Affiliation:
1State Key Laboratory Base of Cell Differentiation and Regulation, College of Life Sciences, Henan Normal University, Xinxiang 453007, Henan, China
Affiliation:
2College of Life Sciences, Henan Normal University, Xinxiang 453007, Henan, China
ORCID: https://orcid.org/0009-0006-2656-7429
Affiliation:
1State Key Laboratory Base of Cell Differentiation and Regulation, College of Life Sciences, Henan Normal University, Xinxiang 453007, Henan, China
Email: yangxg@htu.edu.cn
Explor Dig Dis. 2024;3:443–458 DOI: https://doi.org/10.37349/edd.2024.00060
Received: April 28, 2024 Accepted: September 08, 2024 Published: October 14, 2024
Academic Editor: Jinsheng Guo, Fudan University, China
The article belongs to the special issue Viral Hepatitis
Hepatitis B virus (HBV) infection affects 262 million people worldwide, leading to over 820,000 deaths annually. The reason HBV has been a persistent issue for decades is that it is a non-cytopathic, liver-specific virus with the ability for persistent infection, which cannot be completely eliminated by drugs, eventually progressing to cirrhosis and hepatocellular carcinoma (HCC). Although HBV seems to induce little innate immune activation, adaptive immune responses can mediate viral clearance and liver disease. Here, we review the epidemiology, natural history, lifecycle, and modes of transmission of HBV. We also pay particular attention to the adaptive and innate immune responses to HBV and the research progress on therapeutic vaccines, which may provide new insights for targeted HBV treatment.
HBV is a member of the Hepadnaviridae family and is characterized by its partially double-stranded DNA structure. This virus has a compact genome size of approximately 3.2 kb. HBV is mainly transmitted vertically from mother to child during childbirth (perinatal transmission) or horizontally through blood, damaged skin, or unprotected sexual contact (horizontal transmission), posing a significant threat to global human health. Hepatitis B is an infectious disease caused by HBV, and if not treated promptly, chronic HBV (CHB) infection is highly likely to progress to advanced liver diseases such as cirrhosis and hepatocellular carcinoma (HCC). In recent years, significant progress has been made in both basic and clinical research on HBV, ranging from the biological characteristics of HBV, immunopathological mechanisms, and animal models to the development of new treatment strategies and drugs targeting HBV. In this review, we first summarize the epidemiology and natural history of HBV, followed by discussing the progress in adaptive and innate immune responses to HBV and the development of therapeutic vaccines.
Currently, HBV infection remains a significant global public health issue, affecting an estimated 262 million people worldwide, with 1.5 million new infections annually. Over 820,000 deaths occur each year due to CHB infection, with patients facing significant risks of complications. 15% to 40% of CHB patients may develop cirrhosis, liver failure, and HCC [1].
The World Health Organization (WHO) estimates that about 15%–25% of CHB patients required treatment in 2021 [1]. According to global guidelines, patients with HBV-DNA ≥ 2,000 IU/mL and elevated alanine aminotransferase (ALT) levels should start treatment [2]. There are currently 9 approved drugs for CHB, although only 3 are in active use worldwide. These include 2 interferon (IFN) formulations: conventional and pegylated IFN (pegIFN), and 7 nucleos(t)ide analogs: lamivudine, telbivudine, adefovir, entecavir (ETV), tenofovir disoproxil fumarate (TDF), tenofovir alafenamide fumarate (TAF), and clevudine and besifovir dipivoxil (only in Korea). Although these antiviral drugs show favorable virological responses, patients undergoing IFN treatment may experience flu-like symptoms such as fever, joint and muscle pain, nausea, and vomiting, with fever being the most common adverse reaction during IFN use. Similarly, patients on long-term NAs treatment may develop viral strain resistance and suffer from bone and kidney function damage (Table 1). Therefore, it is urgent to find new drugs to treat hepatitis B and mitigate the adverse reactions caused by the sole use of antiviral drugs. It is surprising that in recent years, research has found that certain traditional Chinese medicine formulations or active ingredients can significantly improve the pathological conditions of patients with hepatitis B, and the combined use of traditional Chinese medicine preparations with antiviral drugs can achieve better therapeutic effects. The latest pharmacological studies indicate that these traditional Chinese medicines and their effective components often have pharmacological functions such as immune regulation, antiviral activity, and protection of liver transaminases. Among them, immune regulation and protection of liver transaminases may be important treatment aspects and advantageous effects of traditional Chinese medicine in the treatment of hepatitis B. For example, in terms of immune regulation, traditional Chinese medicine preparations such as licorice can act on innate immunity or specific immune pathways, regulate immune cells such as Kupffer cells, NK cells, or regulatory T cells (Tregs), inhibit excessive inflammatory reactions, or regulate basic immune functions, thereby effectively improving liver inflammation and reducing liver damage [3]. In addition, some traditional Chinese medicine and effective components have multiple functions, being able to protect liver transaminases while combating viruses, which has filled the gap of single antiviral drugs in the treatment of hepatitis B and its complications, effectively improving the clinical treatment effects. For example, baicalin, licorice, and silymarin preparations can promote liver cell proliferation, inhibit HSC activation, and restore normal levels of liver enzymes, thus improving HBV-related liver fibrosis (Table 2). Therefore, at different stages of HBV infection, traditional Chinese medicine components can be replaced or adjusted in a timely manner according to the symptoms, fully exploiting the unique therapeutic advantages of traditional Chinese medicine.
