Affiliation:
1Key Laboratory of Medical Molecular Virology, Shanghai Institute of Infectious Diseases and Biosecurity, Shanghai Frontiers Science Center of Pathogenic Microbes and Infection, School of Basic Medical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032, China
ORCID: https://orcid.org/0009-0002-5754-7966
Affiliation:
1Key Laboratory of Medical Molecular Virology, Shanghai Institute of Infectious Diseases and Biosecurity, Shanghai Frontiers Science Center of Pathogenic Microbes and Infection, School of Basic Medical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032, China
2Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai Institute of Liver Diseases, Shanghai 200032, China
ORCID: https://orcid.org/0000-0002-9980-8725
Affiliation:
1Key Laboratory of Medical Molecular Virology, Shanghai Institute of Infectious Diseases and Biosecurity, Shanghai Frontiers Science Center of Pathogenic Microbes and Infection, School of Basic Medical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032, China
Email: yhxie@fudan.edu.cn
ORCID: https://orcid.org/0000-0002-2416-7708
Explor Dig Dis. 2025;4:100565 DOI: https://doi.org/10.37349/edd.2025.100565
Received: December 08, 2024 Accepted: January 22, 2025 Published: February 21, 2025
Academic Editor: Esaki M. Shankar, Central University of Tamil Nadu, India
The article belongs to the special issue Viral Hepatitis
Hepatitis B virus (HBV) infection is a major risk factor of cirrhosis and hepatocellular carcinoma (HCC) worldwide. Pathogenesis of HBV-induced cirrhosis and HCC involves viral factors and virus-triggered local inflammatory and immune responses, the latter leading to progressive fibrosis, cirrhosis and carcinogenesis. Antiviral therapeutics suppress HBV replication and reduce the risks of cirrhosis and HCC. We discuss the current knowledge on the pathogenesis of HBV-induced cirrhosis and HCC, focusing on mechanisms of current and emerging antiviral therapeutics.
Chronic hepatitis B (CHB) is caused by hepatitis B virus (HBV) infection. 296 million people are estimated to be chronically infected with HBV worldwide [1], causing 1.1 million deaths in 2022 [2]. CHB is a major risk factor for cirrhosis and hepatocellular carcinoma (HCC). The 5-year incidence of cirrhosis for untreated CHB patients is 8%–20%, while the annual risk of patients with cirrhosis developing HCC is 2%–5% [3].
China represents one-third of the global burden of hepatitis B worldwide [2]. China has established a national plan to eradicate viral hepatitis by 2030, including the hepatitis B vaccine program for newborns and strategies to prevent vertical transmissions of HBV [4]. Nevertheless, incidence rate of HBV infection in China is still the highest among category B infectious diseases [5]. The large sum of chronic HBV-infected people still poses a dire challenge to healthcare providers.
Multiple mechanisms have been identified as potential factors of HBV-induced cirrhosis and HCC. In this review, we introduce the pathogenic pathways potentially leading to HBV-induced cirrhosis and HCC, both virus-related mechanisms and host-related mechanisms. We further discuss current and emerging antiviral therapeutics against HBV infection, focusing on their activities to suppress viral replication and alleviate cirrhosis and HCC risks.
The pathogenic process of CHB and the cirrhogenic and carcinogenic effects of CHB can be described by the interactions of virus-related mechanisms and host-related mechanisms [6]. Virus-related mechanisms include effects of virally expressed proteins and HBV DNA integrations. Host-related mechanisms are mainly the chronic inflammation response triggered by CHB.
HBV is a non-cytopathic, hepatotropic DNA virus, a member of the Hepadnaviridae family. HBV is transmitted through blood or mucosal exposure to infectious blood and bodily fluids. Common transmission routes include sexual transmission, vertical transmission, and nosocomial transmission [7].
