Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical Univerersity of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
Email: mariamozdzan@gmail.com
ORCID: https://orcid.org/0009-0001-3113-9158
Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical Univerersity of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
ORCID: https://orcid.org/0000-0002-1028-8314
Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical Univerersity of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
ORCID: https://orcid.org/0009-0003-5113-2214
Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical Univerersity of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical Univerersity of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
ORCID: https://orcid.org/0000-0002-9755-9761
Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical Univerersity of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
ORCID: https://orcid.org/0000-0003-3659-8115
Affiliation:
Department of Internal Diseases and Clinical Pharmacology, Medical Univerersity of Łódź, Bieganski Hospital, 91-347 Łódź, Poland
ORCID: https://orcid.org/0000-0002-7623-5389
Explor Cardiol. 2023;1:72–87 DOI: https://doi.org/10.37349/ec.2023.00009
Received: March 28, 2023 Accepted: May 24, 2023 Published: October 31, 2023
Academic Editor: Andrea Borghini, Institute of Clinical Physiology-National Research Council (IFC-CNR), Italy
The article belongs to the special issue Common cardiovascular target for a wide gamut of contemporary health problems – thrombotic and arrhythmic sides of an inflammatory coin
Mitral valve prolapse (MVP) is a relatively common mitral valvulopathy and the most common cause of isolated primary mitral regurgitation (MR) requiring surgical repair. It affects about 1–3% of the general population. Although MVP is viewed as a benign condition, the association between MVP and sudden cardiac death (SCD) has been proven. Patients with MVP have a three times higher risk of SCD than the general population. The underlying mechanisms and predictors of arrhythmias, which occur in patients with MVP, are still poorly understood. However, some echocardiographic features such as mitral annulus disjunction (MAD), bileaflet MVP (biMVP), and papillary muscle (PM) fibrosis were frequently linked with increased number of arrhythmic events and are referred to as “arrhythmogenic” or “malignant”. Arrhythmogenic MVP (AMVP) has also been associated with other factors such as female sex, polymorphic premature ventricular contraction (PVC), abnormalities of T-waves, and Pickelhaube sign on tissue Doppler tracing of the lateral part of the mitral annulus. Cardiac magnetic resonance (CMR) imaging and speckle tracking echocardiography are new tools showing significant potential for detection of malignant features of AMVP. This paper presents various data coming from electrocardiography (ECG) analysis, echocardiography, and other imaging techniques as well as compilation of the recent studies on the subject of MVP.
Mitral valve prolapse (MVP) is a relatively common degenerative mitral valve anomaly, which affects about 1–3% of the general population [1]. While it is considered to be a benign condition, MVP patients are likely at increased risk of arrhythmia, leading to sudden cardiac death (SCD) [1, 2]. Patients with MVP are three times more at risk for SCD than the general population [3]. The mechanisms of arrhythmias, which occur in patients with MVP, are still poorly understood; however, some of them such as mitral annulus disjunction (MAD), bileaflet MVP (biMVP) and papillary muscle (PM) fibrosis have been linked to increased arrhythmic events [4]. For this reason, the mitral dysfunction is also referred to as “arrhythmogenic” or “malignant” MVP.
The main target of MVP studies is patients with ventricular arrhythmias (VA), which may be malignant or even lead to SCD. However, there is a possibility that MVP patients in basal ECGs and ECG Holter monitoring as well as during basal transthoracic echocardiography (TTE) may present changes that are predictors of the higher arrhythmia risk. In this review we present non-invasive and simple to gather, yet postulated in the literature as clinically significant, predictors of an arrhythmia in MVP.
MVP can be classified as the primary or the secondary lesion of the mitral valve. The primary form is non-syndromic and degenerative, while the secondary is multifactorial and occurs as a part of syndromic conditions [5]. The primary form occurs more frequently. The major deformities of primary MVP are redundant, hooded valve leaflets, thickened, elongated chordae tendineae (Figure 1), and a dilated mitral annulus typically with MR [6]. The lesion may take an entire valve or a segment of one or both leaflets. About 40% of primary MVP cases are found within families, in a first-degree relative, suggesting a strong genetic factor in its etiology [7, 8].