Current antiviral hepatitis B virus (HBV) drugs have limitations in clinical use
| Types | Names | Notes |
|---|---|---|
| Interferon formulations | IFN-α-1b | The effect of HBeAg seroconversion is poor [4]. |
| PegIFN-α-2aPegIFN-α-2b | Frequent dosing is required, and influenza-like symptoms may occur [5]. | |
| Nucleos(t)ide analogs | Lamivudine | Prone to drug resistance [6] |
| Adefovir | High nephrotoxicity and low genetic barrier to resistance [7] | |
| Telbivudine | High resistance rate [8] | |
| TDF | Long-term use can lead to bone loss [9]. | |
| TAF | Different degrees of dyslipidemia may occur [10]. | |
| ETV | Poor drug compliance [11] |
IFN: interferon; HBeAg: hepatitis B e antigen; pegIFN: pegylated IFN; TDF: tenofovir disoproxil fumarate; TAF: tenofovir alafenamide fumarate; ETV: entecavir
Active ingredients in traditional Chinese medicine that possess immunomodulatory properties and hepatoprotective effects, reducing transaminase activity
| Category | Name | Source | Pathway and mechanism of action | Literature |
|---|---|---|---|---|
| Active ingredients of traditional Chinese medicine | Silymarin | Silybum marianum (L.) Gaertn. | Increase liver glycogen, catalase, and glutathione peroxidase activities. | [3] |
| Glycyrrhizin | Glycyrrhiza uralensis Fisch. | Inhibiting the activation of NKT cells and Th17 cells, downregulating IL-17, and upregulating IL-25. | [12] | |
| Baicalin | Scutellaria baicalensis Georgi. | Induction of Nrf2 accumulation, upregulation of IL-18, PCNA, and cyclinD1 expression, enhancing the proliferation ability of liver cells. | [13] |
Th17: T helper type 17; IL-17: interleukin-17
The functional cure criteria are persistently low hepatitis B surface antigen (HBsAg) < 0.05 IU/mL with HBV-DNA below the detection limit, with or without serological conversion of hepatitis B surface antibodies, considered as a possible achievable goal [14]. Unfortunately, despite the use of INF and nucleos(t)ide drugs for the treatment of HBV infection patients, it is difficult to completely eliminate HBsAg because HBV persists in liver cells as covalently closed circular DNA (cccDNA), which current antiviral drugs cannot clear. HBsAg clearance is influenced by various factors. The probability of achieving HBsAg clearance in CHB patients increases with lower baseline HBsAg levels, a rapid decline of HBsAg early in treatment, lower baseline HBV-DNA levels, and hepatitis B e antigen (HBeAg) negativity [15]. Additionally, indicators such as HBV RNA, HBcrAg, hepatitis B core antibody (anti-HBc), and hepatitis B surface antibody (HBsAb) are also related to HBsAg clearance and are emerging as novel predictors. However, due to the limited research on the correlation between most of these indicators and HBsAg clearance, and the underdeveloped detection techniques for some indicators, their widespread clinical application is not yet realized. Therefore, the predictive value and clinical feasibility of these novel indicators need to be validated through multicenter clinical studies.
The natural history of HBV infection depends on the interaction between viral replication and evolution, host immune response, and environmental factors. The age at which HBV infection occurs is the most important factor influencing chronicity. If infection occurs during the perinatal period, the risk of progression from acute HBV infection to CHB infection is approximately 95%, while the risk for children aged 1–5 years is 20%–30%, and the risk for adults is less than 5%. The natural history of CHB infection can be divided into four stages: (1) HBeAg-positive CHB infection; (2) HBeAg-positive CHB hepatitis; (3) HBeAg-negative CHB infection; (4) HBeAg-negative CHB hepatitis [16]. During the HBeAg-positive CHB infection phase, the characteristics of HBV-infected individuals are high levels of HBV-DNA, HBeAg positivity, normal ALT levels, and no apparent inflammation or fibrosis in the liver. Individuals in this stage of HBV infection can be defined as HBV carriers. During the HBeAg-positive CHB hepatitis phase, the characteristics of HBV-infected individuals are elevated ALT and HBV-DNA levels, accompanied by histological evidence of liver injury. Therefore, these patients are defined as HBeAg-positive CHB patients [16]. The transition from HBeAg seroconversion to antibody to HBeAg, normal ALT levels, low or undetectable HBV-DNA levels reflect the transition from the immune active phase to the immune inactive phase. Finally, there is a portion of patients who undergo HBeAg seroconversion but still exhibit elevated ALT and high HBV-DNA levels, thus being defined as HBeAg-negative CHB patients [16]. Most of these patients were infected with HBV that carried mutations in the precore or core promoter regions. Patients with ongoing liver damage and continuous HBV replication are prone to develop cirrhosis, liver failure, and HCC.