The HBV virion, known as Dane particle, is comprised of an envelope of lipids and hepatitis B surface antigens (HBsAg), a nucleocapsid composed of core proteins, and a partially double-stranded genomic DNA linked to the viral polymerase [8]. When virus particles reach hepatocytes via blood circulation, the HBV virion attaches to the host cell surface by binding to sodium taurocholate co-transporting peptide (NTCP) on the hepatocyte membrane [9]. The relaxed-circular DNA (rcDNA) form of HBV genome contained in the viral capsid is transported into the nucleus, where it is converted by the cellular DNA repair system to the covalently closed circular DNA (cccDNA) which serves as the template for HBV transcription [10]. Transcription products include pregenomic RNA (pgRNA) and mRNAs that are translated into viral proteins. pgRNA undergoes reverse transcription to form progeny viral rcDNA [11] (Figure 1).
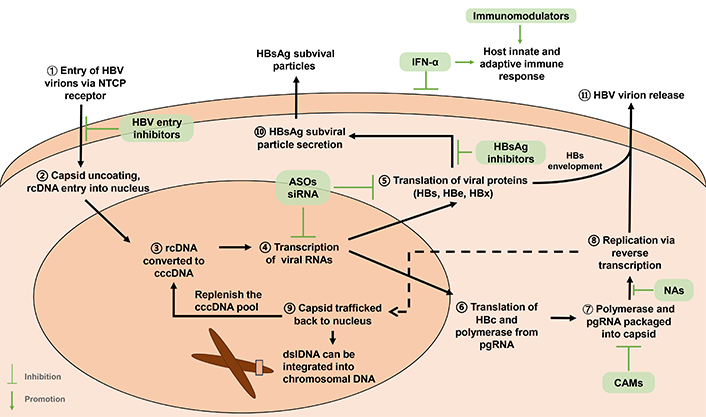
Life cycle of HBV. HBV enters the hepatocyte via NTCP receptor. rcDNA enters the nucleus after uncoating and is converted to cccDNA. cccDNA is the template for transcription of viral mRNAs including pgRNA. HBc and three HBs are reponsible for capsid formation and virion formation, respectively. HBs can also form subviral particles. Both HBs subviral particles and HBe are secreted. HBx modulates viral transcription. New viral nucleocapsids are encapsidated by HBs proteins to become virions. They can also be transported into the nucleus. HBV dslDNA, a by-product of replication, is believed to be the major source of HBV DNA integration. Antiviral targets are shown in green boxes, including NAs, ASOs, siRNA, and CAMs. NAs bind to viral DNA polymerase, causing premature termination of viral DNA transcription; ASOs inhibit transcription by blocking viral mRNA; siRNA induce mRNA degradation; CAMs inhibit encapsidation of pgRNA; HBV entry inhibitors suppress virions entering hepatocytes; HBsAg inhibit HBs protein envelopment of capsid; IFN-α has antiviral and immunomodulatory effect via intercellular and intracellular mechanism, also modulating host immune responses. Immunomodulators alter host innate and adaptive immune responses. ASOs: antisense oligonucleotides; CAMs: capsid assembly modulators; cccDNA: covalently closed circular DNA; dslDNA: double-stranded linear DNA; HBV: hepatitis B virus; HBc: HBV core protein; HBe: HBV e protein; HBeAg: HBe antigen; HBs: HBV surface protein; HBsAg: HBs antigen; HBx: HBV X protein; IFN-α: alpha-interferon; NAs: nucleoside/nucleotide analogs; NTCP: sodium taurocholate co-transporting peptide; pgRNA: pregenomic RNA; rcDNA: relaxed-circular DNA; siRNA: small interfering RNA
HBV X protein (HBx) is an oncogenic protein critically involved in HCC tumorigenesis [12]. HBx is a key driver of HBV infection by mediating cccDNA transcription, modulating cellular and viral promoters and enhancers, and influencing various signal transductions and cell cycle regulation. HBx can promote cell proliferation through the activation of multiple signaling pathways including Wnt/β-catenin, PI3K/AKT, and STAT3 signaling pathways [13]. HBx may inhibit cell apoptosis through various mechanisms, allowing damaged liver cells to survive and accumulate gene mutations, increasing the risk of tumorigenesis [14]. HBx can cause epigenetic changes such as DNA and histone methylation, affecting expression of numerous genes including certain tumor suppressor genes, and promoting liver cell carcinogenesis. HBx can promote cell proliferation signaling in hepatocarcinogenesis, inducing hepatocytes to enter and remain in the G1 phase of the cell cycle [15]. Although it has been reported to have potential effects on numerous tumorigenic pathways, HBx’s precise role in HCC tumorigenesis is yet to be clarified.
