Abstract
Aim:
Selecting patients for immunotherapy in metastatic gastric cancer (mGC) in second and subsequent lines remains challenging. The aim of our study is to assess the feasibility of anti-programmed death-ligand 1 (anti-PD-L1) inhibitors in pretreated patients with mGC, and to determine prognostic and predictive biomarkers.
Methods:
We retrospectively analyzed data of 122 patients treated in five oncology centers in Moscow between 2018 and 2023, who received nivolumab or pembrolizumab for advanced gastric cancer. The primary end-point of our study was 6-month progression-free survival (PFS). For multivariate analysis, variables with a value of p < 0.05 obtained in a univariate analysis were selected. The optimal threshold value of the neutrophil-lymphocyte ratio (NLR) as a predictor of the effectiveness of immunotherapy was determined using receiver operating characteristic (ROC) curve analysis.
Results:
Patients with mGC who received immune checkpoint inhibitors (ICIs) were included. 6-month PFS rate was 31.6%. The median PFS (mPFS) and overall survival (mOS) in patients in the high NLR group (NLR ≥ 1.8) were 2 and 4 months; mOS and mPFS in the low NLR group were not achieved (p < 0.001). The presence of ascites (p < 0.001), the administration of ICIs in III–IV lines (p = 0.004), and NLR ≥ 1.8 (p = 0.006) were independent prognostic factors, associated with decrease of OS. The median OS of patients in favorable and unfavorable prognostic groups were 13 months and 2 months, respectively (p < 0.001).
Conclusions:
Ascites, NLR level of ≥ 1.8, and administration of anti-PD-L1 inhibitors were associated with low efficacy of immunotherapy in patients with microsatellite stable mGC. Further research should be planned including patients who did not receive ICIs to determine the prognostic significance of our model.
Keywords
Gastric cancer, anti-programmed death-ligand 1 (anti-PD-L1), microsatellite stable (MSS), prognostic factorsIntroduction
In 2022, gastric cancer (GC) was the 5th leading cause of cancer death in the Russian Federation, with 38% of cases presenting with metastatic disease at the time of the diagnosis [1]. The median overall survival (mOS) of previously untreated patients with metastatic gastric cancer (mGC), who received platinum agents with fluoropyrimidines, is 8.8–10.7 months [2]. Recent advances in addition of targeted therapy to standard doublet chemotherapy resulted in increasing of long-term outcomes of patients with mGC, whose tumors expressed human epidermal growth factor receptor 2 (HER2), programmed death-ligand 1 (PD-L1), and claudin-18 isoform 2 (CLDN18.2). In the phase III ToGA trial, mOS of patients with HER2-positive mGC who received trastuzumab with chemotherapy was 13.8 months compared to 11.1 months in those assigned to chemotherapy alone [3]. Novel monoclonal antibody zolbentuximab prolongs both progression-free survival (PFS) and OS in patients with CLDN18.2-positive GC up to 8.2 and 14.4 months, respectively [4]. The addition of anti-PD-L1 antibodies to standard oxaliplatin-containing chemotherapy in patients with PD-L1 combined positive score (CPS) ≥ 5 and CPS ≥ 10 showed similar results [5, 6].
Unfortunately, the efficacy of the second line in patients with mGC is lower. A combination of ramucirumab and paclitaxel demonstrated an objective response rate (ORR) of 28% and median PFS (mPFS) of 4.4 months in the RAINBOW trial [7]. In the RAMIRIS trial, the mPFS of patients who received ramucirumab with FOLFIRI was 3.9 months [8]. Approximately 38% of patients who failed second-line therapy, received subsequent treatment for mGC [9]. Regorafenib might be a treatment choice in such patients with mOS 5.8 months, however, 67% of grade 3–5 adverse events (AEs) have been observed [10]. Similarly, mOS in heavily pretreated patients, who received trifluridine/tipiracil for mGC, was 5–6 months with higher toxicity grade—up to 84% grade 3–5 AEs [11].
About half of the patients received combined regimens in the second-line treatment for mGC [12], from which nearly 30% had Eastern Cooperative Oncology Group (ECOG) 2 performance status at the beginning of chemotherapy [12]. Immune checkpoint inhibitors (ICIs) seem to preserve the patient’s quality of life and improve long-term outcomes in certain subsets [13–15].