HBV is a small, hepatotropic DNA virus, spherical in shape, with a lipid envelope, approximately 42 nm in diameter. Mature and infectious HBV particles are also known as Dane particles [17]. The genome of HBV consists of partially double-stranded circular DNA, encoding a total of 7 antigens, namely polymerase P, L, M, S, HBcAg, HBeAg, and X antigen [18]. The envelope of Dane particles contains three surface antigens, L-HBsAg, M-HBsAg, and S-HBsAg, surrounding HBcAg, the virus-encoded polymerase complex, and the viral DNA genome. In addition to Dane particles, the blood of infected individuals also contains numerous spherical and virus-like particles (VLPs), which do not contain HBV genomic DNA and are primarily composed of M-HBs and S-HBs outer membrane proteins. The three outer membrane proteins L-HBs, M-HBs, and S-HBs are all within one open reading frame, transcribed from different promoters but containing the same termination codon. Among them, L-HBs is composed of three structural domains, preS1, preS2, and S, and this protein contains a binding domain for the sodium taurocholate cotransporting polypeptide (NTCP), facilitating HBV infection of liver cells [19]; M-HBs is composed of two structural domains, preS2, and S, does not participate in HBV replication, but is abundantly expressed in hepatitis B patients, and the expression levels of L-HBs and M-HBs in the body are positively correlated with the progression of HCC [20]; S-HBs contains only the S structural domain and is the major component of VLPs. The development of the three HBs proteins laid the foundation for the development of hepatitis B vaccines [21]. In addition, HBcAg is also an important target in the treatment of hepatitis B. Low-level expression of HBcAg in the body can induce sustained cellular and humoral immune responses, playing a role in antiviral activity [22]. Based on the antigenic epitopes presented by its envelope proteins, HBV can be divided into four subtypes, named adw, ayw, adr, and ayr [23]. It is worth mentioning that HBV strains isolated from different regions of the world can be classified into 9 genotypes (A–I) and one putative genotype (J) through genetic sequencing, with differences in the full-length gene sequence at the nucleotide level exceeding 7.5%. Each genotype has a different geographical distribution, which can be used to trace the origin and transmission patterns of the disease [24].
The life cycle of HBV includes virus entry into host cells; rcDNA entry into the nucleus to form cccDNA; expression of viral RNA and proteins; assembly of viral capsids; reverse transcription and formation of rcDNA; and finally, virus packaging, maturation, and budding (Figure 1). Initially, HBV attaches to heparan sulfate proteoglycan (HSPG) via the antigenic loop (AGL) in the HBsAg S domain [25]. Subsequently, through the preS1 region of the L protein, HBV tightly binds to the NTCP on the surface of liver cells [26]. After entry into liver cells, the viral capsid is released, and the rcDNA of HBV enters the nucleus to form cccDNA, which exists in the form of minichromosomes within liver cells [27]. Under the action of host RNA polymerase II, cccDNA serves as a template for transcribing the aforementioned four HBV transcripts and translating seven viral proteins. Of note, in addition to encoding HBcAg, HBeAg, and DNA polymerase, the 3.5 kb pgRNA also serves an essential function as a template for virus reverse transcription and replication. During virus replication, the HBV-DNA polymerase binds to the ε-stem-loop structure near the 5’ end of pgRNA, forming a specific pgRNA-polymerase ribonucleoprotein (RNP) complex, which is enveloped by core antigen peptide dimers, forming an immature core-shell [28]. Within the core-shell, under the catalysis of HBV-DNA polymerase, the 3.5 kb pregenomic RNA becomes a template for reverse transcription (–) strand DNA, which then serves as a template for the synthesis of (+) strand DNA, thereby forming progeny rcDNA. The newly synthesized rcDNA circulates back into the nucleus, generating more cccDNA, thus maintaining the cccDNA pool. Due to errors in viral DNA replication, double-stranded linear (DSL) DNA may be produced [29]. Mature core-shell proteins and envelope proteins aggregate in the endoplasmic reticulum, completing packaging, maturation, and virus budding [30].