Mutations in HBsAg have been associated with HCC [16]. A cross-sectional study of HBsAg-positive patients showed patients with cirrhosis or HCC had a higher frequency of substitution in the major hydrophilic region and immune epitopes in HBsAg [17]. Mutations in antigenic epitopes could impact the conformational structure and alter the immunogenicity of HBsAg in CHB/HCC patients [16].
Gene deletions in HBV pre-S gene segments are valuable biomarkers for higher incidence rates of HCC [18]. HBV pre-S deleted proteins are important oncoproteins that can induce DNA damage and promote growth and proliferation of hepatocytes by activation of endoplasmic reticulum stress-dependent signaling pathways [19]. HBV pre-S2 deleted proteins can further inhibit DNA repair, enhance hepatocyte survival and drug resistance [20].
The integration of HBV DNA into the hepatocyte chromosome is a crucial contributing factor to the development of HCC. Integrated HBV DNA can make stable transcription of viral genes including HBx and HBV surface protein (HBs). HBV integrations can facilitate chromosomal translocations, causing genomic instability [21]. Using long-read DNA sequencing method, multiple unique HBV-associated inter-chromosomal translocations have been identified in early stages of HBV infection in both HCC and non-HCC CHB patients [22]. Collectively, HBV integration may lead to significant transcriptional dysregulation of cellular genes and drive progression to HCC.
HBV genotypes and sub-genotypes are also related to the development of cirrhosis and HCC. Studies suggest genotype C is associated with higher HCC risk than genotype B, and genotype A has a higher HBsAg clearance rate after interferon therapy [23]. Yet, the clinical significance of HBV genotypes is not completely understood.
The antiviral response of the host immune system in chronic HBV infection can cause a series of pathogenic reactions such as liver injury, necrosis, and inflammation, which are manifested by biochemical abnormalities such as elevated serum liver enzymes [24]. Chronic inflammation and fibrogenesis is a dynamic process that involves at least hepatocytes, lymphocytes, macrophages, hepatic stellate cells (HSCs), stromal cells, and their interactions [25]. Persistent viral infection and repetitive inflammatory damage/repair responses lead to irreversible cirrhosis.
HBV-specific CD8+ T cells are the key effector of host adaptive immune response. Patients with CHB have lower levels of HBV-specific CD8+ T cells in blood and liver than those with acute HBV infections [26]. CHB might be viewed as a disease in which HBV-specific CD8+ T cells that escape central tolerance and peripheral CD8+ T cells are not capable of eliminating HBV from the liver [6]. Persistent CD8+ T cell-dependent liver disease leads to continuous episodes of hepatocellular necrosis and regeneration, ultimately resulting in fibrosis, cirrhosis, and HCC [27]. In addition, the process of liver fibrosis and cirrhosis reduces CD8+ T cell-mediated immune surveillance towards infected hepatocytes [28]. Novel epigenomic and transcriptomic methods further clarified T cell responses to HBV and HBV-related HCC. Apart from effector CD8+ T cells, HBV-related tumor-infiltrating regulatory T cells (Tregs) have also shown correlations with viral titer, carcinogenesis, and poor prognosis [29].
