Abstract
Drug-induced liver injury (DILI) poses a complex and heterogeneous clinical challenge, which often resembles non-drug related acute or chronic liver diseases, such as metabolic dysfunction-associated steatotic liver disease (MASLD). Furthermore, certain drugs can induce hepatic steatosis, which is considered a rare variant of hepatotoxicity. Additionally, the detection and diagnosis of DILI in patients with non-alcoholic liver disease present additional challenges that require attention. The importance of achieving an accurate diagnosis is highlighted by the different therapeutic approaches needed for each of these diseases. Nonetheless, as definitive diagnostic tests and distinct biomarkers often remain elusive, the differential diagnosis must rely on a combination of clinical, biochemical, histological, and immunophenotypic profiling. The diagnosis of hepatotoxicity is predicated upon the temporal nexus between the administration of a potentially hepatotoxic drug and the onset of hepatic injury, concomitantly excluding alternative hepatic pathologies. More frequently, this condition presents an acute course, with a more pronounced elevation of cytolytic and cholestatic parameters as compared to fatty liver disease. Advances in elucidating the underlying mechanisms hold promise for bolstering the diagnosis and management of these conditions. This article aims to thoroughly examine and emphasize the currently available scientific evidence to provide valuable insights into the diagnostic strategies for DILI, metabolic-associated liver disease, and drug-induced steatosis (DIS).
Keywords
Drug-induced liver injury, metabolic dysfunction-associated steatotic liver disease, non-alcoholic fatty liver disease, drug-induced steatosisIntroduction
Drug-induced liver injury (DILI) and herb-induced liver injury (HILI) is a growing health problem, often difficult to differentiate from other forms of liver disease [1]. Liver toxicity is classically divided into intrinsic (predictable, dose-related), idiosyncratic (unpredictable, non-dose-related), and more recently an indirect type of liver damage has been described [2]. Although not fully understood, the pathogenesis of idiosyncratic DILI (iDILI) is based on the interplay between drug characteristics and environmental and host factors, where the genetic background and the immune system have a significant role to play [3]. The severity and outcome of the liver damage are highly variable, ranging from an asymptomatic rise of liver enzymes to a more severe injury that can evolve to acute liver failure [4–6].
Diagnosis of iDILI is based on establishing a temporal association between drug exposure and the onset of signs and symptoms of liver disease, and ruling out alternative etiologies of liver injury. The capacity of drugs to induce different phenotypes of liver damage, makes it frequently indistinguishable from other liver injuries such as metabolic dysfunction-associated steatotic liver disease (MASLD), formerly non-alcoholic fatty liver disease (NAFLD) [7].
Up to date, MASLD is a major cause of liver disease worldwide, with an estimated prevalence of 25% [8]. It is characterized by triglyceride deposition in hepatocytes and defined as the presence of macrovesicular steatosis after the exclusion of significant alcohol consumption and other secondary causes as steatogenic drugs [9]. MASLD can lead to metabolic dysfunction-associated steatohepatitis (MASH), former non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma, and diagnosis is based on imaging, serologic, and liver biopsy findings.
The relationship between MASLD and DILI is hard to discern (Figure 1). First, although robust evidence is lacking, MASLD may increase the risk of DILI. What is certain is that a toxic event may induce a more severe liver injury in patients with underlying liver disease, especially in patients with cirrhosis [10]. Second, a variable proportion of patients with iDILI have data of steatosis in combination with other types of histologic findings in liver biopsy. In the US drug-induced liver injury network (DILIN), up to 26% of cases with available histological information showed some degree of steatosis, while this condition was found in less than 2% of the Spanish DILI Registry patients [11, 12]. Whether steatosis in DILI cases is induced by the drug or was previously present is difficult to elucidate if no liver studies were available before DILI onset.
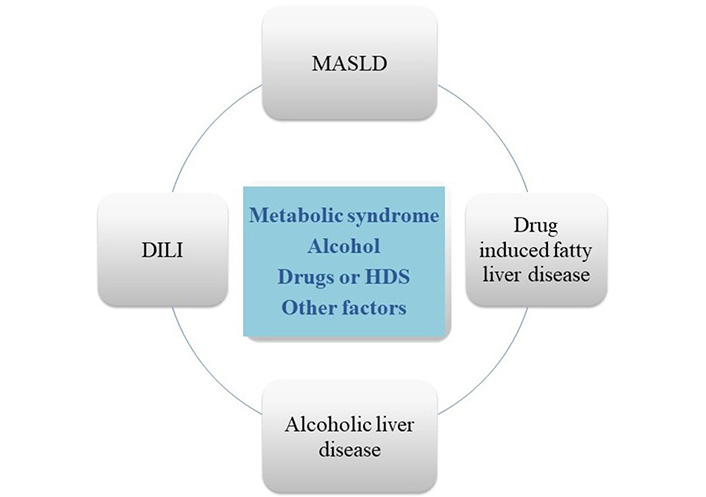
Multidirectional relationship between NAFLD, drug-induced liver disease, drug induced steatosis and alcohol. First, liver steatosis may increase the risk of DILI induced by certain drugs, a variable prevalence of steatosis is present in patients with DILI, and hepatotoxicity may induce a more severe liver injury in patients with MASLD. Besides, some drugs as tamoxifen or methotrexate, can be the cause of drug-induced steatosis/steatohepatitis (DIS/DISH). Finally, alcohol can be another source of confusion as a cause of fatty liver disease, aggravating metabolic steatosis, and as a contributing factor to DILI associated with specific drugs. HDS: herbal and dietary supplements
On the other hand, DIS/DISH is considered as a rare type of DILI. It has been estimated that only 2% of MASLD cases are caused by drugs [13]. This adverse event can induce an acute severe injury or can evolve into a chronic liver disease which can be confused with MASLD [14]. While DIS/DISH may exhibit histological and imaging similarities to MASLD, their natural history, pathophysiology, and prognosis often differ and are influenced by factors such as the specific drug, length of exposure, and individual susceptibility. Nevertheless, the differential diagnosis between iDILI and MASLD is sometimes troublesome [15].