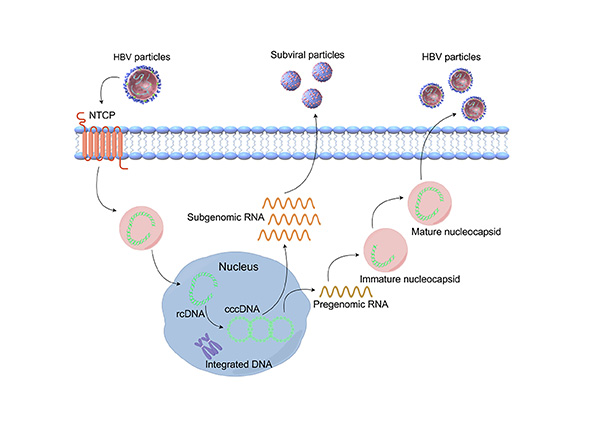
The lifecycle of hpatitis B virus (HBV). The virus enters hepatocytes through the sodium taurocholate cotransporting polypeptide (NTCP) receptor, and then the HBV genome enters the host cell nucleus, forming covalently closed circular DNA (cccDNA) through DNA repair mechanisms. Under the action of regulatory proteins, cccDNA serves as a transcription template to produce pgRNA and mRNA. Subsequently, pgRNA is selectively packaged into the nucleocapsid, undergoes reverse transcription to produce core protein and polymerase, and simultaneously serves as a template for reverse transcription to generate new rcDNA. Finally, virus envelope assembly occurs, and virus particles are secreted. Created by Figdraw
In the current situation, mother-to-child transmission (MTCT) during the perinatal period remains a challenging issue [31]. If a newborn is born to an HBeAg-positive mother and does not receive timely hepatitis B immunoglobulin (HBIG) and hepatitis B vaccine after birth, 70%–90% of newborns will be infected with HBV. If a newborn is born to an HBeAg-negative mother and does not receive HBIG and hepatitis B vaccine, 10%–20% of newborns will develop HBV infection [32]. Even with HBIG and hepatitis B vaccine treatment for newborns, if their mothers have CHB infection, approximately 9% of newborns will be infected with HBV. This is particularly the case when the mother is HBeAg-positive or has a high HBV-DNA level [33]. For example, even after passive-active immunoprophylaxis, when the mother’s viral load is > 8 log10 copies/mL (7.3 log10 IU/mL), the transmission rate is 9% [34]. The latest research suggests that controlling HBV-DNA to < 6 log10 copies/mL (5.3 log10 IU/mL) can reduce the probability of MTCT [35].
In addition, compared to children under 3 years old, the likelihood of newborns developing CHB infection is 90%, while for adults, it is only 5% [33]. In high-prevalence areas such as China, the proportion of women of childbearing age with CHB infection is as high as 17.8% [36]. It can be seen that HBV vaccination is of great significance in reducing and controlling the infection rate of hepatitis B, especially emphasizing the issue of HBV vaccination for newborns.
The risk of infection from blood exposure (such as transfusions, injections, and drug use) depends on the infectious source in circulation, antibodies binding to the virus, the stage of infection in the donor, the amount of blood received, the degree of immune suppression in the recipient, and the type of blood product received [37, 38]. Studies have shown that negativity or low levels of anti-HBc are associated with higher transmission rates. In populations without anti-HBc and HBV < 100 copies/mL, the transmission rate is 50%, while in populations with anti-HBc and HBV < 100 copies/mL, the transmission rate is 3% [37, 38].
It is distressing that patients with occult HBV infection may also present with HBV-DNA positive, anti-HBc positive, and HBsAg-negative statuses. Although the HBV-DNA levels in most patients are approximately 20,000–90,000 copies/mL (approximately 4,000–22,000 IU/mL), lower levels of HBV-DNA titers can also be observed [39]. However, there is no data indicating whether the blood products from these patients are infectious. Meanwhile, there may be a minimal correlation between viral loads in infectious blood and non-infectious blood. Evidence suggests that infection can occur with virus copies as low as 2–5 copies/mL, while it does not occur at 200 copies/mL [38]. Therefore, even low levels of HBV-DNA positivity can potentially transmit HBV infection, making treatment essential and unavoidable for all HBV-DNA-positive individuals.
Sexual contact is also one of the main transmission routes of HBV. From the data, the infection risk is highest among men who have sex with men (MSM). This is associated with anal sex, increasing number of sexual partners, and duration of sexual activity [40]. Approximately 70% of MSM are infected after 5 years of sexual activity [41]. The risk of HBV infection between opposite sexes also increases with the number of sexual partners, duration of sexual activity, and history of sexually transmitted diseases [42].
Firstly, we need to understand that HBV does not directly destroy hepatocytes. The immune response caused by the virus is the main mechanism leading to hepatocyte damage and inflammation necrosis, and the continuous presence or recurrent occurrence of inflammation necrosis is an important factor in the progression of CHB infection to cirrhosis or even HCC. In the pathological development process, innate immune response plays a key role in the early stage of HBV infection and initiates subsequent specific immune responses [43].
The main manifestations of the decline in HBV-specific CD8+ T cell function in CHB patients are antigen-specific loss and proliferation limitation resulting in low frequency, as well as high expression of inhibitory receptors such as programmed death-1 (PD-1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), T cell immunoglobulin and mucin domain-3 (TIM-3) (Figure 2) [44]. Due to the inability to effectively produce cytokines and exert antiviral activity when CD8+ T cells are exhausted during acute immune activity and are re-exposed to HBV, chronic replication of HBV and the occurrence of HCC may result [45]. In HBV-related HCC tumor tissues, CD8+ tissue-resident memory T cells (TRM) are enriched, with high levels of PD-1 expression, leading to TRM functional suppression and depletion [46]. Therefore, it can be seen that functionally impaired or exhausted CD8+ T cells can exacerbate the impact on the occurrence and development of HBV-related HCC.