Chronic liver inflammation and fibrosis cause malignant alterations in liver microenvironment. In general, persistent hepatic inflammation induces an imbalance between liver fibrogenesis and fibrolysis, and promotes cell proliferation and genomic instability. Activation of HSCs is the primary driver of liver fibrogenesis [30]. Acute liver injury activates tumor growth factor β1 (TGF-β1) and platelet-derived growth factor subunit B (PDGFB) signaling pathways to transdifferentiate quiescent HSCs into activated myofibroblast-like HSCs, producing excessive collagens deposited to extracellular matrixes [31]. In chronic liver inflammation, fibrogenesis initiated by activated HSCs results in a favorable microenvironment for tumor development [32]. In addition, single-cell omics have identified CD36+ cancer-associated fibroblast can secrete macrophage migration inhibitory factors, creating an immunosuppressive microenvironment [33]. The crosstalk between hepatic cells and extracellular microenvironment in chronic HBV infection and inflammation remains to be further clarified.
Multiple molecular signaling pathways have been identified in hepatic fibrosis, cirrhosis, and carcinogenesis [34]. TGF-β pathway can activate fibroblasts, induce cell apoptosis, and initiate epithelial-mesenchymal transition (EMT) [35]. Other growth factor-associated pathways such as JAK/STAT pathways [34], PDGF/PDGFRs pathways [36], and PI3K/AKT pathways [37] mainly regulate fibrosis activation and EMT initiation. Pathways regulating inflammation and metabolism can modulate myofibroblasts activation and carcinogenesis. Toll-like receptor 4 (TLR-4) signaling pathway could sensitize HSCs to TGF-β stimulation and activate Kupffer cells to regulate fibrosis [38].
The goal of treating CHB is to achieve a functional cure. Functional cure is defined as sustained undetectable circulating HBsAg (< 0.05 IU per milliliter) and HBV DNA after a finite course of treatment [39, 40]. Timely and effective antiviral therapy can improve the prognosis of patients, resulting in reduction of the progression of liver inflammation, necrosis, and fibrosis in the majority of patients, reduction of the risk of HCC, and improvement of survival and quality of life.
Conducting antiviral therapy requires a comprehensive assessment of the patient’s risk of disease progression based on serum HBV DNA, alanine aminotransferase (ALT) levels, and severity of liver disease, as well as age, family history, and concomitant diseases [41].
The 2022 Guidelines on the Prevention and Control of Chronic Hepatitis B in China recommends screening for HBsAg in high-risk and general populations, emphasizing timely initiation of antiviral treatment in patients at risk for disease progression [42]. It is recommended that antiviral therapy should be initiated in HBsAg-positive patients with one or multiple characteristics including a family history of CHB cirrhosis or HCC; over 30 years of age; significant inflammation (≥ G2) or fibrosis (≥ F2) manifested by non-invasive markers or liver histology examination; HBV-related extrahepatic manifestations such as glomerulonephritis or vasculitis [43]. Serum ALT and HBV DNA levels should be conducted to assess treatment response and/or disease progression [44]. Follow-up and monitoring of HBV reactivation should be conducted once in 6–12 months, including surveillance for alpha-fetoprotein (AFP) and ultrasonography. The process of selecting indications for antiviral therapies in chronic HBV infection patients is shown in Figure 2.
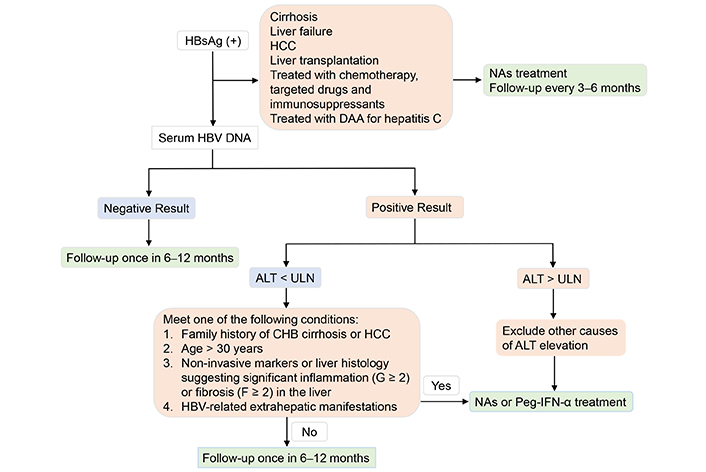
Selecting indications for antiviral therapies in chronic HBV infection patients. ALT: alanine aminotransferase; CHB: chronic hepatitis B; DAA: direct-acting antiviral; HBsAg: hepatitis B surface antigen; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; NAs: nucleoside/nucleotide analogs; Peg-IFN-α: pegylated interferon-α; ULN: upper limit of normal
The two classes of current therapeutic drugs for CHB are oral antiviral nucleoside/nucleotide analogs (NAs) and interferons (IFNs).
