Abstract
Hepatitis B surface antigen (HBsAg) seroclearance is considered the functional cure and the optimal treatment endpoint for chronic hepatitis B (CHB). Patients with CHB who cleared HBsAg generally have a favorable clinical course with minimal risk of developing hepatocellular carcinoma (HCC) or cirrhotic complications. Nevertheless, a minority of patients still develop HCC despite HBsAg seroclearance. While patients with liver cirrhosis are still recommended for HCC surveillance, whether other non-cirrhotic patients who achieved HBsAg seroclearance should remain on HCC surveillance remains unclear. This review provides an overview of the incidence of HBsAg seroclearance, the factors associated with the occurrence of HBsAg seroclearance, the durability of HBsAg seroclearance, the risk of developing HCC after HBsAg seroclearance, the risk factors associated with HCC development after HBsAg seroclearance, the role of HCC risk scores, and the implications on HCC surveillance. Existing HCC risk scores have a reasonably good performance in patients after HBsAg seroclearance. In the era of artificial intelligence, future HCC risk prediction models based on artificial intelligence and longitudinal clinical data may further improve the prediction accuracy to establish a foundation of a risk score-based HCC surveillance strategy. As different novel hepatitis B virus (HBV) antiviral agents aiming at HBsAg seroclearance are under active development, new knowledge is anticipated on the natural history and HCC risk prediction of patients treated with new HBV drugs.
Keywords
Cirrhosis, HBsAg seroclearance, liver cancer, surveillanceIntroduction
Although birth-dose or infant vaccination and effective antiviral therapy have made a big impact on the incidence and natural history of chronic hepatitis B (CHB), chronic hepatitis B virus (HBV) infection still affects 258 million people worldwide and remains a leading cause of cirrhosis and hepatocellular carcinoma (HCC) [1]. Hepatitis B surface antigen (HBsAg) seroclearance is a critical step in the natural history of CHB. Most patients have undetectable viremia and normal liver biochemistry after HBsAg seroclearance, and less than 5% of patients have HBsAg reversion after seroclearance [2]. This translates into a much-reduced risk of HCC and cirrhotic complications [3]. As clearance of intrahepatic covalently closed circular DNA (cccDNA) and integrated HBV DNA is unlikely to be achieved in the foreseeable future using current technologies, sustained HBsAg seroclearance with serum HBV DNA less than the lower limit of quantitation is generally accepted as the functional cure of CHB [4].
That being said, HBsAg seroclearance reduces but does not eliminate the risk of HCC, especially when HBsAg seroclearance occurs later in life and after the development of cirrhosis [5, 6]. HBsAg seroclearance significantly overlaps with occult HBV infection (OBI), which is characterized by undetectable serum HBsAg yet detectable serum and/or intrahepatic HBV DNA [7]. Isolated hepatitis B core antibody (anti-HBc) positivity is also a distinct serological pattern, defined by negative HBsAg and positive anti-HBcs. Although a few instances of OBI can be attributed to mutations in the HBsAg gene, resulting in undetectability through currently available assays, the majority of OBI cases stem from replication-competent viruses that are strongly suppressed in their replicative and transcriptional activities by the immune system of the host [8]. The ongoing low-level replication and transcription of cccDNA may lead to the risk of reactivation and silent progression of chronic liver disease [9]. Current guidelines only provide recommendations on HCC surveillance in patients with positive HBsAg. The optimal management after HBsAg seroclearance is poorly defined. With this background, this review discusses the natural history of CHB after spontaneous and treatment-related HBsAg seroclearance, factors associated with HCC development after HBsAg seroclearance, and how clinicians can apply the knowledge to select patients for surveillance.
Overview of HBsAg seroclearance
Incidence of HBsAg seroclearance
HBsAg seroclearance can occur spontaneously or be induced by antiviral treatment including oral NAs and (pegylated)-interferon. Current first-line NAs including entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide possess a higher genetic barrier to drug resistance, effectively suppress HBV replication, and prevent disease progression. Despite the majority of patients with CHB can achieve sustained suppression of viral replication using current first-line NA treatment, HBsAg seroclearance remains a rare event [7], with an annual rate of about 1% [10].