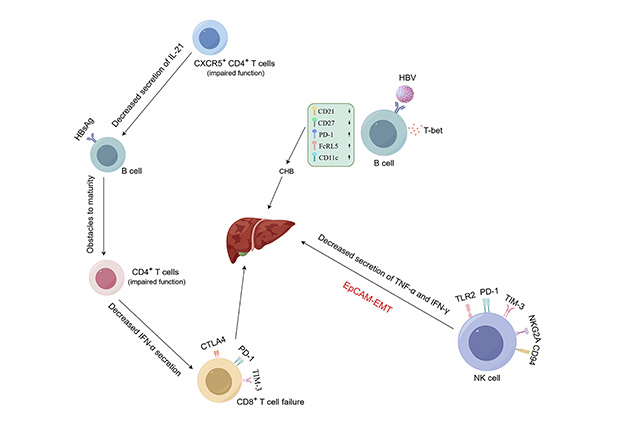
Mechanisms of hepatitis B virus (HBV) involvement in liver disease. (1) The decline in HBV-specific CD8+ T cell function is mainly manifested as antigen-specific loss and proliferation limitation resulting in low frequency, as well as high expression of inhibitory receptors such as programmed death-1 (PD-1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), mucin domain-3 (TIM-3), etc. Meanwhile, functionally impaired CD8+ T cells also accelerate HBV’s erosion of the liver. (2) In NK cells, upregulation of inhibitory receptors NKG2A, TIM-3, Toll-like receptor 2 (TLR2), CD94, and PD-1, as well as decreased secretion of tumor necrosis factor-α (TNF-α) and interferon-gamma (IFN-γ), are all factors promoting the occurrence and development of HCC. (3) Downregulation of CD21 and CD27 expression, along with upregulation of PD-1, FcRL5, and CD11c expression in B cells, increases the probability of CHB occurrence. Created by Figdraw
CD4+ T cells can accelerate antiviral and antitumor immune responses, primarily by producing cytokines that can activate B cells and CD8+ T cells [47]. T helper type 1 (Th1) cells derived from initial CD4+ T cell differentiation produce cytokines such as IFN-gamma (IFN-γ), enhancing immune surveillance by CD8+ T cells [48]. Relevant studies indicate that elevated Tregs in CHB patients not only inhibit specific immune responses induced by HBV antigens but also suppress specific immune responses induced by tumor antigens in HCC patients [49]. The immune escape of HBV-related HCC is related to Tregs, which can lead to the formation of portal vein tumor thrombosis in HCC patients [50]. Therefore, targeted therapy to reduce Tregs activity may be of great help to HCC patients [51].
B cells can mediate adaptive humoral immune responses by producing relevant antibodies that bind to the virus. In the sustained control of HBV, B cells play an important role, and their changes can affect the entire B cell population. It has been reported that HBsAg-specific B cells enriched with T-bet are present in the blood and liver of many CHB patients, which is a characteristic of B cells with antiviral potential. Compared with overall B cells and HBsAg-specific B cells from controls, CD21 and CD27 expression is downregulated, and PD-1, FcRL5, and CD11c expression is upregulated in B cells from CHB patients (Figure 2) [52].
The liver, as an immune organ containing various innate immune cells (NK cells, NKT cells, γδ T cells, etc.), possesses innate immune functions. Early NK cells with antiviral and antitumor activity constitute a relatively large proportion of innate immune cells [53]. Relevant studies have found that in NK cells of CHB patients, the upregulation of inhibitory receptors NKG2A, TIM-3, Toll-like receptor 2 (TLR2), CD94, and PD-1, as well as the decreased secretion of tumor necrosis factor-α (TNF-α) and IFN-γ, are factors that promote the occurrence and development of HCC (Figure 2) [54–56]. In HCC patients, a large proportion of infiltrating CD11b-CD27-NK subset cells in tumor origin exhibit an inactive and immature phenotype, with weak cytotoxicity and decreased IFN-γ production ability, hence closely related to clinical outcomes [46]. Relevant studies have confirmed that excessive secretion of liver cell-specific IFN-γ may promote the occurrence of HCC. In hepatitis virus transgenic mice, IFN-γ secreted by NK cells induces liver cell-related damage through epithelial-mesenchymal transition (EMT), thereby increasing the likelihood of HCC occurrence and development [57].
γδ T cells exert their cytotoxic T cell function by directly recognizing antigens on the surface of invading organisms, making them an important population of innate immune cells in the liver and the first line of defense distributed throughout the body. Research has found that γδ T cells drive the aggregation of myeloid-derived suppressor cells in the mouse HBV-tolerant liver in an interleukin-17 (IL-17)-dependent manner, greatly limiting the function of CD8+ T cells and promoting systemic CD8+ T cell exhaustion [58]. Recent related studies have detected distinct phenotypic and functional characteristics of γδ T cells in acute and CHB infections using multiparameter flow cytometry, suggesting their potential pathogenic relevance [59].