PD-L1 expression and high microsatellite instability (MSI-H) are well-known predictive biomarkers for ICIs in patients with mGC [16, 17]. Nivolumab and pembrolizumab in combination with oxaliplatin-containing chemotherapy are standard first-line therapy of advanced GC in patients with PD-L1 CPS ≥ 5 and CPS ≥ 10, respectively [6, 14]. However, optimal PD-L1 CPS cut-off for GC immunotherapy in patients who failed the first- and second lines of therapy remains challenging. A slight increase in survival rates was observed in patients who received nivolumab for mGC, regardless of PD-L1 expression: mOS was 5.3 months [18]. Similar results were obtained from the KeyNote-059 study with ORR of 15.5% and mOS of 5.6 months in patients with PD-L1 positive mGC [19].
Another predictive biomarker for ICIs is MSI-H: combined analyses of three trials revealed durable responses to anti-PD-L1 antibodies and high survival outcomes in patients with mGC [16]. The prevalence of MSI-H GC is 22%, according to The Cancer Genome Atlas (TCGA) Group [20], however, real-world data suggests the prevalence of MSI-H GC is up to 3–10% [21]. Molecular analyses of gastric adenocarcinoma conducted by TCGA showed a high number of immune signaling pathways not only in MSI-H tumors but in adenocarcinomas with Epstein-Barr virus (EBV), which predicts high sensitivity to ICIs [22, 23]. In Chinese retrospective analyses of patients with mGC who received ICIs, the mPFS of patients with EBV+/MSS was 8.2 months versus 2.0 months in patients with EBV−/MSS (p < 0.001), and mOS in the first group was not reached (p = 0.002) [24].
Tumor mutational burden (TMB) also predicts response to anti-PD-L1 antibodies [25]. In the Korean study, the mOS of patients who received immunotherapy for mGC with high TMB (TMB cut-off was 14.3 Mut/Mb) was 22.4 months compared to 3.6 months in those with low TMB (p < 0.033) [26]. Currently, TMB and EBV determination in tumors is not routinely performed.
Сlinical factors associated with response to ICIs are actively studied in addition to tumor biomarkers [27, 28]. Inflammatory signaling pathways resulting from genomic instability are one of the “Hallmarks of cancer”, and its tumor-induced inflammatory response has been confirmed as an effective prognostic biomarker in many cancers [29]. Neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and lymphocyte-monocyte ratio (LMR) are the most well-studied systemic inflammatory markers with both prognostic and predictive role [30]. Most studies show the prognostic significance of NLR and PLR in patients who received immunotherapy for non-small-cell lung cancer and melanoma treatment [31–34]. In the recent two years, meta-analyses showed an association between low NLR and PLR levels and favorable outcomes with durable responses to ICIs in patients with mGC [35]. Some prognostic scales for patients with mGC have been investigated and validated, however, none include immunotherapy [36, 37].
In real-world data, the mPFS and mOS of patients, who received ICIs for mGC, were 2 and 6 months, respectively, with no statistically significant difference in OS according to microsatellite or PD-L1 status [38, 39]. Given the mentioned above survival rates and lack of prognostic factors, the administration of immunotherapy in pretreated patients with mGC seems challenging. The aim of our study is to assess the feasibility of ICIs in patients with advanced GC, especially in heavily-pretreated populations, and evaluate the prognostic and predictive role of clinical characteristics and tumor biomarkers to identify patients with the most significant benefit from receiving ICIs for the treatment of mGC.
Materials and methods
Patients and treatment
This retrospective multicenter study was conducted in five oncology centers (Moscow, Russia) and included data on 122 patients who received monotherapy of ICIs between January 2018 and February 2023. Participating medical centers are listed in the acknowledgments. Inclusion criteria were as follows: patients 18 years old and older, histologically confirmed gastric adenocarcinoma, ECOG 0–2, administration of nivolumab or pembrolizumab for metastatic GC. Patients who progressed within six months after the completion of adjuvant or perioperative chemotherapy were considered to have failed first-line therapy. Pembrolizumab was administered intravenously at a dose of 200 mg every three weeks or 400 mg every six weeks. Nivolumab was administered intravenously at a dose of 3 mg/kg or 240 mg every two weeks or 480 mg every four weeks. Interchangeability between two antibodies was allowed.