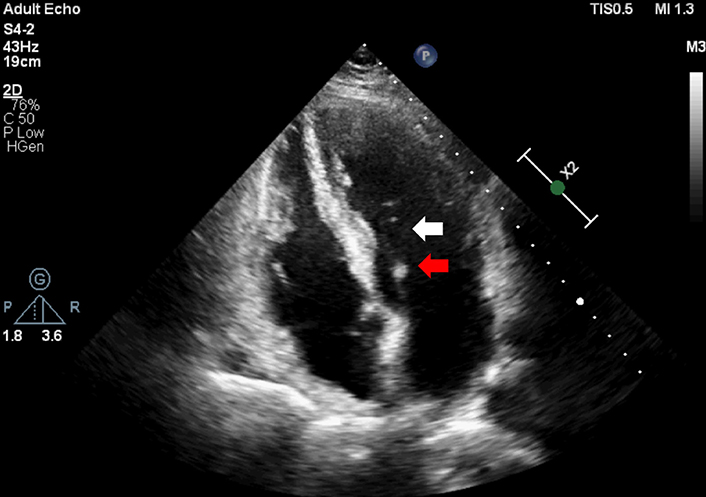
Prolonged chordae tendineae (white arrow) and thickened leaflet (red arrow) in Barlow like MVP
The secondary form of MVP is associated mainly with connective tissue disorders such as Marfan syndrome, Ehlers-Danlos syndrome, osteogenesis imperfecta, and other certain medical conditions such as rheumatic fever, polycystic kidney disease, and Hashimoto’s thyroiditis [9–11].
While the clinical implications of the secondary form of MVP seem to be benign, the primary mitral prolapse is associated with malignant VA, therefore this review will only deal with primary mitral prolapse and its connections with SCD.
Clinicians distinguish between two main types of primary MVP: BD also known as myxomatous degeneration, and fibroelastic degeneration (FED) [12]. Other two less common types are also observed: FED+ and forme fruste [13]. Differences between these types can be determined based on clinical impact, a macroscopic and histological examination, and echocardiographic findings. Morphological differences between those four types are presented in Figure 2.
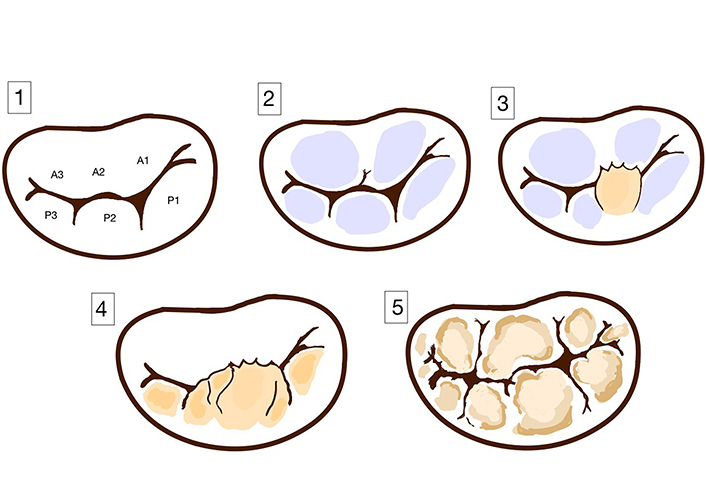
Spectrum of primary mitral valve disease. (1) Normal leaflet; (2) FED; (3) FED+; (4) forme fruste; (5) Barlow’s disease (BD)
In isolated FED there is a deficiency of collagen. The leaflets appear to be normal in height and thickness, only the prolapsing segment is elongated and distended [14, 15]. In contrast to fibroelastic deficiency, BD is characterized by diffuse excess tissue and affects more than one segment of the valve and even can take over the entire leaflet [14, 15].
FED occurs mainly in the elderly patients (> 60 years old), who experience acute symptoms related to chordae rupture, while BD develops earlier in life. BD patients usually have a long history of an asymptomatic mid-systolic click [15, 16]. Dilatation and disjunction of the mitral annulus as well as the prevalence of myocardial fibrosis are more frequent in patients with BD compared to those with FED [17]. FED patients in echocardiography show an isolated segmental prolapse of a valve, leading to the holosystolic MR [18]. Echocardiographic findings in BD patients include a multisegmental prolapse involving one or both leaflets in a valve with a significant fibrosis and a large annular size [13]. Both groups can suffer from VA or SCD, but in BD patients are more likely to suffer from arrhythmias related to severe MR, LV dilatation or dysfunction [19].
FED+ and forme fruste are rare subtypes of MVP. Forme fruste MVP is a subclinical degenerative form of the disease with excess tissue and myxomatous changes in multiple leaflet segments, but not involving a large valve size, which distinguishes it from BD [13, 20]. FED+ is a form of FED in long-standing prolapse [15]. It is characterized by transparent leaflets with the thickening prolapsing segment of valve, which is caused due to secondary myxomatous changes [13, 20].