Alcohol can be another source of confusion as a cause of fatty liver disease, aggravating metabolic steatosis, and as a contributing factor to DILI associated with specific drugs such as halothane, methotrexate and isoniazid [15].
Finally, the detection of DILI in patients with MASLD plays an important role not only in routine clinical practice, but also in the setting of clinical trials, especially in MASLD trials, where baseline abnormal liver tests may confound the detection and risk assessment of DILI [16].
The aim of this article is to review the available evidence and provide guidance for the diagnosis of iDILI, MASLD, and DIS.
DILI
Drugs and HDS can induce a number of changes in the functionality and structure of the hepatobiliary system, most of which are non-specific. Moreover, the clinical presentation of DILI is very heterogeneous and may mirror acute or chronic liver damage due to other aetiologies [3]. As a result, the diagnosis of DILI is particularly challenging, as it requires a comprehensive assessment to rule out alternative etiologies of liver injury (Figure 2) [15].
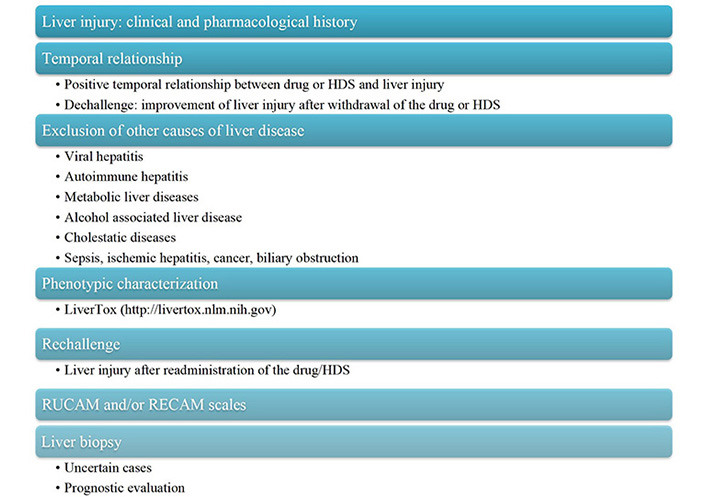
DILI diagnostic algorithm with a point-by-point assessment strategy. RECAM: revised electronic causality assessment method; RUCAM: Roussel Uclaf Causality Assessment Method
Clinical manifestations
Symptoms and signs of DILI, when present, include fatigue, weakness, right upper quadrant pain, nausea, dark urine, pruritus, fever, and rash. Severe cases may manifest with jaundice, encephalopathy, ascites, or bleeding [17, 18]. An exhaustive examination and clinical assessment are necessary to rule out other conditions that may lead to liver damage as alcohol consumption, total parenteral nutrition, heart failure, hypotension, sepsis, or cancer [18].
Medication history
When DILI is suspected, a detailed pharmacological history must be taken of all drugs and/or HDS consumed in the last 6 months; although some drugs have longer latency as nitrofurantoin, methotrexate, minocycline, or statins [18, 19]. In addition, medical history should include the start and stop dates of suspected agents, possible dose changes, and previous exposure. Besides, it is essential to collect the clinical-analytical course after discontinuation of the drug (dechallenge) and possible re-exposure effect (rechallenge). An improvement after dechallenge or worsening after rechallenge would support the diagnosis of DILI [20].
The LiverTox website (https://livertox.nih.gov/) is a useful tool for DILI assessment, providing an updated review of over 1,000 drugs and HDS. In addition, it collects information on latency, the pattern of liver injury, and the LiverTox likelihood scale of liver injury for a particular agent; highly useful data when multiple aetiological agents are involved [21].
Initial laboratory assessment
DILI is generally detected by the presence of abnormal liver biochemical blood tests including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin levels (TBL), and direct bilirubin levels. Additionally, serum albumin levels and the international normalized ratio (INR) serve as indicators of disease severity [20].
For the iDILI case definition, at least one of the following criteria is necessary: ALT elevation exceeding 5 times the upper limit of normal (ULN), ALP levels surpassing 2 times the ULN, or ALT increase exceeding 3 times the ULN in conjunction with total TBL levels exceeding two times the ULN. In cases where ALT values are unavailable during the recognition of iDILI, AST values can be employed as a reliable substitute. In patients with underlying liver injury, ULN must be replaced by the baseline mean values prior to the initiation of the hepatotoxic agent [15].
The biochemical pattern of liver injury may be of help in the characterization and differential diagnosis of DILI. For this aim, the ratio value (R-value) between ALT and ALP is used, which is defined as the result of dividing ALT/ULN-ALT by ALP/ULN-ALP, using the first serum values available during the event. Liver injury is considered “hepatocellular” if there is an ALT elevation ≥ 5 ULN or if the R-value is ≥ 5. Alternatively, when there is an ALP elevation ≥ 2 ULN or the R-value is ≤ 2, the liver injury is termed “cholestatic”. The term “mixed” liver injury is used for R values between 2 and 5 [15]. While delineating the specific pattern of DILI aids in differential diagnosis, it is important to note that certain drugs have been linked to multiple clinical profiles [20].
