19 patients with suspected cardiac involvement plays an important role. The first and most widespread method is TTE, which, in the hand of an experienced physician, can visualize the many abnormalities of cardiac structure and function in COVID-19 and therefore can direct the diagnosis and initiate specific treatment. However, TTE has its limitations, including the difficulty of obtaining good-quality imaging in patients who are obese, mechanically ventilated, or have severe lung diseases. Because of the possibility of transmission of infection due to close contact with the subject, it is necessary to precisely establish the indications for the test, postponing its performing, whenever possible, to the phase with a negative antigen test in patients.
According to current findings, COVID-19 can also lead to cardiac involvement and implications in patients without respiratory symptoms. The cited results of CMR studies render caveats of not ignoring the symptoms reported by patients even with a mild course of infection. CMR is an imaging method that allows to characterize the properties of cardiac tissues in vivo, the assessment, and the consequences of cardiac damage, including features of myocarditis. The limitations of CMR are the inability to perform the test in acute COVID-19, critical condition, the high cost of the test, and low availability as well as long examination time and the need for patient cooperation.
Chest CT as the primary method of diagnosing COVID-19 pneumonia can provide information on cardiac involvement, and CAC, which is not routinely determined especially in patients without known coronary artery disease. The published reports of a high risk of thromboembolic complications in COVID-19 detected with CTPA are of the highest clinical utility, despite the limitations such as the difficulty of contrast administration in patients with kidney disease and the radiation dose the patient receives.
It is worth emphasizing that the clinical course, especially chest pain, fluctuations in blood pressure, elevated levels of biomarkers such as troponin, CK-MB mass, and NT-proBNP, especially with concomitant ECG changes, should prompt the physician to reach for imaging methods of cardiac involvement in the course of COVID-19, including TTE, CT, CMR, or even coronary angiography.
ACE2: angiotensin-converting enzyme 2
CAC: coronary artery calcium
CMR: cardiovascular magnetic resonance
COVID-19: coronavirus disease 2019
CRP: C-reactive protein
CT: computed tomography
CTPA: computed tomography pulmonary angiography
ECG: electrocardiogram
LGE: late gadolinium enhancement
PCR: polymerase chain reaction
PE: pulmonary embolism
RV: right ventricular
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
SPECT: single photon emission computed tomography
TTE: transthoracic echocardiography
ID: Conceptualization, Data curation, Resources, Writing—original draft, Writing—review & editing. MK and AP: Writing—original draft. KWD: Conceptualization, Methodology, Supervision, Writing—original draft, Writing—review & editing.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