A high mid-systolic click belongs to the typical auscultation signs of MVP, which is caused by a sudden tension of the mitral leaflets as the valve prolapse into the left atrium in systole [21]. The study of Perazzolo Marra et al. [4] has shown a myocardial scarring correlates with both the presence of MVP’s murmur and late gadolinium enhancement (LGE) distribution. In this study, patients with MVP and the confirmed LGE presence were twice as likely to have a mid-systolic click on auscultation compared to patients with no such findings. Additionally, patients with MVP and a mid-systolic click were more likely to develop stress-induced inferobasal lesions [22], in which the risk of severe VA was particularly high [1]. Based on those studies, the authors have hypothesized that the presence of click in patients with MVP can suggest the malignant prognosis and it should be recommended to take special note of the possible risks of VA occurrence. VA severity and its risk for mortality among MVP patients are presented in Table 1.
Arrhythmia severity classification [23]
| VA | Arrhythmia burden rate (bpm) | Typical examples | Risk of mortality (hazard ratio, 95% Confidence Interval) |
|---|---|---|---|
| Mild | PVC ≥ 5% VT runs 120 |
| 1.2 [0.63–2.14] |
| Moderate | VT runs 120–179 | 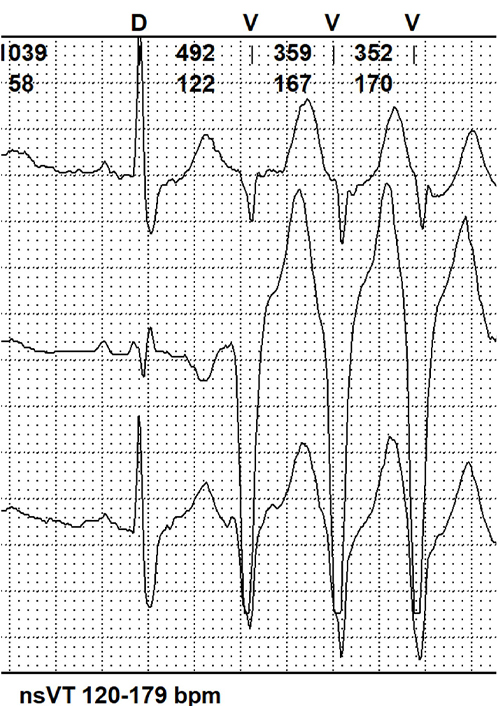 | |
| Severe | VT runs ≥ 180 or history of sustained VT | 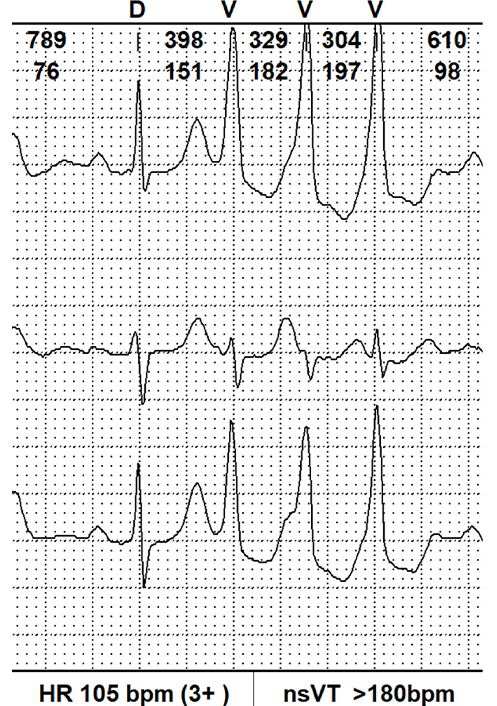 | 2.94 [1.36–6.36] |
bpm: beats per minute; VT: ventricular tachycardia; nsVT: non-sustained VT
In 2022 experts of EHRA published a consensus statement [24] that included a review of current literature focused on arrhythmic MVP. This consensus outlines the primary areas of knowledge deficiency concerning the recognition, risk stratification, and management of arrhythmogenic MVP (AMVP). Additionally, it offers practical recommendations for diagnosing and treating patients with AMVP. The document emphasizes the crucial elements involved in diagnosing the arrhythmic form of MVP: the presence of MVP (with or without MAD) and VA that is frequent [≥ 5% total premature ventricular contraction (PVC) burden] or complex (nsVT, VT, VF) and the absence of any other well-defined arrhythmic substrate.