On the other hand, apart from the initial approach, additional laboratory investigations, and imaging studies are needed to exclude alternative etiologies of hepatic injury. This work-up should include the determination of hepatitis B surface antigen, anti-hepatitis B core antibody immunoglobulin M (IgM), hepatitis C ribonucleic acid (RNA), hepatitis A IgM antibody, and hepatitis E IgM antibody (and/or RNA). In addition, the evaluation of ceruloplasmin levels plus 24-hour urine copper (Wilson’s disease), autoantibodies and immunoglobulin G (IgG, autoimmune hepatitis), and antimitochondrial antibody levels (primary biliary cholangitis) is recommended [15].
Imaging
Abdominal imaging, such as ultrasonography, may exclude bile duct obstruction, evidence of chronic liver disease or other parenchymal changes (e.g., hepatic steatosis), vascular thrombosis or tumors. In most cases of iDILI, the abdominal ultrasound is normal. In certain clinical situations, such as persistent abdominal pain or jaundice, further imaging tests, such as computerized tomography (CT) or magnetic resonance cholangiography, should be recommended despite normal abdominal ultrasonography [3].
Liver biopsy
Given the clinical similarity between iDILI and other liver diseases, coupled with the lack of definitive diagnostic tests, liver biopsy serves as a valuable tool in discerning certain cases of hepatotoxicity. This is relevant in the presence of autoimmune features [22, 23] and when no improvement or worsening of the liver injury is observed despite the withdrawal of the culprit drug or HDS. Histological findings may support the diagnosis and/or rule out other possibilities [15]. Furthermore, the biopsy is useful in establishing prognosis, as certain histopathological features, such as ductular reaction, microvesicular steatosis and advanced stages of necrosis, and fibrosis, have been linked to an elevated risk of liver failure and mortality [11].
Causality assessment tools
An objective validated and structured approach would be of help to confidently attribute liver injury to a particular drug. Several causality assessment scales and methods have been developed for this purpose [24]. The DILIN structured expert opinion causality scale categorizes DILI likelihood as “unlikely”, “possible”, “probable”, “very likely” or “definitive”, which limitations are poor reproducibility and the limited number of DILI experts [3, 21].
On the other hand, the RUCAM is a well-validated scale that is widely applicable in clinical practice and provides a probability category based on the scoring of different items. Therefore, this scale can be used by experts as well as practicing clinicians [21]. Subsequently, the updated RUCAM was developed and it has been enhanced through the provision of a refined definition of the elements to be considered and greater precision in data elements, thereby facilitating the exclusion of alternative causes [25].
In addition, a revised electronic version of the RUCAM scale, the RECAM scale, has recently been described (http://gihep.com/dili-recam/) [26]. This scale might offer certain advantages over RUCAM, as its computerized format, risk factors elimination, simplifies the latency and dechallenge fields, and incorporates the possibility of including data from liver biopsy, rechallenge, and other diagnostic tests. However, in contrast to the RUCAM scale, this scale has not yet been validated. Pending future studies demonstrating its efficacy, it may serve as an additional tool in the assessment of causality in DILI [21].
Genetic testing
In recent years, due to genome-wide association studies (GWAS) and transcriptome-wide association studies (TWAS), several genetic variants related to iDILI have been identified, mainly haplotypes and genotypes of human leucocyte antigen (HLA) (Figure 3) [27–33]. HLA genotyping is available and can be highly helpful in specific clinical situations, since certain HLA alleles have a high negative predictive value (> 95%), allowing a particular drug to be ruled out when multiple agents are involved [3]. Furthermore, thanks to the international collaborative work, a 5-locus genetic risk score has been developed and tested in amoxicillin-clavulanic acid-related DILI patients with a high specificity, supporting a future role for genetic testing in DILI causality assessment and potential risk management [28].
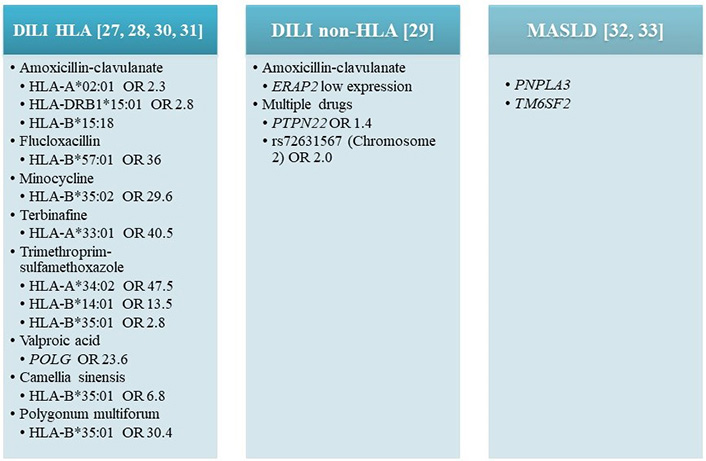
Genetic polymorphisms associated with DILI and MASLD development [27–33]. DILI induced by specific drugs, has been associated with several genetic variants, mainly haplotypes, and genotypes of HLA with different odds ratios. Non-HLA related with DILI. OR: odds ratio; PNPLA3: patatin-like phospholipase domain-containing 3; PTPN22: protein tyrosine phosphatase non-receptor type 22; TM6SF2: transmembrane 6 superfamily member 2; POLG: ploymerase gamma gene; ERAP2: endoplasmic reticulum aminopeptidase 2
MASLD
MASLD, former NAFLD, represents the prevailing liver disorder on a global scale, impacting 17–46% of adults in Western populations, and its incidence is on the rise [34, 35]. MASLD encompasses a wide spectrum of histopathological features spanning from simple steatosis to varying degrees of inflammation (MASH) and fibrosis [34]. Obesity stands as the primary risk factor for developing MASLD, and it is associated with a multitude of comorbidities including cardiovascular disease, type 2 diabetes mellitus, and malignancy [36].