NAs are essential as the first-line antiviral therapy for CHB. NAs with a high genetic barrier [45], which includes entecavir (ETV), tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), tenofovir amibufenamide (TMF), is strongly recommended. NAs with a low genetic barrier, such as lamivudine (LAM), adefovir dipivoxil (ADV), and telbivudine (LdT), are no longer recommended in the latest guidelines [46]. The 2022 edition of the Guidelines on the Prevention and Control of Chronic Hepatitis B in China includes ETV, TDF, TAF, and TMF as the first-line recommended drugs for CHB antiviral treatment [47]. ETV can inhibit HBV viral replication potently with a good safety profile and a low resistance rate [48]. ETV’s 5-year cumulative resistance incidence rate is 1% [48]. ETV is suitable for the treatment of CHB with active viral replication in adults, persistently elevated ALT levels, or liver histology showing active lesions [49]. However, an increased risk of spontaneous abortion has been found in patients with HBV infection taking ETV or ADV, in comparison with those prescribed with LAM [50]. TDF also has a potent antiviral effect and an extremely low resistance rate [51]. However, TDF has a certain degree of nephrotoxicity. Its long-term use produces a slight risk of kidney damage and reduced bone density and is not suitable for patients with renal insufficiency and osteoporosis [52]. The 2020 WHO guideline recommends administering antivirals during pregnancy, specifically TDF, for the prevention of HBV mother-to-child transmission [53]. Compared with TDF, TAF has almost no nephrotoxicity and less ill-impact on bones. Drug resistance to TAF has yet to be found. The dosage of TAF is lower than TDF at the same efficacy, with 25 mg of TAF being comparable to 300 mg of TDF [51]. TMF is non-inferior to TDF in terms of anti-HBV efficacy and showed better bone and renal safety [54]. Nucleoside analog therapy can safely and effectively inhibit HBV replication. However, NAs affect the viral life cycle by inhibiting viral reverse transcription and do not target cccDNA which is the key to the risk of recurrence of HBV replication in patients with CHB after discontinuing NA treatment [49].
Interferon acts on HBV transcription and replication by enhancing the function of immune cells and promoting the expression of cytokines and interferon-stimulated genes (ISGs), thus exerting the dual roles of immune regulation and antiviral therapy [55]. IFNs can inhibit HBV transcription and reduce the expression of viral proteins by promoting the degradation of HBV pgRNA and core particles, or by epigenetic modification of cccDNA [55]. Several studies have shown the advantage of IFN-α therapy over nucleoside analogs in reducing the incidence of HCC in high-risk HBV carriers [56]. Nevertheless, the use of interferon therapy is limited by its various side effects [57].
Several studies have been conducted to find new classes of antiviral drugs aimed at reducing the incidence of HCC by completely eliminating all HBV antigens and genetic materials in the body with a limited course of therapy [49]. To achieve the goal of a complete cure for HBV infection, future new drugs will need to break through the following bottlenecks: complete elimination of HBV DNA replication, complete inhibition of HBsAg production, and restoration of a normal immune response against HBV [58]. It is thought that only a combination of multiple types of drugs can achieve several of these goals simultaneously [59].
During antiviral therapy, it is important to follow medical advice, adhere to medication, and do regular monitoring to determine the efficacy of antiviral therapy, medication adherence, drug resistance, and adverse effects [60].