This document has several aims based on the latest knowledge advancements in SCD and AMVP. First, it seeks to define the specific characteristics and phenotype of the AMVP complex. Additionally, it proposes various approaches for effectively screening and diagnosing the AMVP complex. Moreover, the document puts forth strategies for risk stratification in order to better understand the severity and prognosis of AMVP.
Furthermore, comprehensive imaging protocols are recommended, utilizing transthoracic Doppler-echocardiography and cardiovascular magnetic resonance (CMR), to thoroughly assess the AMVP complex and gather valuable diagnostic information. The document also includes management approaches, offering practical suggestions for the treatment and care of patients with AMVP.
Echocardiography has been recognized as the gold standard for a diagnosis of patients with MVP. Figure 3 shows an example of a TTE examination of a patient with MVP and co-occuring MR.
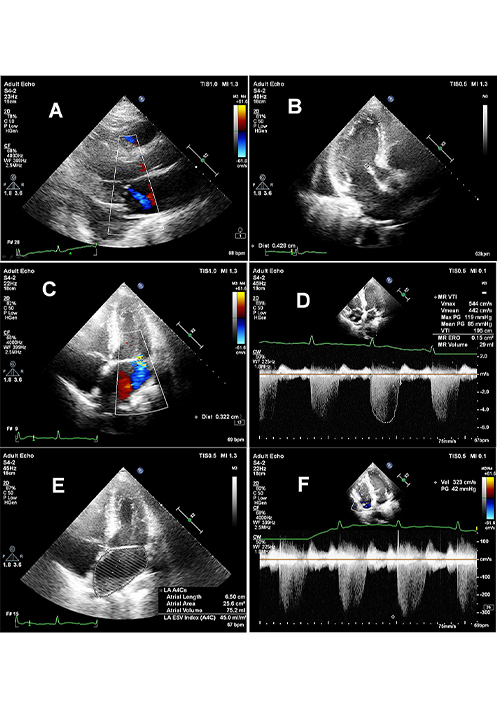
MVP with small MR. Panel A: long axis view with blue colored central jet of first grade MR; panel B: thickened mitral valve, with anterior leaflet thickness exceeding 4 mm; panel C: vena contracta width of 3 mm indicating I grade of MR; panel D: quantitative assessment of MR with effective orifice area (ERO) = 0.15 cm2 and regurgitant volume (RV) = 29 mL; panel E: moderate enlargement of left atrial volume LAV = 75 mm and LAVI = 45 mL/m2; panel F: tricuspid regurgitation indicating indirectly on moderately increased systolic pulmonary pressure with TRV = 3.2 m/s and tricuspid gradient of 42 mmHg
Lastly, an important aspect is highlighting the critical gaps in knowledge that currently exist, aiming to guide future clinical trials and collaborative research endeavors. By identifying these gaps, the document encourages further investigation and research to fill in the missing pieces and enhance our understanding of AMVP.
ECG changes in MVP entail impaired repolarization, including T-wave inversion [25] and ST-segment elevation [26], most often visible in inferior leads. In bileaflet mitral prolapse, T-wave inversion is more common than in other patients with MVP [27]. A new finding was observed on ECGs of patients with MVP, a notch (reverse Δ-wave) in the descending arm of QRS complex, which was observed in 79% (237/300) of patients (Alizadeh-Asl et al. [28]). A notch in the descending arm of QRS, indicative of early repolarization, is more frequent in young patients with history of unexplained syncope or those one who experienced idiopathic ventricular fibrillation (IVF) in the past [29, 30]. Furthermore, an increased corrected QT dispersion (QTcD) is claimed to have a significant correlation with VA in MVP [31]. This large difference between the maximum and minimum QT corrected (QTc) for heart rate reflects both the reduction and increase of repolarization intervals. It could be explained by increased wall stress or regional ischemia [32]. Other ECG changes were observed in Rs or rS waves, visible in inferior or in I and aVL leads, however, they require further research [28].
Significantly lower number of the scientific reports presented an association between supraventricular arrhythmia and MVP, especially atrial fibrillation (AF) in patients with MR due to MVP. As the degree of MR increases in MVP patients, the volume of the left atrium enlarges, leading to an increase in the frequency of AF. AF is associated with adverse cardiovascular events and decreased survival rate. The risk of stroke among those patients is significantly higher [33]. In the study of Berbarie et al. [34] 28% of patients referred for mitral valve repair or replacement due to MR (secondary to MVP) presented AF before operation (69 of 249 patients). The study showed that the frequency of AF in patients with significant MR secondary to MVP is relatively low (with persistent AF achieving 15% and paroxysmal AF 13%). According to the study of Essayagh et al. [23], eighteen percent of the overall population included in their research had AF. Among them, 54 out of 107 patients had various severity of VA events.