Factors associated with HBsAg seroclearance
Lower HBsAg level [10–12], a strong kinetic of HBsAg level decline [11, 13, 14], older age [12, 15, 16], negative hepatitis B e antigen (HBeAg) [10, 16], and lower HBV DNA [10, 12, 17, 18] are associated with an increased likelihood of HBsAg seroclearance. The Asian population with CHB also has a lower incidence of HBsAg seroclearance than the non-Asian population [11, 19], which may be related to a different mode of infection and duration of chronic infection. The impact of HBV genotype remains less apparent [20]. While previous meta-analysis suggests no major difference in the rate of HBsAg seroclearance among different HBV genotypes [10], some studies showed a higher incidence of HBsAg loss among patients with HBV genotype A or D relative to those with HBV genotype B, C, or E [19], while HBV genotype C was associated with a higher chance of HBsAg seroclearance than genotype B among the Asian population [19]. Still, as the prevalence of HBV genotype was different in the Asian population (mainly genotypes B and C) and non-Asian population (mainly genotypes A, D, and others), it remains hard to segregate the effect of genotype from the effect of race as well as the mode and duration of HBV infection on the rate of HBsAg seroclearance. Co-infection of hepatitis C virus (HCV) is also associated with a higher chance of HBsAg seroclearance [21]. HBsAg level is usually lower in patients with HCV co-infection than those with HBV mono-infection [22]. Some previous studies also documented a relatively high incidence of HBsAg seroclearance in individuals with HBV/human immunodeficiency virus (HIV) co-infection [23–25], although some studies also reported a similar incidence [26–28]. More studies with formal comparisons would be necessary to confirm the observations. On the other hand, a recent individual patient data meta-analysis suggested that the presence of hepatic steatosis is associated with an increased chance of HBsAg seroclearance, though the exact mechanism remains unclear [29]. Previous studies suggested that compared to patients with CHB and no steatosis, patients with CHB and steatosis have a lower viral load [12], which is a factor contributing to a higher chance of HBsAg seroclearance (Table 1).
Factors associated with HBsAg seroclearance
| Factors | Descriptions |
|---|---|
| Factors associated with a higher likelihood of HBsAg seroclearance | Low quantitative HBsAg [10–12, 19], e.g., < 100 IU/mL for Asians and < 1,000 IU/mL for Caucasians [12, 30] |
| Strong kinetic of HBsAg level decline [11, 13] | |
| Low HBV DNA level, e.g., HBV DNA level < 2,000 IU/mL in untreated patients [12]; pretreatment HBV DNA level < 20,000 IU/mL [17]; HBV DNA level < 100 IU/mL at week 24 after antiviral treatment cessation [18] | |
| Negative HBeAg [10, 16] | |
| Older age [11, 12, 16] | |
| Non-Asian population [11, 19, 30] | |
| HCV co-infection [21] | |
| Novel HBV treatments such as siRNA and ASO [31] | |
| Interferon treatment [10] | |
| Factors that may be associated with HBsAg seroclearance | HBV genotype A or D [16, 19] |
| Presence of hepatic steatosis [12, 29] | |
| HIV co-infection [23–25] |
ASO: antisense oligonucleotides; HBeAg: hepatitis B e antigen; HBsAg: hepatitis B surface antigen; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; siRNA: small interfering RNA
There is no major difference in the rate of HBsAg seroclearance in patients with and without nucleo(s)tide analogues (NA) treatment history [10]. However, among patients who received antiviral treatment, the incidence of NA-induced HBsAg seroclearance is lower than that of interferon-induced HBsAg seroclearance [10], which may be due to the immunomodulatory effect of interferon as well as patient selection. In an international multicenter cohort of 4,769 NA-treated patients with CHB, a low annual rate of HBsAg seroclearance of 0.22% was observed [32]. Also, both entecavir and tenofovir disoproxil fumarate contribute to a similarly low rate of HBsAg loss [33]. Patients who achieved NA-induced HBsAg seroclearance include those who achieve HBsAg seroclearance on treatment and those who achieve it after cessation of NA. Stopping NA among non-cirrhotic patients with CHB who have long-term viral suppression has been a hot area of research in recent years. Emerging evidence suggests that stopping NA is associated with a higher chance of HBsAg seroclearance or transitioning to HBeAg-negative chronic infection, i.e., inactive carrier phase. Factors associated with HBsAg seroclearance after stopping NA include low HBsAg levels, low HBV DNA levels, negative HBeAg, and non-Asian population, which are similar to those for other types of HBsAg seroclearance [30]. Caution should however be made before stopping NA in the Asian population as hepatitis flares and the need for retreatment are likely more common [34].