Complement receptors (CR), and regulatory protein groups are another important component of the innate immune system. Increasing evidence suggests that C5a is involved in the pathogenesis of liver diseases such as liver damage, repair, and fibrogenesis. Using the Luminex screening system, it was found that the serum concentration of C5a was significantly decreased in CHB patients with severe fibrosis and early liver cirrhosis [60]. In studies comparing HBV-related liver disease patients with healthy controls, genetic polymorphisms of CR1 were associated with the risk of male HBV-related HCC [61]. In addition, TLR-4 in TLRs can directly mediate the body’s response to pathogens, promoting the release of inflammatory factors such as IL-6, IL-8, and IL-10, and participating in the occurrence of HBV.
Although a considerable amount of research has been conducted on the pathogenesis, epidemiology, immunology, and prevention of hepatitis B, and preventive hepatitis B vaccines are widely used globally, many chronic hepatitis B patients and HBV carriers still cannot be cured. Currently, nucleoside analogs used to treat hepatitis B patients can inhibit virus replication but have poor immune-modulating capabilities, thus cannot completely suppress the liver damage caused by the subsequent development of hepatitis B. Therefore, hepatitis B patients must use these drugs long-term or even for life [62]. However, extensive research has found that therapeutic immunization is a promising treatment strategy. By enhancing the body’s immune response to HBV, it effectively combats the virus and ultimately cures the disease. Early studies on therapeutic vaccination have focused on preventive recombinant vaccines, such as hepatitis B vaccines containing HBsAg. The primary goal of anti-HBV therapy is to remove HBsAg and produce protective HBsAb. This strategy seems feasible, but with research progress, it has been found that preventive recombinant vaccines cannot sufficiently induce the body’s specific T cell response to suppress HBV-DNA replication. Therefore, there is still a long way to go in exploring other vaccine technologies [63].
Some studies have shown that the therapeutic effect of anti-HBV drugs is closely related to the host’s immune response. However, the primary purpose of antiviral drugs is not to regulate the host’s immune response but to only temporarily suppress virus replication. Once discontinued, HBV-DNA will still replicate massively in the body of hepatitis B patients, so antiviral drugs cannot achieve the goal of cure [64]. On the other hand, the method of administering therapeutic hepatitis B vaccines may induce HBV-specific immune responses, regulate the immune capacity of hepatitis B patients, and thus restore their specific immune response to HBV.
Initially, scientists chose to use HBsAg-based vaccines to treat hepatitis B patients, but these vaccines had poor immunogenicity. Wang et al. [63] designed a ferritin nanoparticle that could deliver HBsAg to dendritic cells and macrophages, inducing high levels and persistent immune responses, providing a feasible strategy for the functional cure of chronic hepatitis B patients. Although the use of HBsAg induces the production of HBsAb and activates CD4+ T cells, the sole use of HBsAg still cannot achieve the goal of virus clearance and liver damage inhibition, possibly because there are specific immune responses to multiple antigens of HBV in hepatitis B patients. For therapeutic hepatitis B vaccines, the activation of CD8+ T cells is crucial, so it is necessary to develop other targets [65, 66]. Whitacre et al. [67] found that in the HBV transgenic mouse model, cytotoxic T lymphocytes (CTLs) specific for HBcAg are crucial for controlling HBV replication and preventing liver damage in patients with chronic hepatitis B. HBcAg and B cell epitopes from the preS1 region integrate to induce HBsAg and HBcAg-specific immunity in HBV transgenic mice, activating endogenous dendritic cells without inducing liver damage. This indicates that HBcAg is a component of therapeutic HBV vaccines. The distinct immunological roles of HBsAg and HBcAg in the body provide a new approach for their combined use. On one hand, HBsAg induces protective antibodies against HBV, necessary for blocking HBV replication; detection of HBs antibodies in serum of hepatitis B patients implies recovery [68]. On the other hand, HBcAg can induce HBV-specific immunity without causing liver damage. Combining these two antigens can lead to the development of effective therapeutic vaccines. Surprisingly, Su et al. [69] developed a novel HBsAg and HBcAg combined vaccine, which was evaluated for its immunogenicity and therapeutic effects in HBV transgenic mice using different adjuvants. The results showed that the water-in-oil emulsion adjuvant formulation containing Quillaja saponaria-21 and monophosphoryl lipid A not only stabilized HBsAg and HBcAg for at least 12 weeks but also promoted dendritic cell activation in mice. This adjuvant-antigen combination induced high levels of HBV-specific antibodies and cytotoxic T cell responses in mice, with over 90% of CD8+ T cells being multifunctional T cells, inducing a balanced Th1/Th2 response and inhibiting HBV-DNA replication in mice. This demonstrates that multi-target antigens combined with adjuvants or carrier immunotherapy can serve as a therapeutic vaccine option for curing CHB.