In the presence of environmental and genetic factors, the interaction between the liver, adipose tissue, and the gastrointestinal system gives rise to systemic inflammation and insulin resistance. Consequently, there is an augmented supply of fatty acids to the liver and an increased activation of de novo lipogenesis. Criteria for NAFLD diagnosis are imaging or biopsy evidence of hepatic steatosis, the exclusion of substantial alcohol consumption (more than 20 g in women and 30 g in men per day) and alternative causes of hepatic steatosis (Figure 4).
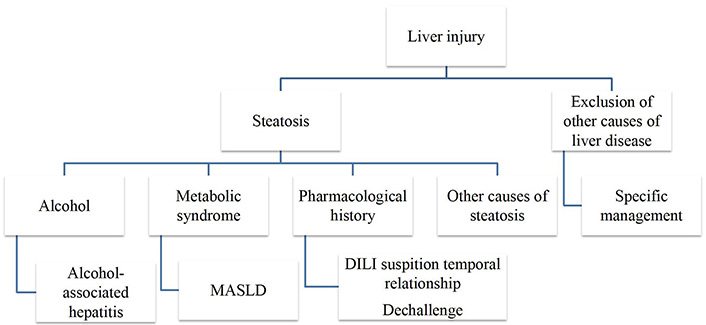
Causes of liver steatosis. Causes of liver steatosis, other than MASLD and alcohol include drugs, autoimmune hepatitis, haemochromatosis, Wilson’s disease, coeliac disease, hepatitis C virus, starvation, A/hypo-betalipoproteinaemia, lipoatrophy, hypopituitarism, hypothyroidism, parenteral nutrition or inborn errors of metabolism
In a recent pan-national consensus of hepatology societies, the terms NAFLD and NASH have been redefined. They have been replaced, respectively, by MASLD and MASH to avoid using confusingly exclusive terms such as “non-alcoholic” and potentially stigmatizing terms as “fatty”.
Thus, the presence of MASLD should be considered in a patient with established hepatic steatosis and at least one of these five cardiometabolic criteria: (1) body mass index ≥ 25 kg/m2 (23 Asia) or waist circumference > 94 cm (male), 80 cm (female) or ethnicity adjusted; (2) fasting serum glucose ≥ 5.6 mmol/L (100 mg/dL) or 2-hour post-load glucose levels ≥ 7.8 mmol/L (≥ 140 mg/dL) or HbA1c ≥ 5.7% (39 mmol/L) or type 2 diabetes or treatment for type 2 diabetes; (3) blood pressure ≥ 130/85 mmHg or specific antihypertensive drug treatment; (4) plasma triglycerides ≥ 1.70 mmol/L (150 mg/dL) or lipid-lowering treatment; (5) plasma HDL-cholesterol ≤ 1.0 mmol/L (40 mg/dL) (male) and ≤ 1.3 mmol/L (50 mg/dL) (female) or lipid-lowering treatment. In the event that an additional cause of hepatic steatosis was identified, a combined aetiology would then be considered. A new category, outside of pure MASLD, called MetALD (MASLD and alcohol liver disease) was selected to describe people with MASLD who consume larger amounts of alcohol per week (> 140 g per week in females and > 210 g per week in males) [7].
Less frequent causes of liver steatosis, other than MASLD, alcohol or drugs, include autoimmune hepatitis, haemochromatosis, Wilson’s disease, coeliac disease, hepatitis C virus-associated fatty liver (genotype 3), starvation, A/hypo-betalipoproteinaemia, lipoatrophy, hypopituitarism, hypothyroidism, parenteral nutrition or inborn errors of metabolism (such as lysosomal acid lipase deficiency) [15]. A liver biopsy is necessary for the definitive diagnosis of MASH [37].
Clinical manifestations
Most patients with MASLD are asymptomatic, although occasionally may refer to asthenia, malaise, and discomfort in the right upper quadrant of the abdomen [38]. In the cases where MASH has progressed to cirrhosis, patients may debut or suffer from any of its complications including hepatocellular carcinoma (HCC). Most often, diagnosis is made after detection of elevated hepatic aminotransferase levels or hepatic steatosis on imaging.
Initial laboratory assessment
Patients with fatty liver disease usually exhibit mild to moderate elevations in ALT or AST levels [38]. When aminotransferases are increased, they are usually two to five times the ULN, with an AST to ALT ratio of less than one [39]. It should be noted that a normal ALT level does not rule out clinically significant histologic injury and the magnitude of aminotransferase elevation does not serve as a predictor of hepatic inflammation or fibrosis severity [40]. ALP levels may be increased less than two to three times the ULN. Ferritin levels or transferrin saturation may be also increased in MASLD patients, and the former has been associated with a higher nonalcoholic fatty liver disease activity score, and with liver fibrosis [41].
Some of the most used serological tests and scoring systems to evaluate MASLD include the Fatty Liver Index (FLI) for evaluation of steatosis, the NAFLD Fibrosis Score (NFS), Fibrosis-4 (FIB-4) Index, and the Enhanced Liver Fibrosis (ELF) test for the assessment of the presence and severity of liver fibrosis in MASLD patients [42].
Imaging
Steatosis must be identified by imaging modalities such as ultrasound, CT or magnetic resonance imaging (MRI).
Qualitative assessment
A meta-analysis comprising 49 studies and 4,720 patients reported a sensitivity of 85% and specificity of 94% for ultrasound in diagnosing steatosis, with liver biopsy serving as the gold standard [43]. However, it has limited sensitivity and does not reliably detect fatty liver disease when less than 20% of steatosis is present or in obese patients [44]. While CT and MRI can effectively identify steatosis, they lack sufficient sensitivity in detecting inflammation or fibrosis.