Study endpoints
The primary endpoint of this study was 6-month PFS. Secondary endpoints included overall survival (OS), ORR, disease control rate (DCR), and safety. PFS was the time from the date of first-cycle immunotherapy to the date of disease progression or death from any cause. OS was the time from the date of first-cycle immunotherapy to the date of death from any cause. Patients who were lost to follow-up and were alive at the last data cut-off were censored. ORR was defined as the proportion of patients whose overall response was complete response (CR) or partial response (PR) for measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. DCR was the proportion of patients whose overall response was CR, PR, or stable disease (SD). Pretreatment NLR was calculated as the absolute number of neutrophils in blood before the first cycle of immunotherapy divided by the absolute number of lymphocytes in blood before the first cycle of immunotherapy. PD-L1 CPS was assessed by immunohistochemistry (IHC) up to local pathologists. MSI status was tested by polymerase chain reaction or IHC. Toxicity was assessed for each immune-related adverse event (irAE) according to the Common Terminology Criteria for Adverse Events ver. 5.0.
Statistical analyses
The data cut-off for survival analyses was 13 March 2024. To detect an increase of 15% in 6-month PFS, a minimum of 105 patients should be included in this study (power 90%, alpha 0.05). Survival outcomes were estimated using the Kaplan-Meier method and compared via a log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard model. A two-sided p-value < 0.05 was considered statistically significant. The cut-off value for NLR was determined using ROC analysis. The difference in category variables of characteristics among the low NLR group and high NLR was compared by χ2 tests. All statistical analyses were carried out using Statistical Package for the Social Sciences for Windows (v.23.0; IBM Corp. Armonk, NY, USA) and Prism 10 (10.1.1; GraphPad Software, Boston, USA).
Results
Patient characteristic
Between 1 January 2018 and 28 February 2023, 122 patients who received ICIs for mGC treatment were included (Table 1). The median age was 64 years (range 26–84): 62% male and 38% female. ECOG 1 performance status was in 56.2% of patients, ECOG 2 was in 35% of patients, and ECOG 3 was in two patients (1.7%). Twenty-seven patients (22%) had MSI-H adenocarcinoma. In patients with known PD-L1 expression levels, PD-L1-positive adenocarcinoma has been observed in 71 (58.2%) of patients. All patient characteristics are described in Table 1.
Patient characteristics
| Characteristics | Number (%) | |
|---|---|---|
| Age | < 65≥ 65 | 66 (54.1)56 (45.9) |
| Gender | MaleFemale | 75 (61.5)47 (38.5) |
| ECOG | 0–12–3 | 77 (63.1)45 (36.9) |
| Lauren type | ColonicDiffuseMixedNot known | 29 (23.8)53 (43.4)15 (12.3)25 (20.5) |
| MSI status | MSI-HMSSNot known | 27 (22.1)71 (58.1)24 (19.8) |
| PD-L1 (CPS) | PD-L1 negativePD-L1 CPS 1–9PD-L1 CPS ≥ 10Not known | 7 (5.7)57 (46.7)14 (11.5)44 (36.1) |
| HER2 | PositiveNegative Not known | 19 (15.7)88 (72.7)15 (11.6) |
| Primary tumor | PresenceSurgically removed | 70 (57.4)52 (42.6) |
| Number of organs with metastases | 1–2≥ 3 | 77 (63.1)45 (36.9) |
| Metastatic sites | LiverLungsPeritoneumLymphatic nodesBonesOther | 46 (38)16 (12.4)54 (44.6)64 (52.1)12 (9.9)15 (12.4) |
| Line of immunotherapy | First-lineSecond-lineThird-lineFourth-line and subsequent | 6 (4.9)58 (47.5)40 (32.8)18 (14.8) |
| Number of lines after immunotherapy | 012≥ 3 | 78 (64.5)22 (18.2)17 (13.2)5 (4.1) |
ECOG: Eastern Cooperative Oncology Group; MSI: microsatellite instability; MSI-H: high microsatellite instability; MSS: microsatellite stable; PD-L1: programmed death-ligand 1; CPS: combined positive score; HER2: human epidermal growth factor receptor 2
ICIs were administered in different lines of therapy for mGC (Table 2).