Myocardial fibrosis seems to be strongly associated with severe VA in MVP. It can be described as interstitial, compact, focal, diffuse or patchy [23, 25, 35]. Focal localization of fibrosis in MVP patients has been attributed to a regional strain of the myocardium caused by prolapsing leaflets [2].
To detect local fibrosis LGE-CMR uses gadolinium-based contrast, which accumulates in the extracellular matrix. It shows the presence of myocardial replacement fibrosis in patients with MVP involving the PM but LGE-CMR is not as sensitive as CMR T1 mapping for detection of an early stage of fibrosis [17, 36]. Furthermore, CMR-T1 mapping assesses quantified myocardial and interstitial fibrosis without using gadolinium and extracellular volume (ECV) fraction quantification. Noninvasive CMR-T1 mapping techniques allow the measurement of a surrogate marker of the myocardial interstitial fibrosis.
Bui et al. [37] discovered a connection between post-contrast T1 times and arrhythmias in MVP patients. They found that in those patients with VA, only 36% had been detected with LGE in LV myocardium, while a shorter post-contrast T1 time remained the only factor predicting the risk of arrhythmias.
Several studies have raised an issue of correlation between fibrosis and SCD in MVP. The study of Kitkungvan et al. [38] included 356 patients with primary MR, in which 177 had MVP. All patients underwent contrast CMR examination to assess cardiac remodeling, severity of MR, and LV replacement fibrosis. The MVP patients had the strongest connection with LV fibrosis, which was six times more common than in non-MVP patients. During follow-up, the rate for events of arrhythmia was higher in MVP patients.
Forty-three patients with MVP after SCD were enrolled in the study of Basso et al. [2]. In all of the cases, fibrosis has been found in PM of the left ventricle (LV), and among 88% of patients it was detected in the inferobasal wall. A biMVP was found in 70%. 10 from 12 cases in ECG had inverted T waves on inferior leads, and all had right bundle-branch block VA.
One of the best-documented causes of malignant MVP is MAD, which was first described in 1981 Bharati et al. [39]. MAD is an anatomic abnormality of the mitral annular structure, which includes the atrial-valvular-ventricular junction, where the point of insertion of the posterior mitral leaflet is dislocated, leading to a wide separation between the mitral leaflet insertion point and left ventricular attachment. The visualization of MAD is based on non-invasive imaging including TTE [40], transesophageal echocardiography (TEE) [41], and cardiac magnetic resonance (CMR) [19, 42]. MAD is best visible on TTE in the parasternal long‐axis view in the systolic phase [43]. CMR, apart from the detection of the presence, extent, and location of MAD, has additional benefits of fibrosis assessment, which is also associated with arrhythmic events among MAD patients [44].
In 1986, a study of Hutchins et al. [45] based on pathology assessment described anatomic variations of mitral annulus morphology and revealed an association between MAD and MVP. MAD was found in 6% of the hearts, particularly in the patients with MVP.
Dejgaard et al. [46] observed that patients with isolated MAD suffer more frequently from severe VA than patients with MVP without MAD. Another finding also proves that even a small disjunction (< 4 mm) can cause malignant ventricular events, which suggest MAD as a clear risk marker of arrhythmic events. Moreover, it seems that the length of MAD correlates with the severity of prolapse and myocardial fibrosis [43]. Several studies support the idea that the incidence of VA increased with greater extent of MAD distance [23]. However, MAD by itself is a significant risk factor of arrhythmia. Groeneveld et al. [47] research also support that hypothesis. In their study, they included 72 subjects with an IVF and 72 healthy controls. 56% of patients with IVF had MAD and among them, 43% of patients with MAD and IVF did not have a co-existing MVP, which also confirms the arrhythmogenic effect of MAD. In this study, MAD was commonly measured in a different location of the heart in both groups of patients, with or without IVF. The MAD occurred in the inferolateral wall only in patients with IVF. MAD is only detectable, when the mitral annulus presents itself abnormally during ventricular systole. It appears as separated from the ventricular myocardium by a variable distance, from a few to more than 10 mm [48]. MAD, also known as the atrialization of the posterior leaflet base, can involve either a small or large portion of the mitral annulus, typically in the region of the P1 and P2 mitral valve scallops [49]. MAD makes a contribution to the elongation of the LV myocardium at the inferobasal region as well as the PM, leading to hypertrophy and fibrosis of the inferobasal LV [50]. This process entails the Purkinje fibres and PM, which leads to electrical instability. It creates the substrate and triggers malignant VA, in particular Purkinje-triggered ventricular fibrillation (VF), and sustained, monomorphic VT from the inferobasal LV [22, 51].