Quantitative assessment
1H magnetic resonance spectroscopy (1H-MRS) and MRI-proton density fat fraction (PDFF) are the primary techniques for hepatic steatosis quantification and monitoring changes over time [45]. Another imaging technique that allows the evaluation of steatosis is the controlled attenuation parameter (CAP). Although it has limited ability to discriminate histological grades, it has demonstrated a very good accuracy in assessing steatosis compared with liver biopsies [46]. Ultrasound attenuation imaging (ATI) is an emerging quantitative approach for evaluating hepatic steatosis. In comparison to CAP, this novel technology enables the simultaneous display of B-mode ultrasound images and ATI images on a single screen. This combined visualization facilitates the assessment of liver structure and assists the operator in identifying the optimal measurement zone, thereby enhancing accuracy in steatosis measurement [47].
Liver biopsy
Liver biopsy is still the gold standard for MASLD and MASH diagnosis, and severity prediction. Diagnosis of fatty liver disease is based on the presence of steatosis in more than 5% of hepatocytes, while steatohepatitis is defined by the appearance of hepatocyte ballooning and lobular inflammation. Different histologic patterns have been described in patients with MASLD, zone 3 injury of adult steatohepatitis, zone 1 steatosis with fibrosis observed in children, and steatosis without steatohepatitis criteria [48]. The best histologic predictors of outcome are the fibrosis stage and the presence of ballooning and portal inflammation [49, 50]. To standardize the histologic evaluation of MASLD, the “NAFLD Activity Score” and the “Steatosis Activity Fibrosis” classification methods are recommended to assess disease activity [51, 52]. Albeit this is important information, liver biopsy is an invasive and not risk-free technique. Thus it is indicated for patients with uncertain diagnosis of MASLD or in the setting of clinical trials [53].
Genetic testing
Numerous genetic modifiers of MASLD have been identified [54], yet only a fraction have undergone robust validation. The most well-established genetic association is with PNPLA3, the gene responsible for encoding PNPLA3. This association was initially discovered through genome-wide association studies and subsequently confirmed in diverse cohorts, and ethnicities as a modulator of MASLD severity across the entire histological spectrum [32]. Another gene, TM6SF2, has been reported as an additional disease modifier [33], offering potential utility in risk stratification for liver-related and cardiovascular morbidity (Figure 3). However, genetic variants only account for 10–20% of overall heritability [55].
DIS
Definition
DIS refers to the abnormal accumulation of fat in the liver as a result of drug use. It is characterized by an excessive deposition of triglycerides within hepatocytes, leading to hepatocellular lipidosis [56].
DIS is considered a rare DILI phenotype whose incidence and prevalence rates are uncertain. It is estimated that up to 2% of cases diagnosed as MASLD are actually caused by drugs. However, this phenotype may be underdiagnosed, given that differentiation between the two entities can only be made by identifying exposure to a steatogenic drug [13, 15]. Indeed, within the Spanish DILI Registry, the predominant occurrence of steatosis was observed in a limited number of cases with histological data [12], whereas the US DILIN reported steatosis in as many as 26% of cases, varying in severity [11].
Pathogenesis
Hepatocytes have a key role in lipid metabolism. In steatosis liver disease there is an increase in free fatty acids within the hepatocyte. This may be caused by increased uptake (mainly from peripheral tissue, especially adipose tissue, and to a lesser extent from dietary sources), increased de novo lipogenesis within the hepatocyte or reduced utilization (either through β-oxidation or secretion) [56]. Although it has been poorly investigated, it is believed that drugs can activate fat accumulation in the liver via different pathways, which include inhibition of mitochondrial function (β-oxidation), increasing insulin resistance, promoting hepatic de novo lipogenesis or increasing free fatty acid uptake [57].
Causative agents and patterns of damage
DIS includes pure microvesicular steatosis, mixed microvesicular and macrovesicular, pure macrovesicular steatosis, and steatohepatitis [58].
Microvesicular fatty liver is related to mitochondrial injury mainly associated with drugs such as acetylsalicylic acid (AAS, Reye syndrome), valproic acid, glucocorticoids, NSAIDs, tetracycline, NRTI, and cocaine.
Glucocorticoids, methotrexate, NSAIDs, metoprolol, chlorinated hydrocarbons, 5‐fluorouracil, cisplatin, irinotecan, and tamoxifen can induce macrovesicular steatosis by altering lipid metabolism and promoting lipid accumulation in the liver. Besides, amiodarone, valproic acid, and methotrexate can induce mixed macro and microvesicular steatosis. Finally, DISH has been associated with amiodarone, methotrexate, 5‐floururacil, cisplatin, irinotecan, and tamoxifen [20].
Metabolic syndrome and its components have been considered as risk factors for the development and severity of DIS in patients treated with methotrexate and tamoxifen [15]. Preventing DIS involves careful monitoring of patients upon treatment with the above-mentioned drugs and treatment is mainly based on the withdrawal of the offending medication, which can lead to the resolution of the liver disease.
Differential diagnosis between DILI, DIS and MASLD
The absence of definite diagnostic methods and biomarkers together with the limits of the causality scales in the assessment of idiosyncratic hepatotoxicity makes diagnosis frequently troublesome and is mainly based on the exclusion of alternative causes of liver disease [11]. This makes differential diagnosis between this entity and nonalcoholic steatohepatitis sometimes extremely difficult, since the intake of drugs is widespread among the general population and the prevalence of metabolic syndrome causing fatty liver in developed countries is increasing. Thus, both processes may overlap. Furthermore, it is not known exactly to what extent the presence of metabolic syndrome with or without nonalcoholic fatty liver disease may increase the risk or influence the prognosis and evolution of an episode of hepatotoxicity. On the other hand, certain agents have the potential to induce or exacerbate fatty liver disease, further contributing to the development or progression of steatosis [58]. Clinical, biochemical, and histological features of DILI, DIS, MASLD, and alcohol-associated hepatitis are described in Table 1 [7, 15, 20, 38, 58–61].