The method designed to optimize the specific immune response in hepatitis B patients also includes DNA vaccines. The DNA vaccine platform features rapid construction, easy replication, and high stability at room temperature. This vaccine technology is based on genetically engineered plasmids containing potent promoters, which can increase in vivo transcriptional activity. The corresponding plasmids are administered intramuscularly systemically or primarily locally. Currently, DNA vaccines have been widely studied in the field of chronic hepatitis B infection [70]. A phase I clinical trial showed that using DNA vaccines expressing hepatitis B envelope protein can activate T cells to produce IFN-γ and suppress HBV-DNA replication. In clinical trials, 10 patients with chronic hepatitis B received injections of DNA vaccines encoding HBV envelope protein. Among them, 5 patients showed a decrease in serum HBV-DNA levels after 3 injections, and HBV-DNA was completely cleared in one patient. The patients tolerated the treatment well, but the persistence was not strong. After the third immunization, the HBV-DNA levels in the patients increased again after 10 months, proving that DNA vaccines encoding a single antigen can only transiently activate the body to produce a specific immune response and cannot exert a long-lasting effect in the body [71]. Based on this issue, the team led by Wang et al. [70] utilized a novel dendritic cell-targeting protein coupled with DNA vaccines. This protein delivers DNA to dendritic cells through electrostatic coupling to prolong the action time of DNA vaccines. Studies have shown that protein/DNA complexes can induce a stronger specific immune response. Compared with the single vaccine group, mice vaccinated with protein/DNA complexes can secrete higher levels of IFN-γ and IL-4. The serum HBV-DNA and HBsAg levels in HBV transgenic mice significantly decreased, with HBsAg expression levels approaching zero and HBV-DNA replication levels dropping to 50% of the original levels. This indicates that the complex of targeted protein and DNA vaccine can induce both humoral and cellular immune responses in the body. This method can be used not only to treat HBV-infected patients but also for the treatment of other viral infectious diseases or cancer patients [72].
Plasmids or viruses are commonly used as vectors to deliver specific DNA segments to host cells, and many vectors have been utilized in vaccine preparation. Among the most effective delivery systems are viral vectors, as they possess strong immunogenicity, capable of eliciting long-lasting immune responses, with adenoviral vectors being the most commonly used among all viral vectors [73]. Currently, in the development of therapeutic hepatitis B vaccines, there has been some progress in research on adenoviral vectors. It has been found that TG1050 is a therapeutic hepatitis B vaccine based on non-replicating human adenovirus 5 (Ad5). This vaccine expresses a fusion protein composed of truncated HBcAg, HBV polymerase, and partial HBsAg structural domains. A single immunization of mice with this vaccine can induce persistent and specific T cell responses, and specific CD8+ T cells can still be detected one year after immunization, which may be related to the ability of Ad5 to induce a lasting effector period [74]. The generated T cells can simultaneously target three antigens: HBcAg, HBV polymerase, and HBsAg, and can produce cytokines such as IFN-γ and TNF-α, while significantly reducing HBV-DNA levels. However, the potential drawback of using human adenoviral vectors is that they may induce the production of anti-adenovirus antibodies, which could to some extent affect the immunogenicity of therapeutic hepatitis B vaccines [12]. Additionally, Cargill et al. [74] designed an immunotherapeutic candidate vaccine, ChAdOx1-HBV, which induced strong CD8+ HBV-specific T cell responses in mice after immunization. This may be because the chimpanzee Adenoviruses vector can induce CD8+ T cell responses, and ChAdOx1-HBV immunogen contains the full sequence of HBV. The response of HBV-specific T cells to multiple HBV antigens is necessary for successful clearance of acute HBV infection [13]. In summary, due to the strong affinity of adenovirus for the liver, there is a high demand for adenoviral vectors in the development of HBV-targeted gene therapy. By utilizing adenoviral vectors, an effective method for eliminating HBV infection can be created.
Vaccination is the most effective method to prevent HBV infection. The recommended standard schedule for hepatitis B vaccination includes three doses, administered within 24 hours of birth (timely birth dose), at 1 month, and at 6 months. In 1992, the World Health Organization recommended the global administration of the hepatitis B vaccine as part of the Expanded Program on Immunization [75].
China’s hepatitis B vaccination strategy has achieved significant success, with over 200 million newborns vaccinated. The vaccination rate gradually increased after 2005 and reached a high level after the implementation of a new policy, which provides free hepatitis B vaccine for infants. Prior to the introduction of this policy, families were required to pay for vaccination services (2002–2004) or both service fees and vaccine doses (1992–2001) [76]. Due to relatively low early birth cohort vaccination rates, a catch-up plan was implemented for non-immunized children born from 1994 to 2001 in 2009–2010. Currently, adult hepatitis B vaccination is not a priority. The incidence of hepatitis B in the adult population remains high, and with increasing age, the infection rate continues to rise while the hepatitis B vaccination rate sharply declines [77]. Studies have shown that new hepatitis B patients are mainly concentrated in the 15–44 age group, and the prevalence of HBsAg carriers among adults has not significantly decreased. An adult hepatitis B vaccination program would have significant health benefits and is likely to be cost-effective [78]. Additionally, providing free vaccines to the general adult population would help promote health equity, as there are clear social gradients in the burden of hepatitis B-related diseases and hepatitis B vaccination coverage.