In another study of Pavon et al. [36] assessing MAD length and LV fibrosis, patients with MVP underwent CMR using LGE and basal segments myocardial ECV by T1 mapping. The authors in this retrospective study included 30 patients with MVP and MAD (MVP-MAD). The maximum MAD length was 9.4 mm (7.1–12.3). The MAD length was most strongly associated with a higher ECV, but not with a higher native T1 or LGE extent. Furthermore, a study has shown MVP-MAD patients had a higher ECV, which indicates a higher amount of interstitial fibrosis, even in the absence of detectable LGE. The presence of LGE was registered in 47% respondents with MVP-MAD patients. LGE was most frequently detected in the vicinity of the posterior mitral leaflet attachments. In conclusion they propose ECV by T1 mapping as a new risk marker of arrhythmia in MVP patients.
In patients with MVP-MAD have been observed the medical conditions known as tricuspid valve prolapse (TVP) and tricuspid annular disjunction (TAD). However, it seems that these conditions are not linked to a higher occurrence of VA [52].
During CMR exam, in patients with MVP systolic curling was also noted. It is presented in Figure 4. It refers to the downward movement bigger than 3.5 mm of the posterior mitral valve annular during systole, which causes the adjacent myocardium to curl and appear curved on imaging of the myocardium [4]. It has also been associated with the myocardial fibrosis. According to a study of Perazzolo Marra et al. [4] the occurrence of posterior systolic curling in MVP patients, assessed in LGE-CMR, was found to be more prevalent (94% versus 19%; P < 0.001) when compared to those without the LGE enhancement. The ratio of wall thickness between the basal to mid-LV regions exceeding 1.5 was also more common (61% versus 25%; P = 0.016) among patients with MVP.
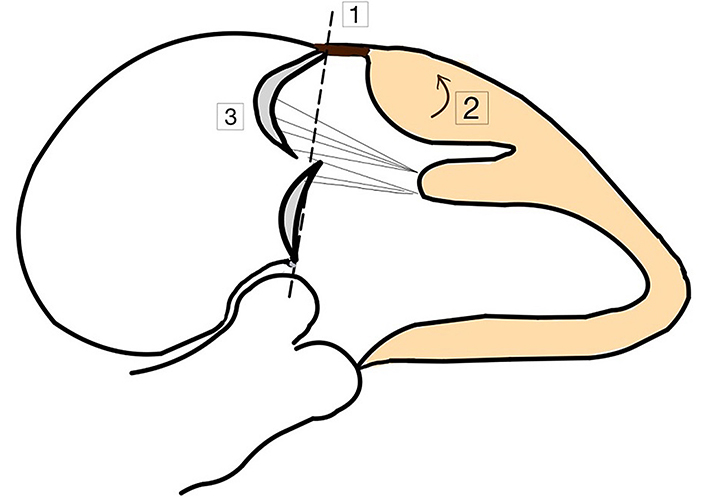
Morphofunctional abnormalities among AMVP patients. (1) MAD; (2) curling of LV posterior basal wall; (3) prolapse of posterior mitral valve
In patients with MVP-MAD, LV undergoes a remodelling process and both the focal replacement and interstitial myocardial fibrosis can be detected by CMR. In comparison to LGE extent, the ECV of the basal LV segments has the significant impact on the severity of MAD and the occurrence of OHCA [44]. Therefore, including ECV in the CMR examination of MVP patients may provide a better assessment of a fibrous remodeling and provide further information beyond the evaluation of LGE for SCD risk.
Another pathology in MVP patients, which promotes VA is a biMVP, presented in Figure 5. In the study Basso et al. [2] observed, that patients with MVP and complex VA showed a biMVP involvement in 70% of cases. The retrospective study of Garbi et al. [53] was based on pathology detection of 68 hearts from the patients with MVP as the lone abnormal finding, who died due to SCD. Bileaflet involvement, extensive leaflet thickening, thickened and elongated chords without rupture, and permanent leaflet displacement into the left atrium were found in all of the cases with the malignant MVP.
In another pathology analysis Han et al. [54] evaluated 376 cases with MVP, of which only 71 had isolated MVP. Among those 71 hearts, in 74% fibrosis was detected and 87% had a bileaflet valve, although no cases had documented severe MR.