Durability of HBsAg seroclearance
Both spontaneous and antiviral treatment-induced HBsAg seroclearance is considered durable with a low risk of HBsAg seroreversion [2, 16, 35]. A durable HBsAg loss at 6 years and 10 years was observed in 93% of patients with HBsAg loss and 95% of patients with HBsAg loss and a confirmation test six months after the initial negative HBsAg test respectively [36]. While HBsAg seroclearance may not immediately be accompanied by hepatitis B surface antibody (anti-HBs) seroconversion, patients after HBsAg seroclearance will gradually develop anti-HBs seroconversion over time [36]; up to 81% of patients can develop detectable anti-HBs 10 years after the initial HBsAg seroclearance. Anti-HBs seroconversion is associated with a lower risk of HBsAg seroreversion. The impact of anti-HBs seroconversion appears to be more obvious among patients with spontaneous HBsAg seroclearance and interferon-induced HBsAg seroclearance [37], as compared to patients with NA-induced HBsAg seroclearance [2]. As HBsAg seroclearance is the optimal treatment endpoint for current NA treatment, clinicians can consider stopping the NA treatment in patients who achieved HBsAg loss. As NA-induced HBsAg loss is uncommon, the necessity and the optimal duration of NA consolidation therapy remain to be defined [2, 38].
NA cessation after HBsAg seroclearance
Most international guidelines recommend discontinuing NAs after confirmed HBsAg loss sustained for 6–12 months [2], with or without anti-HBs seroconversion [39–41]. While HBV relapse, hepatitis flare and retreatment after NA cessation were common in CHB patients [34], these unfavourable outcomes rarely occur in patients who have achieved HBsAg seroclearance. Risk factors for hepatitis flares after stopping NA after HBsAg loss include potent immunosuppressive agents (namely B cell depletion therapies or high-dose corticosteroid therapy) [42], consolidation antiviral treatment less than 6 months post-HBsAg loss [2], and detectable HBsAg with ultrasensitive HBsAg assay (detection limit 0.005 IU/mL) [43]. The risk of hepatic decompensation decreases over time after HBsAg seroclearance because of the halt of liver injury secondary to the virus [7]; whereas a low yet steady risk of HCC remains even up to 12 years after HBsAg seroclearance, likely because of the persistence of viral cccDNA and integrated HBV in the hepatocytes [6]. HBsAg seroclearance, with or without anti-HBs seroconversion, is the optimal endpoint for the current HBV cure programmes. Hence, per-protocol NA cessation after HBsAg seroclearance is the common practice in these programmes. HBsAg seroreversion may occur in some patients if HBsAg seroclearance resulted from the inhibition of HBsAg expression, namely small interfering RNA (siRNA), or antisense oligonucleotides (ASO) [44] (Table 1). Long-term follow-up data from these programmes would be valuable to ascertain the minimal risk of disease progression in patients who achieved functional cure with these novel HBV therapeutics.
Since overt hepatitis flares and life-threatening episodes have been reported in patients with pre-existing cirrhosis who discontinue NAs, treatment discontinuation is currently discouraged in patients with cirrhosis [45], and similarly not preferred in patients with HCC and decompensated disease [40]. Furthermore, if cirrhosis has developed before HBsAg seroclearance, patients remain at risk of HCC, therefore HCC surveillance should continue [3, 5]. Immunosuppression, especially with B-cell depleting agents like rituximab may lead to HBV reactivation even after HBsAg seroclearance [46].