Although potent low-resistance oral NA therapy results in strong suppression of HBV replication, some patients still show poor response and hypoviremia, leading to disease progression. It is recommended that potent low-resistance drugs should be preferred for patients on initial therapy; for patients on treatment, HBV DNA quantification should be performed periodically, and for patients with virological breakthroughs and low viremia [58]. HBV drug-resistant mutations should be tested and the patients should be given salvage therapy as early as possible [41], as shown in Table 1.
Drug resistance mutations in HBV polymerase gene and rescue anti-HBV therapies after developing drug resistance
| Type of drug resistance | Drug resistance mutations in HBV polymerase gene [61] | Recommended treatment [47] |
|---|---|---|
| LAM resistance | rt80V/I, rtl169T, rtV173L, rtL180M, rtA181T, rtT184S, rtM204V/I, rt236T | Change to TDF or TAF |
| Telbivudine resistance | rtM204I | Change to TDF or TAF |
| Adeforvir resistance | rt181T/V, rt236T | Without LAM/telbivudine resistance: change to ETV, TDF or TAF;With LAM/telbivudine resistance: change to TDF or TAF |
| Tenofovir resistance | rtA194T | No resistance detected to date |
| ETV resistance | rtl169T, T184A/C/F/G/I/L/M/S, rtM204V/I, rt202G/C/I, M250I/L/V | ETV + TDF or ETV + TAF;Change to TDF or TAF |
ETV: entecavir; LAM: lamivudine; TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate
For patients who discontinue medication at the end of treatment, liver function, HBV serum markers, and HBV DNA should be tested once a month for 3 months after discontinuation of medication, regardless of whether or not the patient has achieved a response during antiviral therapy; and every 3 months thereafter, in order to detect recurrence of hepatitis and deterioration of liver function in a timely manner [61]. AFP and ultrasonography should be examined every 3 to 6 months. For patients with cirrhosis, AFP and abdominal ultrasonography should be tested every 3 months, and CT and MRI should be done if necessary for early detection of HCC [62].
Current antiviral therapy faces low effectiveness for functional cure of chronic HBV infection and does not completely eradicate the risk of HBV-induced cirrhosis and HCC. NAs are effective in suppressing HBV replication by inhibiting the reverse transcription step in viral life cycle. However, they do not directly target HBV cccDNA in the nucleus of infected hepatocytes. In addition, reservoir HBV DNA inside hepatocyte nucleus can integrate into host chromosomal DNA which is a crucial process of hepatocarcinogenesis [63]. Numerous investigational therapies are underway in order to reach a functional cure for chronic HBV infection, as shown in Table 2. Two major categories of new anti-HBV therapies are direct-acting antivirals (DAAs) and host-directed antivirals (HDAs). DAAs target proteins that are essential in viral replication or interfere expression of viral antigens. HDAs mainly modulate the immune system [64]. Some early-stage clinical trials have achieved functional cure in a small portion of patients, but an optimal strategy of combination therapy for the large part of patients remains challenging [65]. Nevertheless, combining different antiviral agents in one therapy is likely the answer for a functional cure for chronic HBV infection.