Patient characteristics according to the line of immunotherapy, MSI, and PD-L1 CPS
| Line of immunotherapy | Median number of cycles | Patients with MSI-H, n (22.3%) | Patients with MSS and PD-L1 CPS ≥ 1, n (51.2%) | All patients, n (100%) |
|---|---|---|---|---|
| 1 | 20 | 5 | 2 | 6 |
| 2 | 12 | 17 | 24 | 58 |
| 3 | 6 | 5 | 25 | 40 |
| 4 | 3 | 0 | 5 | 11 |
| 5 | 3 | 0 | 5 | 6 |
| 6 | 2 | 0 | 1 | 1 |
MSI-H: high microsatellite instability; MSS: microsatellite stable; PD-L1: programmed death-ligand 1; CPS: combined positive score
Survival analyses
Eleven patients continued receiving anti-PD-L1 antibodies at the data cut-off (13 March 2024). Six-month PFS was 31.6%. Median OS in all patients was seven months (95% CI: 2.0–20.0), mPFS was three months (95% CI: 1.5–9.5) (Figure 1).
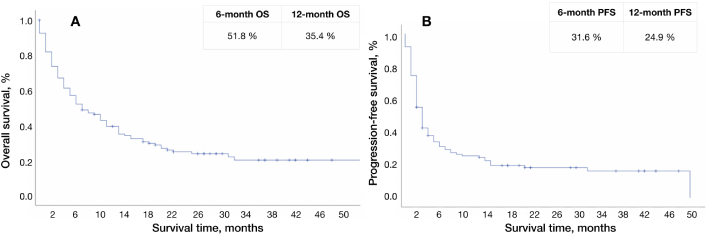
Survival curve of all patients. А: Survival curve for overall survival, B: survival curve for progression-free survival. OS: overall survival; PFS: progression-free survival
Statistically significant differences in OS have been observed according to MSI status (25 months in patients with MSI-H vs. six months in patients with MSS; 95% CI: 0.21–0.86; HR: 0.43). A trend towards better mPFS was found in MSI-H patients (10 months in patients with MSI-H vs. three months in patients with MSS; 95% CI: 0.26–1.01; HR: 0.51) (Figure 2).
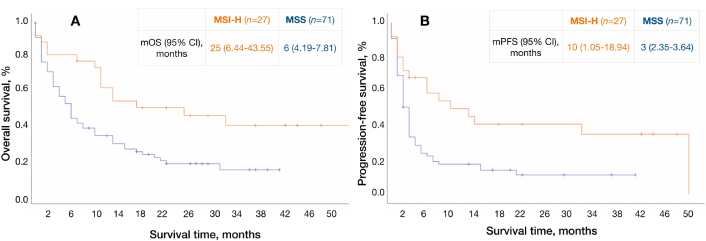
Survival curves according to MSI status. А: Survival curves for overall survival, B: survival curves for progression-free survival. MSI-H: high microsatellite instability; MSS: microsatellite stable; mOS: median overall survival; mPFS: median progression-free survival
No statistically significant differences were found according to PD-L1 CPS in patients with MSS tumors with known PD-L status (Table 3). Response assessment was available in 51 (42%) patients. DCR and ORR were 36.6% and 10.6%, respectively. The median time from the first cycle of anti-PD-L1 inhibitors to radiologically confirmed treatment response was three months (range 1–14). The median duration of response was 12 months (range 3–17). No cases of pseudoprogression were observed.