Differences between NAFLD/MAFLD, iDILI, DIS, and alcohol associated hepatitis [7, 15, 20, 34, 38, 58–61]
| Differential diagnosis | DILI [15, 59] | DIS/DISH [20, 58] | NAFLD/MAFLD [15, 34, 38] | Alcohol-associated hepatitis [61] |
|---|---|---|---|---|
| Clinical presentation | Acute or chronic Multiple phenotypes | Acute or chronic | Chronic | Acute or chronic |
| Associated signs and symptoms | Fatigue, weakness, right upper quadrant pain, nausea, jaundice, dark urine, pruritus, fever, and rash | Fatigue, weakness, right upper quadrant pain, nausea, jaundice, dark urine, pruritus, fever, and rash AAS | Asymptomatic Asthenia, malaise, and right upper quadrant pain | Jaundice Ascites, edema, malaise, fever, hepatomegaly, confusion |
| Drugs, HDS, and alcohol | More than 1,000 drugs and HDS More frequent antibiotics (amoxicillin-clavulanic acid) Check-in LiverTox (https://livertox.nih.gov/) [60] | Acute fatty liver: amiodarone, didanosine, stavudine, valproate, and zalcitabine Drug-associated fatty liver disease: methotrexate, 5-fluorouracil, irinotecan, tamoxifen, corticosteroids, lomitapide, and mipomerson | No suspected drugs Alcohol intake: NAFLD: < 20 g/d (F), < 30 g/d (M) MASLD: < 140 g/w (F), < 210 g/w (M) | Alcohol consumption of more than 40 g/d in women and 50–60 g/d for men, with less than 60 days of abstinence before the onset |
| Biochemical parameters | ALT > 5 × ULN ALT > 3 × ULN + TB > 2 × ULN ALP > 2 × ULN | Not established | AST and ALT < 5 × ULN AST/ALT < 1 Normal ALP and TB | TB > 3 mg/dL (> 50 μmol/L) AST/ALT > 1.5 AST, and ALT > 400 UUI/L GGT > 100 U/L |
| Radiological findings | No specific findings | Liver steatosis | Liver steatosis | Liver steatosis Hepatomegaly |
| Liver biopsy | Acute hepatocellular liver injury: lobular inflammation, portal inflammation, interface hepatitis, apoptosis, granulomas, coagulative necrosis, and confluent or bridging necrosis Cholestatic DILI: acute cholestasis, chronic cholestasis, and acute cholestatic hepatitis | Steatosis, steatohepatitis Microvesicular (mitochondrial injury) Macrovesciular Mixed | Steatosis > 5% of hepatocytes, ballooned hepatocytes, lobular inflammation, apoptotic bodies, portal inflammation, perisinusoidal collagen, portal fibrosis, Mallory-Denk, megamitochondria, glycogenated nuclei in periportal hepatocytes, lobular lipogranulomas, PAS-diastase-resistant Kupffer cells, and hepatic siderosis | Ballooned hepatocytes, Mallory-Denk bodies, neutrophil infiltration, ductular reaction, bilirubinostasis, and pericellular, and sinusoidal fibrosis |
d: day; F: female; GGT: gamma-glutamyl transpeptidase; M: male; TB: total bilirubin; w: week; PAS: periodic acid-schiff; ×: times
Clinical manifestations
DILI and MASLD can be asymptomatic or paucisymptomatic, thus differential diagnosis based on clinical signs is difficult to make. MASLD is less likely to produce jaundice, choluria or acholia than DILI. In addition, patients with cholestatic DILI may have pruritus, which can be severe, leading to excoriations from scratching. In severe DILI cases, hepatic encephalopathy may develop, indicating acute liver failure [62]. DILI patients may develop hypersensitivity signs and symptoms, such as a fever and rash, Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms (DRESS) or a mononucleosis-like illness (pseudo mononucleosis). Finally, both MASH and chronic DILI may go on to develop advanced fibrosis or cirrhosis and have signs, and symptoms associated with advanced liver disease or hepatic decompensation.
Initial laboratory assessment
Regarding biochemical blood tests, serum aminotransferase increase in acute DILI can be marked (≥ 25 times the ULN), while elevation of AST and ALT is generally mild or moderate (two to five times the ULN) in MASLD patients. Even more, in general, MASLD does not present as flares of raised liver enzymes over 5 times the ULN or as an acute hepatitis. The study published by Shamseddeen et al. [63] showed a high prevalence of spontaneous liver enzyme abnormalities among patients with cirrhosis due to MASH participating in clinical trials. However, these abnormalities rarely fulfilled the criteria for suspicion of DILI. Besides, total bilirubin may be increased in both hepatocellular and cholestatic hepatotoxic DILI. In contrast, in patients with MASLD, bilirubin is not increased until advanced liver disease stages. Cholestatic DILI can produce an increase of more than two times the ULN of ALP in the absence of other causes (e.g., bone pathology), as occurs in patients with MASLD. Once both entities develop cirrhosis, the analytical alterations resulting from cirrhosis are indistinguishable.