Investigational novel therapies for HBV infection (selected)
| Categories ofnovel therapies | Types and compounds | Clinical trial (Phase) | References |
|---|---|---|---|
| Direct-acting antivirals (DAAs) | Capsid assembly modulators (CAMs) | ||
| Bersacapavir (JNJ-6379) | Phase 2b | [67] | |
| Morphothiadin (GLS4) | Phase 2 | [68] | |
| EDP-514 | Phase 1 | [69] | |
| siRNA agents | |||
| JNJ-3989 | Phase 2b | [67] | |
| RG6346 | Phase 2 | [70] | |
| AB7-29-001 | Phase 2 | [71] | |
| Antisense oligonucleotides (ASOs) | |||
| Bepirovirsen (GSK32228836) | Phase 2 | [72] | |
| RO7062931 | Phase 1 | [73] | |
| HBV entry inhibitors | |||
| Bulevirtide | Phase 2 | [78] | |
| HBsAg inhibitors | |||
| REP2139 | Phase 2 | [73] | |
| Host-directed antivirals (HDAs) | Immunomodulators, innate immune response | ||
| RO7020531 | Phase 1 | [74] | |
| Selgantolimod | Phase 2 | [75] | |
| Immunomodulators, adaptive immune response | |||
| Epcoritamab | Phase 2a | [68] | |
| Therapeutic vaccines | |||
| BRII-179 | Phase 2 | [76] |
Capsid assembly modulators (CAMs) are a class of antiviral agents that target the assembly of HBV capsid, inhibiting encapsidation process of HBV pgRNA. Oral small-molecule CAMs are capable of decreasing serum HBV DNA and HBV RNA levels. CAMs are not capable of reducing HBV cccDNA or transcription. Therefore, CAMs are used in combination with other agents in investigational therapies [66]. A phase 2b, double-blind, placebo-controlled randomized trial investigates a combination therapy of bersacapvir (CAM) + JNJ-3989 (siRNA) + NA in CHB patients [67]. All patients had significant declines in HBsAg after 48 weeks of the therapy. However, no functional cure was achieved in this study.
Antisense oligonucleotide (ASO) can act as an inhibitor for virus mRNA, blocking viral protein translation. Bepirovirsen (BPV) is a 2′-O-methoxyethyl modified ASO that targets all HBV RNAs. A phase 2b, random, investigator-unblinded trial evaluating the efficacy and safety of BPV in patients with chronic HBV infection has resulted in a sustained HBsAg and HBV DNA loss in 9%–10% of participants [68]. However, patients with cirrhosis and HCC are excluded from this trial, and no further analysis of cirrhosis and HCC risks has been reported.
siRNA, or small interfering RNA, is a type of double-stranded RNA molecule that can induce the degradation of mRNA, thereby silencing the expression of target genes. Xalnesiran (RG6346) is a GalNAc-conjugated, double-stranded siRNA targeting the S-region of HBV genome, leading to degradation of HBV mRNA and reducing production of HBV antigens including HBsAg [69]. A combination of xalnesiran plus an immunomodulator has been proposed as a promising therapeutic approach to achieve a functional cure. A phase 2, randomized, open-label study of efficacy and safety of xalnesiran with and without a pegylated interferon-α (Peg-IFN-α) in participants (NCT04225715) has been conducted [66]. Xalnesiran combined with a Peg-IFN-α resulted in higher rates of HBsAg loss than without a Peg-IFN-α. No progression of cirrhosis and HCC was reported.
HBV entry inhibitors target NTCP on hepatocyte membrane, inhibiting HBV virion from entering hepatocytes. Bulevirtide, a selective NTCP receptor currently marketed as hepatitis delta virus (HDV) treatment in Europe, is in phase 2 clinical trial stage to treat HBV [70]. Several monoclonal antibodies against NTCP have been tested on in vitro and animal in vivo models [71].
HBsAg inhibitors disrupt HBsAg secretion processes, which can effectively decrease HBsAg availability [72]. REP2139 is a nucleic acid polymer that blocks the release of HBsAg. An open-label, phase 2 study (REP401) evaluating the safety and efficacy of the combination therapy of TDF + Peg-IFN-α + REP2139 did not result in an increased rate of functional cure compared with TDF + Peg-IFN-α therapy [73]. Further clinical investigation is needed to assess the efficiency of HBsAg inhibitors.
Immunomodulatory agents in combination therapy can restore innate immune responses and HBV-specific adaptive immune responses. Novel immunoregulatory agents in development include selgantolimod, a TLR-8 receptor antagonist [74]; RO7020531, a TLR-7 receptor antagonist [75]; epcoritamab, a bispecific T-cell-engaging antibody [76], and many others. In addition, immunogenic therapeutic anti-HBV vaccines are being investigated at an early stage of development.