Survival of patients with MSS according to PD-L1 CPS
| Known PD-L1 CPS | N, % | PFS | OS | ||
|---|---|---|---|---|---|
| Median, months | HR, 95% CI | Median, months | HR, 95% CI | ||
| < 1≥ 1 | 4 (5.7)67 (94.3) | 13 | 0.32 (0.10–0.96) | 26 | 0.54 (0.19–1.53) |
| < 5≥ 5 | 43 (60.5)28 (39.4) | 25 | 0.62 (0.30–1.43) | 615 | 0.53 (0.24–1.14) |
| < 10≥ 10 | 47 (66.1)24 (33.8) | 25 | 0.44 (0.71–1.70) | 613 | 0.26 (0.25–1.45) |
| < 20≥ 20 | 51 (71.8)20 (28.1) | 33 | 0.88 (0.32–2.59) | 613 | 0.77 (0.86–2.42) |
MSS: microsatellite stable; PD-L1: programmed death-ligand 1; PFS: progression-free survival; OS: median overall survival; CPS: combined positive score; HR: hazard ratio
Toxicity
All irAEs are described in Table 4. The grade 3–4 irAEs were observed in 3 patients (2.5%). No fatal irAEs were observed.
Immune-related adverse events
| Immune-related adverse events | Patients (N, %) |
|---|---|
| Leads to treatment delay | 5 (4.1) |
| Leads to treatment discontinuation | 1 (0.8) |
| Fatal irAEs | 0 (0) |
| Grade 1 total bilirubin increase | 2 (1.6) |
| Grade 1 itching | 1(0.8) |
| Grade 3 nephritis | 1 (0.8) |
| Grade 2 hypothyroidism | 3 (2.5) |
| Grade 2 ALT/AST increase | 4 (3.3) |
| Grade 3 ALT/AST increase | 1 (0.8) |
| Grade 2 skin rash | 2 (1.6) |
| Grade 2 asthenia | 5 (4.1) |
| Grade 2 colitis | 1 (0.8) |
| Grade 2 diarrhea | 1 (0.8) |
| Grade 3 arthritis | 1 (0.8) |
irAEs: immune-related adverse events; ALT: alanine transaminase; AST: aspartate transaminase
Prognostic and predictive significance of NLR
NLR analysis was available in 71 patients (58%). The median NLR was 2.36 (range 0.41–10.00). The cut-off value of NLR for death prediction was 1.8 (AUC 0.81, p < 0.001) (Figure 3).
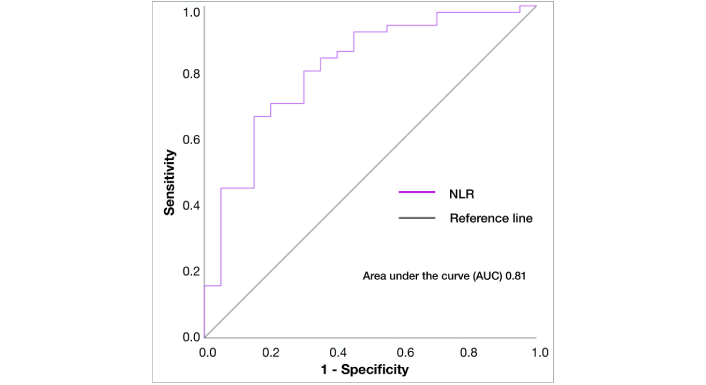
ROC-curve of NLR for predicting death in all patients. NLR: neutrophil-lymphocyte ratio; AUC: area under the curve
The mPFS and mOS in patients with high NLR (NLR ≥ 1.8) were two months and four months, respectively; mPFS and mOS in patients with low NLR (NLR < 1.8) were not reached (р < 0.001) (Figure 4). Few patient characteristics were imbalanced between NLR groups: patients with low NLR predominantly had ECOG 0–1 (75%) and 1 to 2 metastatic sites (92%), while patients with high NLR had more bulky disease (43%) and the primary tumor (72%) (Table 5).