HCC after HBsAg seroclearance
Incidence of HCC after HBsAg seroclearance
HBsAg seroclearance confers a substantially reduced risk of development of HCC compared to that with complete viral suppression, however, the risk is not eliminated. A retrospective cohort study in Korea involving 829 patients with HBsAg seroclearance reported a 2.3% incidence of HCC over a median follow-up of 3.2 years, with an estimated annual incidence of 0.55% during 3,464 person-years of follow-up [47]. Another retrospective study with a longer follow-up duration suggested a 0.6% 8-year cumulative incidence for HCC development in 376 patients with NA-induced HBsAg seroclearance [3].
While the long-term HCC risk after HBsAg seroclearance was not well elucidated before, a recent study involving more than 9,700 patients has shed light on that by showing that the cumulative incidence of HCC remains steady 0–7 years and 8–12 years after HBsAg seroclearance, with the 7-year and 12-year cumulative incidences of 1.3% and 2.2% respectively [6]. This shows the persistent risk of HCC over time despite HBsAg seroclearance being achieved.
In line with the American Association for the Study of Liver Diseases (AASLD) guidelines suggesting the cost-effectiveness of HCC surveillance when the annual HCC risk exceeds 0.2% in CHB patients [48], the current evidence substantially indicates a non-negligible risk of HCC despite HBsAg seroclearance and thus may still benefit from regular HCC surveillance.
Factors associated with HCC after HBsAg seroclearance
The risk of HCC persists after HBsAg seroclearnace owing to HBV-to-human DNA integration in hepatocytes leading to genomic instability and production of pro-oncogenic viral proteins [49]. Nonetheless, not all patients have the same HCC risk. Various factors have been identified for association with HCC despite HBsAg seroclearance being achieved, and they can be categorized into patient and disease factors (Figure 1).
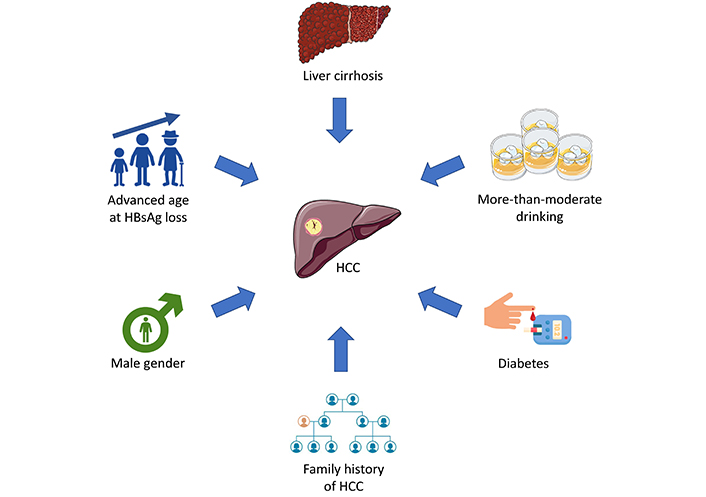
Risk factors of HCC after HBsAg seroclearance. HBsAg: hepatitis B surface antigen; HCC: hepatocellular carcinoma. Parts of the figure were used from or adapted from pictures provided by Servier Medical Art, licensed under CC BY 4.0
For patient factors, sex and age do not only implicate the HCC risk in general but are also associated with HCC after HBsAg seroclearance. Consistent data over the years have shown that male sex is an independent factor associated with HCC after HBsAg seroclearance. One study confirmed the finding of male sex and age > 50 years at the time of HBsAg seroclearance being independent risk factors of HCC after HBsAg seroclearance with an adjusted hazard ratio of 2.47 and 4.31, respectively [5]. Combining sex and age, the risk of HCC was highest in male patients achieving HBsAg seroclearance at age > 50 years with a 5-year cumulative incidence of 2.5%. Male patients attaining HBsAg seroclearance at age ≤ 50 years, as well as female patients attaining HBsAg seroclearance after age > 50 years shared similar and intermediate risk of HCC with a 5-year cumulative incidence of around 1.0%. Nevertheless, female patients with HBsAg seroclearance at age ≤ 50 years had zero risk of HCC for up to 5 years [5]. Apart from sex and age, a positive family history of HCC also increases the risk of HCC after HBsAg seroclearance. For patients with HBsAg seroclearance who have a family history of HCC, a 2.4 times risk of developing HCC was reported compared to those without a family history with a significantly higher cumulative incidence of HCC for those with a positive family history [50]. More-than-moderate alcohol consumption, defined by men drinking more than 2 standard drinks or women drinking more than 1 standard drink per day, is associated with a 2.6 times higher risk of developing HCC despite HBsAg seroclearance [50].