Bileaflet anatomic defect of MVP can be the cause of the abnormal elevated tension on the PM and on inferolateral myocardium. This results in long-term mechanical stress, leading to inflammation or localized ischemia and eventually to myocardial fibrosis [1]. Other studies also supported the hypothesis of biMVP contributing to the occurrence of arrhythmias [23]. Moreover, both histological studies and CMR images confirmed the more frequent occurrence of fibrosis in SCD patients bileaflet involvement [2]. However, this finding remains controversial. According to Nordhues et al. [55], biMVP is not associated with an increased risk of SCD and does not account for the poor forecast. In this large retrospective cohort study, isolated biMVP was only associated with VT and did not appear to significantly increase the risk of cardiac arrest (CA), VF, or ICD implantation compared with monoleaflet MVP.
Vairo et al. [56] conducted a study, in which they specified some of echocardiographic parameters, which could predict malignant events in arrhythmic MVP patients. They have focused on high-risk features, calculated through spackle tracking analysis, which include: anteroposterior (AP) diameter/AM leaflet (AML) length ratio, the mitral valve annulus indexed area, the inferolateral basal S3 velocity, the AML length, and the basal/mid-ventricular segments mechanical dispersion (DM). These variables, which are both anatomical and functional in nature, appear to be interrelated, thus supporting the idea that arrhythmic events may be triggered by mechanical stretching. In particular, these findings suggest that VA may be caused by sudden tugging of the PM on the inferolateral wall. The last three variables had the best area under the curve (AUC) values in their respective receiver operating characteristic (ROC) curves, and the last two variables provided cut-off values with the best sensitivity and specificity. These findings suggest that echocardiography may be useful in identifying patients with MVP who are at higher risk of major arrhythmic events. Additional studies are needed to further investigate the mechanisms of MVP-related arrhythmias and SCD, as well as to identify additional risk factors.
The higher risk was related with the bigger length of the anterior mitral (AM) leaflet and the greater DM of the basal and mid-ventricular segments measured with strain analysis. The study of Ermakov et al. [57] revealed that individuals with an AMVP exhibit a greater degree of a DM compared to those with a nonarrhythmic MVP (59 milliseconds versus 43 milliseconds). This discrepancy serves as an indicator of an irregular ventricular contraction. The velocity of inferolateral basal segment is also significant, but has a lower specificity than DM and AM leaflet length.
Another important finding in TTE examination is Picklehaube’s sign, presented in Figure 6. It is defined as a peak systolic velocity of the lateral mitral valve annular, measuring more than 16 cm/s with tissue Doppler imaging [58]. This sign, resembling a Prussian military helmet, indicates hypermobility of posterolateral LV wall during systole and illustrates the arrhythmogenic phenotype of MVP. It has been proven that patients with Pickelhaube’s sign are in higher risk group, which encounters VAs and has a visible focal fibrosis on CMR.
The study of Zienciuk-Krajka et al. [59], examined patients with malignant type of MVP. They were divided into two groups based on the occurrence of CA in their medical history. All of them underwent a TTE examination. They have shown that AMVP patients after the CA had a higher E/e’ ratio, while the value of the parameter e’ remained the same in both groups. The E/e’ ratio gives a non-invasive insight into diastolic LV pressures in echocardiography. It might reflect the more extensive fibrosis in the CA patients. It is believed to result in a spontaneous diastolic depolarization and an abnormal automaticity. The study of Zienciuk-Krajka et al. [59] was the first research, which noted the association between a high E/e’ ratio and the SCD risk.
A noteworthy recent cohort study was conducted by Essayagh et al. [23] in 2020. This retrospective research aimed to specify VA’s prevalence, severity, determinants, and impact on outcomes in patients with MVP. The study included 595 MVP patients, who had undergone clinical assessment, continuous electrocardiographic monitoring, and echocardiography prospectively for a fifteen-year period. VA in this group appeared among 257 patients, the most common was that one of medium moderation (VT 120–79 beats/min). Severe VA was diagnosed in 9% of patients. Arrhythmia severity was associated with more frequent MAD presence and length, leaflet redundancy, and ST-T changes. The study also confirmed many features previously associated with arrhythmia in MVP, such as left atrial volume index (LAVI), LV end-systolic diameter (LVESD), and ECG QTc duration, whereas sex, severe MR, and bileaflet prolapse were not confirmed as independent predictors. Prospective and retrospective studies summarizing all VA and SCD risk factors in patients with MVP are shown in Table 2 and Table 3.