Various studies have analyzed the factors related to adult hepatitis B vaccination coverage and found that vaccination-related costs (such as user fees and travel expenses) and socioeconomic status (measured by income, education, and occupation) are important factors influencing adult hepatitis B vaccination. Recent research indicates that lower socioeconomic status is associated with relatively lower vaccination rates, which is a major cause of health inequality [15]. Zhu et al. [77, 78] confirmed this view through an individual-level survey conducted in rural areas of eight provinces in China, revealing the socioeconomic determinants of hepatitis B vaccination and vaccination inequality in rural China. Their data analysis found that vaccine and vaccination service fees, travel and waiting times, and travel costs reduced the probability of vaccination. These impacts can be interpreted as the direct and indirect vaccination costs to households. Meanwhile, socioeconomic and demographic variables such as income, age, education level, and occupation were associated with the utilization of vaccination. Additionally, the number of identified true transmission routes and perceived protection were related to the utilization of vaccination. The influence of income increased the probability of vaccination because the ability to pay for vaccines and vaccination services increased. Age had a negative effect on vaccination. One possible reason is that younger individuals are better educated and have better jobs, which increases the likelihood of vaccination. Highly educated groups have more knowledge about hepatitis B infection, such as the number of identified true transmission routes; they better understand the perceived protection of vaccination and are more health-conscious, thus are more proactive in getting vaccinated. This indicates that the contributions of income, education level, true transmission routes, and protection are positive, and these variables increase the inequality in vaccination rates among affluent populations [66].
Since scientists discovered HBsAg in the last century, the progress of hepatitis B research has been demonstrated through improvements in serology and molecular diagnostics, as well as the development of antiviral therapies that effectively control viral replication. However, even with widespread use of vaccines and antiviral treatments worldwide, low- and middle-income countries still face challenges in preventing or treating this infection due to the high costs associated with achieving high vaccine coverage or effective long-term treatment. Although current therapies cannot truly cure HBV infection, researchers have not given up on finding new treatment methods. The biggest challenge in curing hepatitis B at present is eradicating cccDNA and preventing HBV genome integration into the human genome. Therefore, targeting the interference of virus entry, lifecycle, and replication processes has become a research hotspot for the treatment of HBV infection, with important progress made in clinical studies of siRNA drug ALG-125755, RNA interference drug RG6346, and monoclonal antibody KW-027, among others. This brings hope for a functional cure to many hepatitis B patients. However, these drugs still need further investigation of their safety and efficacy in more clinical studies.
We believe that therapeutic vaccines are a very promising immunotherapy strategy in the treatment of hepatitis B. So far, therapeutic hepatitis B vaccines have been developed through different routes, including recombinant protein vaccines, DNA vaccines, and adenovirus vector vaccines. However, there are no hepatitis B vaccines used for therapeutic purposes in clinical practice mainly because most of the recombinant protein vaccines currently under development target HBsAg as a single antigen, which cannot induce a multi-antigen-specific immune response, thus failing to activate low-reactive specific T cells that persist in the subjects’ bodies. DNA vaccines have a short duration of action in vivo and require effective delivery vectors to function. Single DNA vaccines make it difficult to suppress HBV-DNA replication and induce specific immune responses. Although adenovirus vector vaccines can induce high levels of CD8+ T cell production, adverse reactions to this vaccine in humans remain unclear. If the body produces specific antibodies against adenovirus after vaccination, it may reduce the immunogenicity of the vaccine. Therefore, maintaining long-term protective effects of vaccines, clarifying clinical adverse reactions, and curing asymptomatic carriers of hepatitis B are still urgent issues that need to be addressed.
Ad5: adenovirus 5
ALT: alanine aminotransferase
anti-HBc: hepatitis B core antibody
cccDNA: covalently closed circular DNA
CHB: chronic hepatitis B virus
CR: complement receptors
HBeAg: hepatitis B e antigen
HBIG: hepatitis B immunoglobulin
HBsAb: hepatitis B surface antibody
HBsAg: hepatitis B surface antigen
HBV: hepatitis B virus
HCC: hepatocellular carcinoma
IFN: interferon
IFN-γ: interferon-gamma
IL-17: interleukin-17
MSM: men who have sex with men
MTCT: mother-to-child transmission
NTCP: sodium taurocholate cotransporting polypeptide
PD-1: programmed death-1
Th1: T helper type 1
TIM-3: T cell immunoglobulin and mucin domain-3
TNF-α: tumor necrosis factor-α
Tregs: regulatory T cells
TRM: tissue-resident memory T cells
VLPs: virus-like particles
CL: Writing—review & editing, Investigation. CW: Investigation. XY: Writing—review & editing, Supervision.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Hao Xiong, Jinsheng Guo
Laura C. McCoullough ... Peter A. Revill
Juntian Yao ... Youhua Xie