Cirrhosis is the most important disease factor contributing the HCC development after HBsAg seroclearance with unifying evidence showing cirrhotic patients have a 2–10 times risk of HCC than non-cirrhotic patients despite HBsAg seroclearance from large-scale retrospective studies [3, 6, 47, 50]. One of the studies indicated an annual risk of 0.54% of the development of HCC in cirrhotic patients [6], necessitating regular HCC surveillance despite HBsAg seroclearance.
Non-hepatic diseases also implicate the HCC risk. Emerging evidence over the years has shown that diabetes mellitus (DM) is an independent risk factor for the development of HCC in CHB patients [51]. DM affects not only CHB patients but also those with HBsAg seroclearance in the risk of HCC development. In a retrospective study of 4,568 patients with HBsAg seroclearance including 1,560 (34%) patients with DM, DM was found to be an independent risk factor of HCC after adjustment of age, male sex, presence of cirrhosis, use of statins, and antiviral treatment before HBsAg seroclearance [52]. Nonetheless, those with good glycemic control [i.e., average hemoglobin A1c (HbA1c) < 6.5–7%] had a significantly lower risk of HCC compared to those, with a 5-year cumulative incidence of 4% in those with HbA1c ≥ 7% compared to 1.8% in those with HbA1c < 7% [52]. This stresses the importance of identifying and adequately treating patients with DM to prevent HCC development.
While HBsAg seroclearance can happen spontaneously or be induced by HBV treatment, studies have consistently shown that the risks of HCC are comparable between spontaneous and NA-induced HBsAg seroclearance [53–55]. Yet it is worth mentioning that the difference in HCC risk between spontaneous and (pegylated)-interferon-induced HBsAg seroclearance has not been well elucidated and further studies are required to confirm whether the same conclusion applies to (pegylated)-interferon-induced HBsAg seroclearance.
HCC prediction models
Previous literature suggests that patients with CHB who achieve HBsAg seroclearance can still develop HCC [6]. Therefore, it is essential to assess the HCC risk in patients who have experienced HBsAg loss. Even after clearing HBsAg, patients should receive HCC surveillance if they are at risk. A more accurate risk estimation could result in a more precise recommendation for HCC surveillance.