The summary of original retrospective studies on predictors of VAs or SCD in MVP patients
| Study | Year | No. of AMVP patients | Female (%) | Age (years) | AMVP risk factors |
|---|---|---|---|---|---|
| Akcay et al. [31] | 2010 | 30 | 72% | 42 ± 15 36 ± 13 | Mitral anterior leaflet length Enhanced QTcD |
| Sriram et al. [50] | 2013 | 10 | 90% | 33 ± 16 | BiMVP Female sex PVC Biphasic/inverted inferior T waves |
| Basso et al. [2] | 2015 | 30 | 73% | 41 ± 12 | Fibrosis of PMs and inferobasal LV free wall, detected by LGE on CMR |
| Bui et al. [37] | 2017 | 14 | 29% | 55 ± 13 | Reduced postcontrast T1 times suggestive of diffuse myocardial fibrosis |
| Garbi et al. [53] | 2018 | 68 | 48% | 38 ± 15 | Bileaflet prolapse Mitralannulus dilatation Degenerative features of the myocytes Right ventricular fibrosis |
| Ermakov et al. [57] | 2019 | 32 | 53 | 57 ± 13 | Increased DM by speckle-tracking echocardiography |
| Essayagh et al. [23] | 2020 | 257 | 39 | 68 ± 15 | ST-T changes MAD LAVI LVESD QTc interval BiMVP MR grade |
| Pavon et al. [36] | 2021 | 30 | 40 | 50 ± 17 | MAD ECV > 27% |
| Vairo et al. [56] | 2023 | 22 | 86 | 44 ± 14 | AP diameter/AML length ratio The mitral valve annulus indexed area The inferolateral basal S3 velocity The AML length The basal/mid-ventricular segments DM |
The summary of original prospective studies on predictors of VAs or SCD in MVP patients
| Study | Year | No. of AMVP patients | Female (%) | Age (years) | AMVP risk factors |
|---|---|---|---|---|---|
| Turker et al. [60] | 2010 | 20 | 60% | 36 ± 13 | Moderate to severe MR |
| Narayanan et al. [61] | 2016 | 17 | 41% | 61 ± 16 | Young age |
| Perazzolo Marra et al. [4] | 2016 | 33 | 75% | 44 ± 10 | MAD Curling LV fibrosis detected by CMR |
| Constant Dit Beaufils et al. [62] | 2021 | 125 | 45% | 53 ± 15 | Replacement myocardial fibrosis, located mainly on the basal inferolateral midwall or PM |
MVP is a common valvulopathy, which affects up to 3% of the general population, with a small but clinically relevant subgroup of patients at risk for malignant arrhythmic events, including SCD. The association between MVP and SCD has been proved, but the more accurate identification of patients with risk factors demands further studies. MAD and fibrosis, occurring in MVP are significantly more prevalent with patients experiencing IVF compared with healthy controls. Anatomical and functional features related to the increased arrhythmic risk have been documented in high-risk MVP patients, based on the hypothesis of a mechanical stretch-induced origin of arrhythmic events. Echocardiography and CMR, but also a prolonged ECG monitoring during 24-hour Holter examination, may identify patients at higher risk of major arrhythmic events. Further studies are crucial to investigate additional risk factors. Better risk stratification and intervention strategies are needed for higher risk MVP patients.
AF: atrial fibrillation
AM: anterior mitral
AML: anterior mitral leaflet
AMVP: arrhythmogenic mitral valve prolapse
AP: anteroposterior
biMVP: bileaflet mitral valve prolapse
CA: cardiac arrest
CMR: cardiovascular magnetic resonance
DM: mechanical dispersion
ECG: electrocardiography
ECV: extracellular volume
IVF: idiopathic ventricular fibrillation
LAVI: left atrial volume index
LGE: late gadolinium enhancement
LV: left ventricle
MAD: mitral annulus disjunction
MR: mitral regurgitation
MVP: mitral valve prolapse
QTc: QT corrected
SCD: sudden cardiac death
TTE: transthoracic echocardiography
VA: ventricular arrhythmias
VT: ventricular tachycardia
Maria Możdżan: Writing—original draft, Investigation, Writing—review & editing. KWD: Investigation, Writing—review & editing, Supervision. Monika Możdżan: Conceptualization, Investigation, Writing—review & editing. MB, ID, ZM and MS: Writing—review & editing.
The authors declare that they have no conflicts of interest.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Lodz (approve number: RNN/73/22/KE).
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from all participants.
Not applicable.
Not applicable.
©The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Bogusława Nowak ... Karina Wierzbowska-Drabik
Piotr Hamala, Karina Wierzbowska-Drabik
Barbara Uznańska-Loch ... Tomasz Rechciński
Annamaria Del Franco ... Iacopo Olivotto
Miłosz Broncel, Marlena Broncel