Liver biopsy
As mentioned above, histologic diagnosis of MASLD is based on the presence of more than 5% of steatosis in liver tissue, while MASH necessitates the presence of hepatic steatosis accompanied by hepatocyte ballooning degeneration and hepatic lobular inflammation (typically in acinar zone 3). Fibrosis is not an obligatory diagnostic characteristic but may be observed. The morphological appearance of MASH may be histologically indistinguishable from alcoholic steatohepatitis. Additional histological findings in MASH encompass apoptotic (acidophilic) bodies, mild chronic portal inflammation, perisinusoidal collagen, portal fibrosis devoid of perisinusoidal or pericellular fibrosis, Mallory-Denk bodies, megamitochondria, glycogenated (vacuolated) nuclei in periportal hepatocytes (rarely observed in alcoholic steatohepatitis), lobular lipo granulomas, PAS-diastase-resistant Kupffer cells, and hepatic siderosis [64]. Histologic findings in patients with DILI include acute or chronic hepatocellular injury, acute or chronic cholestasis, steatosis, and steatohepatitis, granulomas, zonal necrosis, signs of hepatic venous outflow obstruction, sinusoidal obstruction syndrome, nodular regenerative hyperplasia, phospholipidosis or peliosis hepatis [20].
New biomarkers
Genetic, epigenetics, and mechanistic biomarkers
Different studies have been conducted to identify new biomarkers for the improvement of DILI and MAFLD detection, and diagnosis [15]. Bonkovsky et al. [65] evaluated non-HLA-related genetic variants associated with common liver diseases as MAFLD (PNPLA3 and TM6SF2) in iDILI patients, finding that none of the genetic polymorphisms tested were significantly associated with the risk of development, severity, or outcome of DILI.
The consortium known as the International Safer and Faster Evidence-based Translation Consortium conducted an evaluation of various biomarkers, leading to the discovery of several potentially valuable candidates for assessing DILI. These include osteopontin (OPN), cytokeratin-18 [total keratin-18 (K18) and caspase cleaved K18 (ccK18)], α-glutathione-S-transferase (α-GST), and miRNA-122 (miR-122), among others. These promising biomarkers show great potential for DILI assessment [66]. Among epigenetic markers, miRNAs are single-stranded RNAs of 21–23 nucleotides that regulate gene expression at epigenetic, transcriptional, and post-transcriptional levels. They in turn influence cell proliferation, differentiation, metabolism, infectious, and non-infectious disease progression, apoptosis, and metastasis. Among these miRNAs, miR-122 is specific to vertebrate species and is highly expressed in the liver, accounting for 70% of all miRNAs, and is important for hepatocyte function. It is also minimally expressed in other organs [67]. miR-122 has been implicated in fatty acid metabolism in mouse studies and its increase in plasma has been linked to the degree of fibrosis in fatty liver disease, viral hepatitis B and C virus hepatitis, and as a marker of liver damage in mice, and in humans with hepatotoxicity [68]. miR-122 has been described as one of the most promising biomarkers in the detection of DILI, whose levels appear to be more specific for liver damage than AST or ALT, with a large intra-individual and inter-individual variability [56]. On the other hand, only miR-122 has been accepted as a circulating biomarker for the diagnosis and prognosis of MASLD. Other studies have found a set of candidate miRNAs to distinguish DIS from MAFLD in animal and in vitro studies, that could be used in drug screening during preclinical development [69–71]. In this way, López-Riera et al. [71] showed that 10 miRNAs [miR-22-5p, -3929, -24-2-5p, -663a, -29a3p, -21 (5p and 3p), -27a-5p, -1260, and -202-3p] were induced in human HepG2 cells and secreted to the culture medium upon incubation with model steatogenic drugs (valproate, doxycycline, cyclosporine A and tamoxifen). Although promising, these findings need to be confirmed in prospective clinical studies.
K18, a structural protein of the cytoskeleton that has a full-length form, and ccK18 are considered as mechanistic biomarkers of liver injury. During hepatocellular necrosis, K18 is passively released from necrotic cells into the blood, while when apoptosis occurs, caspases cleave K18, and release it into the blood. Therefore, early hepatocyte damage could be detected by measuring the levels of K18 (an indicator of necrosis) and ccK18 (an indicator of apoptosis) [72]. Both forms are elevated in the circulation after DILI, showing potential for diagnosis and prognostic use. Although proportional serum levels of K18 and ccK18 may be useful indicators of DILI, elevated levels of these proteins have also been found in patients with hypoxic hepatitis, alcoholic steatohepatitis, MASLD, and other related disorders [73]. In MASLD patients, ccK18 levels have been found to be significantly higher in MASH than in patients with simple steatosis [74] thus ccK18 has been extensively validated as a marker for MASH, with a pooled receiver operating characteristic curve (AUROC) of 0.82 [95% confidence interval (CI), 0.76–0.88], and has been recognized by MASH guidelines as the most promising non-invasive test for the diagnosis and treatment of MASH [75, 76].
Cueto-Sanchez et al. [77] compared the performance of OPN, total K18 and ccK18, α-GST and miR-122 among patients with DILI and other forms of acute liver injury, finding limited specificity of these biomarkers for DILI, except ccK18, that presented the highest potential in distinguishing DILI from autoimmune hepatitis.
Other potential biomarkers for DILI are glutamate dehydrogenase, high mobility group protein B1, and macrophage colony-stimulating factor receptor; however further studies are needed for the validation of these markers [3]. Finally, a recent prospective cohort study has identified several protein biomarkers that could be useful to diagnose and distinguish DILI from any other acute liver injury in clinical practice, with fructose-1,6-bisphosphatase 1 being of particular interest [78].
Immunological biomarkers
Caballano-Infantes et al. [79] analyzed the activation profile [cluster of differentiation 69 (CD69), CD25, and HLA-DR] and natural killer group 2 member D (NKG2D) on iNKT cells and CD4/CD8 T cells in peripheral blood from prospectively collected MASLD and DILI patients compared to controls. This study showed an increase in iNKT cells in MASLD patients compared to DILI subjects. Besides, a positive correlation between CD69+iNKT, insulin resistance, AST level, FIB4, and AST to Platelet Ratio Index (APRI) was detected in these patients. DILI cases showed an increase in CD69+ and HLA-DR+ in both CD4+ and CD8+ T cells, detecting the most relevant difference in CD69+CD8+ T cells, concluding that this could be a potential distinctive biomarker for differentiating DILI from MASLD [79].