So far, there are only a few published HCC risk scores for patients after HBsAg seroclearance [50, 56]. Existing HCC risk scores for patients with CHB, including the CU-HCC [57], REACH-B [58], PAGE-B [59], and mPAGE-B scores [60] have also been evaluated and compared with the novel HCC risk score in patients after HBsAg loss [50]. Yang et al. [50] found that their new score had a 5-year area under the receiver operating characteristic curve (AUROC) of 0.80 (0.72–0.88) and a 10-year AUROC of 0.84 (0.75–0.92), which performed better than the CU-HCC scores of 0.74 (0.66–0.82) for 5 years and 0.76 (0.68–0.84) for 10 years, and the REACH-B scores of 0.70 (0.63–0.78) for 5 years and 0.73 (0.65–0.81) for 10 years, in predicting HCC development over the respective periods (Table 2). Lee et al. [56] showed that a simple CAMP-B score (cirrhosis, age ≥ 50 years, male sex, and platelets < 1.5 × 1011/L) achieved a 5-, 10- and 15-year AUROC of 0.79 (0.74–0.84), 0.77 (0.72–0.82), and 0.80 (0.74–0.85) respectively, which were comparable to that of PAGE-B and mPAGE-B scores. In CU-HCC and REACH-B scores, some weights are given to the HBV viral markers including HBV DNA and HBeAg status, which will not apply to patients after HBsAg seroclearance. The mPAGE-B score performed better than the PAGE-B score in Yang et al. study [50]. This can be because the PAGE-B score was developed in a Caucasian population while the mPAGE-B score was derived in a Korean population, which is expected to perform better in another Korean cohort from Yang et al. study [50]. The novel risk score highlighted that older age, cirrhosis, significant alcohol intake, and a family history of HCC remain important risk factors after HBsAg seroclearance. While the novel risk score performed better than existing HCC risk scores for CHB, it is important to notice that the comparison was based on internal validation. One may expect that the novel risk score can be less predictive in an external validation cohort. Also, information on significant alcohol intake and family history of HCC may be less readily available in some of the patients. In that situation, mPAGE-B score can be a good alternative as it requires only objective demographic and laboratory data and achieved an acceptably good performance in predicting 5-year and 10-year HCC development in Yang et al. study [50].
Performance of HCC risk scores in patients with chronic hepatitis B after hepatitis B surface antigen seroclearance in Yang et al. study [50, 57–60]
| Parameters | Novel score | CU-HCC score | REACH-B score | PAGE-B score | mPAGE-B score |
|---|---|---|---|---|---|
| Age (years) | < 40 = 040–49 = + 150–59 = + 2≥ 60 = + 3 | ≤ 50 = 0> 50 = + 3 | + 1 for every 5 years from 35 to 65 years old | 16–29 = 030–39 = + 240–49 = + 450–59 = + 660–69 = + 8≥ 70 = + 10 | < 30 = 030–39 = + 340–49 = + 550–59 = + 760–69 = + 9≥ 70 = + 11 |
| Gender | Male = + 2Female = 0 | Male = + 6Female = 0 | Male = + 2Female = 0 | ||
| Cirrhosis | Yes = + 2No = 0 | Yes = + 15No = 0 | |||
| Family history of HCC | Yes = + 1No = 0 | ||||
| More-than-moderate drinking | Yes = + 2No = 0 | ||||
| Albumin (g/L) | ≤ 35 = + 20> 35 = 0 | < 30 = + 330–34 = + 235–39 = + 1≥ 40 = 0 | |||
| Total bilirubin (µmol/L) | > 18 = + 1.5≤ 18 = 0 | ||||
| ALT (IU/L) | ≥ 45 = + 215–44 = + 1< 15 = 0 | ||||
| HBV DNA (log copies/mL) | ≤ 4 = 04–6 = + 1> 6 = + 4 | ≥ 6 = + 45–6 = + 54–5 = + 3< 4 = 0 | |||
| HBeAg status | Positive = + 2Negative = 0 | ||||
| Platelet (× 109/L) | < 100 = + 9100–200 = + 6≥ 200 = 0 | < 100 = + 5100–149 = + 4150–199 = + 3200–249 = + 2≥ 250 = 0 | |||
| Discriminatory performance by time-dependent AUROC (95% CI) | |||||
| Prediction for HCC in 5 years | 0.799(0.722–0.877) | 0.739(0.656–0.822) | 0.704(0.627–0.781) | 0.657(0.564–0.745) | 0.732(0.642–0.823) |
| Prediction for HCC in 10 years | 0.835(0.751–0.919) | 0.763(0.682–0.844) | 0.734(0.653–0.814) | 0.705(0.608–0.802) | 0.790(0.714–0.867) |
ALT: alanine aminotransferase; AUROC: area under the receiver operating characteristic curve; CI: confidence interval; HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; HCC: hepatocellular carcinoma