Effectiveness of novel anti-HBV therapeutics in reducing HBV-induced cirrhosis and hepatocarcinoma risks requires long-term evaluation. High level of HBV DNA level (> 2,000 IU/mL) is a strong predictor of cirrhosis and HCC [66], and there is a linear relationship between HBV DNA and risk of cirrhosis and HCC in hepatitis B e antigen (HBeAg)-negative patients. However, in HBeAg-positive patients, a relatively higher HBV DNA level is associated with a lower immediate risk of developing cirrhosis and HCC [58]. Reaching a functional cure for HBV with a lower risk of cirrhosis and HCC would benefit millions of patients with CHB.
The major challenge of developing novel therapeutics is to overcome cccDNA in HBV infection as well as maintain a life-long adaptive immune response without inducing excessive cytopathic effects. Apart different therapeutic approaches discussed above, we suggest mRNA therapeutic vaccines might be an effective strategy to achieve a functional, even complete cure. mRNA vaccines have shown stronger ability to induce desired immune responses and lower production costs compared to traditional vaccines [77]. Currently, only animal models have been utilized to test the potential effect of mRNA vaccine in HBV therapeutics. A type of lipid nanoparticle-formulated mRNA vaccine optimized for the expression of HBV core, polymerase, and surface antigens was tested on CHB mouse models. After three immunizations, a significant drop in HBsAg and HBeAg serum levels was observed [78]. This emerging field of therapeutics may have the potential to greatly improve HBV prevention and treatment efficacy.
Managing chronic HBV infection would alleviate the risk of developing cirrhosis and HCC. In-depth studies on virus life cycles, hepatic immune response, and inflammatory and fibrosis processes provide insights into infection-cirrhosis-carcinoma transition, as well as potential therapeutic targets.
Current antiviral therapies with NAs and IFNs are essential for preventing cirrhosis and HCC in chronic HBV infection patients. Close monitoring for drug resistance and improving compliance would benefit treatment outcomes.
Investigational therapies for chronic HBV infection have shown potentials to achieve a functional cure. Combination of different categories of anti-HBV agents is likely to be more effective. Effectiveness of these novel therapies to reduce and prevent the development of cirrhosis and HCC remains to be answered.
ADV: adefovir dipivoxil
AFP: alpha-fetoprotein
ALT: alanine aminotransferase
ASO: antisense oligonucleotide
BPV: bepirovirsen
CAMs: capsid assembly modulators
cccDNA: covalently closed circular DNA
CHB: chronic hepatitis B
DAAs: direct-acting antivirals
EMT: epithelial-mesenchymal transition
ETV: entecavir
HBeAg: hepatitis B e antigen
HBsAg: hepatitis B surface antigen
HBV: hepatitis B virus
HBx: hepatitis B virus X protein
HCC: hepatocellular carcinoma
HDAs: host-directed antivirals
HSCs: hepatic stellate cells
IFNs: interferons
IFN-α: alpha-interferon
LAM: lamivudine
NAs: nucleoside/nucleotide analogs
NTCP: sodium taurocholate co-transporting peptide
PDGFB: platelet-derived growth factor subunit B
Peg-IFN-α: pegylated interferon-α
pgRNA: pregenomic RNA
rcDNA: relaxed-circular DNA
TAF: tenofovir alafenamide
TDF: tenofovir disoproxil fumarate
TGF-β1: tumor growth factor β1
TLR-4: toll-like receptor 4
TMF: tenofovir amibufenamide
JY: Writing—original draft. JG and YX: Writing—review & editing, Investigation, Supervision. All authors agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Prof. Jinsheng Guo and Youhua Xie who are Guest Editors of Exploration of Digestive Diseases, had no involvement in the decision-making or the review process of this manuscript.
Not applicable.
Not applicable.
Not applicable.
All the citations included in this manuscript are available upon request by contact with the corresponding author.
This work was funded by the National Fund of Nature Science of P.R. China [91129705, 81070340, 81971921] and Shanghai Pujiang Talent Program [09PJ1402600]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Hao Xiong, Jinsheng Guo
Laura C. McCoullough ... Peter A. Revill
Chunzheng Li ... Xianguang Yang