Microbiota
The potential impact of gut microbiota on the progression of MASLD remains a subject of considerable interest, yet its relevance in DILI is still poorly comprehended. A recent investigation undertook a metagenomic analysis of gut microbiota in MASLD, DILI, and control cohorts, unveiling the key bacterial metabolic pathways associated with these conditions [80]. Notably, MAFLD patients (referring to those recruited with the old NAFLD criteria) exhibited reduced abundances of Alistipes, Barnesiella, Eisenbergiella, Flavonifractor, Fusicatenibacter, Gemminger, Intestinimonas, Oscillibacter, Parasutterella, Saccharofermentans, and Subdoligranulum compared to DILI patients. Conversely, DILI patients demonstrated diminished abundances of Acetobacteroides, Blautia, Caloramator, Coprococcus, Flavobacterium, Lachnospira, Natronincola, Oscillospira, Pseudobutyrivibrio, Shuttleworthia, Themicanus, and Turicibacter in comparison to the MASLD group. Furthermore, the study identified seven bacterial metabolic pathways that were exclusively impaired in DILI, with the majority being associated with metabolic biosynthesis. In the MASLD group, variations in bacterial metabolic pathways in relation to DILI and control groups primarily pertained to fatty acid and lipid biosynthesis. Further confirmation of these findings in larger cohorts of patients could be the basis for the development of individual or panels of microbiome biomarkers to distinguish MASLD and DILI [80].
Detection of DILI on MASLD patients
DILI can occur with lower than stipulated levels of laboratory abnormalities, the reason for the current higher transaminase level threshold for hepatotoxicity diagnosis, is that up to 20% of the general population has mildly elevated liver biochemistries due to alcohol consumption, MASLD or other common disorders [20, 81].
The following criteria are used to make a DILI diagnosis in patients with MASLD: time from exposure to the beginning of the first signs of hepatic impairment, biochemical, and histological markers of hepatic impairment and information on improvements in hepatic function upon therapy discontinuation [82]. Among all the scoring systems, RUCAM is the most accurate to establish the diagnosis of DILI in MASLD patients [83].
The increase of MASLD clinical trials and the higher inclusion of patients with MASLD in trials of therapies for other diseases as diabetes mellitus, has advocated to the development of consensus guidelines for detecting DILI in these patients [84].
Treem et al. [16] published a consensus guideline for the assessment and management of DILI during clinical trials in adults with MASLD and other chronic liver diseases. These authors recommend the establishment of laboratory criteria for DILI detection, knowledge of the underlying disease behavior, to use more sensitive liver function tests and being aware of potential confounders related to complications of the disease. Additionally, they advocate for the establishment of pretreatment laboratory values as a basis for increased monitoring and for discontinuing drugs if potential DILI arises, using multiples of baseline liver test values and/or a specific threshold value, rather than multiples of ULN.
Conclusions
The distinction between DILI, MASLD, and DIS is crucial for establishing the correct diagnosis and providing the appropriate treatment. Misdiagnosis can lead to inappropriate management, unnecessary tests, and delays in the treatment of these conditions. Actual limitations in the differentiation between these three entities call for studies of clinical, biochemical, and histological data, along with biomarkers and immunophenotyping profiles for a comprehensive phenotyping of DILI, MASLD, and DIS. Nowadays, using clinical evaluation along with the available tests mentioned above, an accurate diagnosis can be reached in most cases. Further comprehension of insights into the underlying mechanistic pathways will lead to the discovery and validation of novel biomarkers that will contribute to improving the diagnosis of these diseases.
Abbreviations
| ALP: |
alkaline phosphatase |
| ALT: |
alanine aminotransferase |
| AST: |
aspartate aminotransferase |
| CCK18: |
caspase cleaved keratin 18 |
| CD69: |
cluster of differentiation 69 |
| CT: |
computerized tomography |
| DILI: |
drug-induced liver injury |
| DILIN: |
drug-induced liver injury network |
| DIS: |
drug-induced steatosis |
| DISH: |
drug-induced steatohepatitis |
| HDS: |
herbal and dietary supplements |
| HLA: |
human leucocyte antigen |
| iDILI: |
idiosyncratic drug-induced liver injury |
| IgM: |
immunoglobulin M |
| K18: |
keratin-18 |
| MASH: |
metabolic dysfunction-associated steatohepatitis |
| MASLD: |
metabolic dysfunction-associated steatotic liver disease |
| MRI: |
magnetic resonance imaging |
| NAFLD: |
non-alcoholic fatty liver disease |
| OR: |
odds ratio |
| PNPLA3: |
patatin-like phospholipase domain-containing 3 |
| RECAM: |
revised electronic causality assessment method |
| RNA: |
ribonucleic acid |
| RUCAM: |
Roussel Uclaf Causality Assessment Method |
| TB: |
total bilirubin |
| TM6SF2: |
transmembrane 6 superfamily member 2 |
| ULN: |
upper limit of normal |
Declarations
Author contributions
MGC: Conceptualization, Writing—original draft, Writing—review & editing. JPTO and AGG: Conceptualization, Writing—original draft.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This research was supported by grants from Instituto de Salud Carlos III, cofounded by the Fondo Europeo de Desarrollo Regional—FEDER contract numbers: [FIS 21-01248; PI18/00901; UMA18-FEDERJA-193]. Cofunded by European Union. CIBERehd is funded by the Instituto de Salud Carlos III (ISCIII). MGC is a member of the COST ACTION “CA-17112”, Prospective European DILI Network, supported by European Cooperation in Science and Technology (COST). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2023.