We recently explored machine learning as an alternative to traditional regression methods for HCC risk prediction. Machine learning provides a comprehensive methodology, allowing for direct parameter selection, optimizing data use, and minimizing bias. We developed models using machine learning techniques that included 46 clinical and laboratory parameters. Notably, the ridge regression model, named HCC-RS, achieved an AUROC of 0.840 and outperformed existing HCC risk scores including CU-HCC (0.672), GAG-HCC (0.745), REACH-B (0.671), PAGE-B (0.748), and REAL-B scores (0.712). These results highlight the potential of machine learning to improve HCC prediction [61]. Kim et al. [62] deployed the gradient-boosting machine algorithm on a dataset of 13,508 patients to predict HCC risk in CHB patients undergoing antiviral treatment. Impressively, this model outperformed traditional predictors, such as PAGE-B, mPAGE-B, REACH-B, and CU-HCC, achieving C-indices ranging from 0.79 to 0.81 in both Korean and Caucasian validation cohorts. This was significantly higher than the C-indices of 0.57 to 0.79 observed with the previous models [62]. In a more recent study [63], an ensemble method-based machine learning technique was introduced for HCC risk evaluation in CHB patients treated with entecavir or tenofovir disoproxil fumarate. This new approach significantly surpassed the earlier models, with an AUROC of 0.9 compared to the 0.772 of REAL-B in the training cohort. Its predictive accuracy consistently remained high with an AUROC of 0.9, in stark contrast to traditional models, such as mPAGE-B, PAGE-B, REAL-B, HCC RESCUE, and CAMD, which achieved AUROCs between 0.721 and 0.801 [63]. These results highlight the potential of machine learning to improve HCC prediction in patients with CHB.
Establishing a threshold for HCC surveillance is crucial because of the extensive resources it requires and its role in early detection and management. Its cost-effectiveness, especially for low-risk patients, is debated. HCC surveillance’s cost-effectiveness is primarily influenced by the annual incidence rate. In essence, a higher HCC incidence results in a reduced cost for each detected HCC [64]. Traditionally, the recommendation has been to initiate HCC surveillance when CHB patients have an annual risk exceeding 0.2%, and 1.5% for those diagnosed with cirrhosis [65]. Renowned organizations like AASLD [65], the Asian Pacific Association for the Study of the Liver (APASL) [66], and the European Association for the Study of the Liver (EASL) [67] have issued HCC surveillance guidelines. AASLD and EASL recommend using both abdominal ultrasonography (US) and serum alpha-fetoprotein (AFP), while APASL advises only US. Recent research pointed out that surveillance through US with AFP emerged as a cost-effective strategy in 80.1% of simulations, given a willingness-to-pay benchmark of $100,000 per quality-adjusted life-year. The study further highlighted that an HCC incidence exceeding 0.4% annually, coupled with a biannual surveillance adherence above 19.5%, is crucial for the US with AFP strategy to be more cost-effective than no surveillance at all [68].
The significance of HCC surveillance in improving early detection and management of HCC is undeniable. Guidelines from key organizations, including AASLD, APASL, and EASL, unanimously support HCC surveillance for patients with cirrhosis, specifically tailored to factors like age, ethnicity, and family history of HCC, citing substantial benefits in terms of early detection, availability of curative treatments, and enhanced overall survival [69]. Furthermore, the timing of surveillance is a crucial element of effective HCC management, with current recommendations endorsing a six-month interval [70]. This recommendation is supported by evidence linking it to heightened detection rates of early-stage HCC tumors, thereby facilitating improved treatment outcomes and prognoses. While the increased frequency of imaging has not demonstrated additional advantages, integrating modalities such as US and AFP testing has been shown to amplify the sensitivity for detecting early-stage HCC [71]. A meta-analysis highlighted US sensitivity at 94% for HCC at any stage and 63% for early-stage tumors, noting that biannual screening boosts early-stage detection sensitivity to 70% [72]. The incorporation of AFP with US has been observed to elevate early-stage HCC detection sensitivity from 32% to 65% [73]. Despite AFP’s variable efficacy, it is endorsed as a complementary tool for cirrhosis screening alongside the US, with AFP levels above 20 ng/mL achieving approximately 70% sensitivity and 90% specificity for HCC [74]. Nevertheless, EASL guidelines remain cautious regarding AFP usage due to a propensity for false positives [75]. The advent of novel biomarkers and predictive models, such as GALAD, heralds a promising future for more precise HCC detection [76].
Existing HCC risk scores are accurate for identifying patients with low HCC risk who may be exempted from HCC surveillance with a high negative predictive value of over 95% [77, 78]. However, they usually underestimate the risk of HCC among patients with high risk [78]. Thus, their utility in guiding HCC surveillance strategy remains limited. With the advancement in risk prediction using artificial intelligence and longitudinal clinical data [79], artificial intelligence-based HCC risk models in the future may better estimate the HCC risk among patients with intermediate to high risk and facilitate a risk score-based surveillance strategy. New biomarkers for HCC are also under active investigation, which may further improve the accuracy of the current risk scores [80].
Future perspectives
Although a number of HCC risk scores have been developed for patients with CHB, few have catered for patients who have achieved HBsAg seroclearance. Furthermore, most HCC risk scores were based on baseline clinical and laboratory parameters. In the real world, however, clinicians see patients not once but repeatedly over many years. Thus, it is important to have dynamic models that cater for changes in clinical and laboratory parameters over time. Given the complexity of such data, artificial intelligence will probably be the way forward [61, 81]. As clinicians at busy clinics are unlikely to input data and apply complex models, innovative methods to draw relevant data automatically from the electronic health record and streamline the workflow will be needed.
On a separate note, a number of agents are undergoing active development with the aim of achieving functional cure of CHB [44]. Whether and how these novel therapies will impact on HCC risk is currently unknown. For example, some studies suggest that patients previously treated with peginterferon have a lower risk of HCC than those receiving NAs for CHB [82]. Therefore, it is important to examine the natural history of patients treated with new HBV drugs and determine how best to predict outcomes in such patients.
Conclusions
HBsAg seroclearance, also known as functional cure of CHB, reduces the risk of HCC, cirrhosis, and mortality. Nonetheless, a small proportion of patients may still develop HCC after HBsAg seroclearance. In this article, we summarized the natural history of CHB after HBsAg seroclearance, risk factors of HCC, performance of various HCC risk scores, and the way forward. To cater for this special population, separate HCC risk models are likely required, and artificial intelligence holds promise in improving prediction through handling complex and dynamic clinical data.
Abbreviations
| AASLD: | American Association for the Study of Liver Diseases |
| AFP: | serum alpha-fetoprotein |
| anti-HBs: | hepatitis B surface antibody |
| APASL: | Asian Pacific Association for the Study of the Liver |
| AUROC: | area under the receiver operating characteristic curve |
| cccDNA: | covalently closed circular DNA |
| CHB: | chronic hepatitis B |
| DM: | diabetes mellitus |
| EASL: | European Association for the Study of the Liver |
| HbA1c: | hemoglobin A1c |
| HBeAg: | hepatitis B e antigen |
| HBsAg: | hepatitis B surface antigen |
| HBV: | hepatitis B virus |
| HCC: | hepatocellular carcinoma |
| NA: | nucleo(s)tide analogues |
| OBI: | occult HBV infection |
| US: | ultrasonography |
Declarations
Author contributions
JCTL and VWKH: Writing—original draft, Writing—review & editing. GLHW, VWSW, and TCFY: Conceptualization, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
JCTL and VWKH declare that they have no conflicts of interest. GLHW has served as an advisory committee member for AstraZeneca, Gilead Sciences, and Janssen, and as a speaker for Abbott, AbbVie, Ascletis, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen, and Roche. She has also received a research grant from Gilead Sciences. VWSW has served as a consultant or advisory committee member for AbbVie, Boehringer Ingelheim, Echosens, Intercept, Inventiva, Novo Nordisk, Pfizer, and TARGET PharmaSolutions; and a speaker for Abbott, AbbVie, Gilead Sciences, and Novo Nordisk. He has received a research grant from Gilead Sciences, and is a cofounder of Illuminatio Medical Technology Limited. TCFY has served as an advisory committee member and a speaker for Gilead Sciences.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.