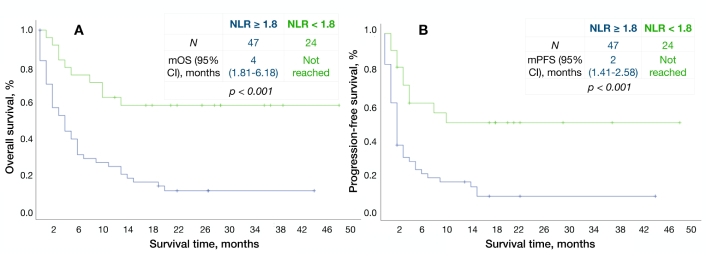
Kaplan-Meier survival curves according to NLR. A: Overall survival, B: progression-free survival. NLR: neutrophil-lymphocyte ratio; mOS: median overall survival; mPFS: median progression-free survival
Patient characteristics according to NLR
| Risk factor and category | NLR < 1.8n, (%) | NLR ≥ 1.8n, (%) | P | |
|---|---|---|---|---|
| All | 24 | 47 | ||
| Age | < 65≥ 65 | 8 (33)16 (67) | 26 (55)21 (45) | 0.079 |
| Gender | MaleFemale | 17 (71)7 (29) | 28 (60)19 (40) | 0.352 |
| ECOG | 0–1 2–3 | 18 (75)6 (25) | 23 (49)24 (51) | 0.035 |
| Histology grade | G1–G2G3NA | 7 (29)16 (67)1 (4) | 17 (36)26 (55)4 (9) | 0.464 |
| MSI status | MSI-H MSSNA | 6 (25)13 (54)5 (21) | 8 (17)29 (62)10 (21) | 0.716 |
| Signet-ring cell carcinoma | YesNoNA | 3 (13)6 (25)15 (62) | 13 (27)9 (20)25 (53) | 0.193 |
| Primary tumor | PresenceSurgical removal | 10 (42)14 (58) | 34 (72)13 (28) | 0.012 |
| Number of organs with metastases | 1–2≥ 3 | 22 (92)2 (8) | 27 (57)20 (43) | 0.003 |
| Ascites | YesNo | 9 (37)15 (63) | 28 (60)19 (40) | 0.078 |
| Peritoneal carcinomatosis | YesNo | 11 (46)13 (54) | 30 (64)17 (36) | 0.146 |
| Bone metastases | YesNo | 1 (4)23 (96) | 7 (15)40 (85) | 0.176 |
| Pain | YesNo | 8 (33)16 (67) | 24 (51)23 (49) | 0.156 |
| Line of immunotherapy | I–IIIII–IV | 13 (54)11 (46) | 19 (40)28 (60) | 0.271 |
NLR: neutrophil-lymphocyte ratio; ECOG: Eastern Cooperative Oncology Group; MSI: microsatellite instability; MSI-H: high microsatellite instability; MSS: microsatellite stable; NA: not available
Treatment response was assessable in 49 patients (70%) with known NLR with target lesions via CT scans according to iRECIST criteria. ORR was in 6 patients (35%) in the low NLR group with one CR and five PRs. No CRs were observed in patients with high NLR. DCR was 71% and 38%, respectively. Patients with low NLR had higher DCR (p = 0.027) and ORR (p = 0.002) compared to the counterpart group (Table 6).
Treatment response in the low NLR group and high NLR group
| Response | NLR < 1.8n, % | NLR ≥ 1.8n, % | P |
|---|---|---|---|
| N | 17 | 32 | |
| CR | 1 (6) | 0 (0) | 0.159 |
| PR | 5 (29) | 1 (3) | 0.008 |
| SD | 6 (35) | 11 (34) | 0.949 |
| PD | 5 (29) | 20 (62) | 0.70 |
| ORR | 6 (35) | 1 (3) | 0.002 |
| DCR | 12 (71) | 12 (38) | 0.027 |
NLR: neutrophil-lymphocyte ratio; objective response rate (ORR) = complete response (CR) + partial response (PR); disease control rate (DCR) = CR + PR + stable disease (SD); PD: progressive disease
Univariate and multivariate analyses for OS in patients with MSS
We performed a Cox regression analysis in patients with MSS and MSI-H separately due to significant differences in survival rates between them, which are described above.
Univariate analyses for OS revealed eight prognostic factors in patients with MSS: ECOG performance status (0–1 vs. 2–3), the presence of signet-ring cells in histology, the presence of primary tumor, the number of organs with metastases (1–2 vs. ≥ 3), ascites, pain, line of immunotherapy administration (I–II vs. III–IV), and NLR group (Figure 5).
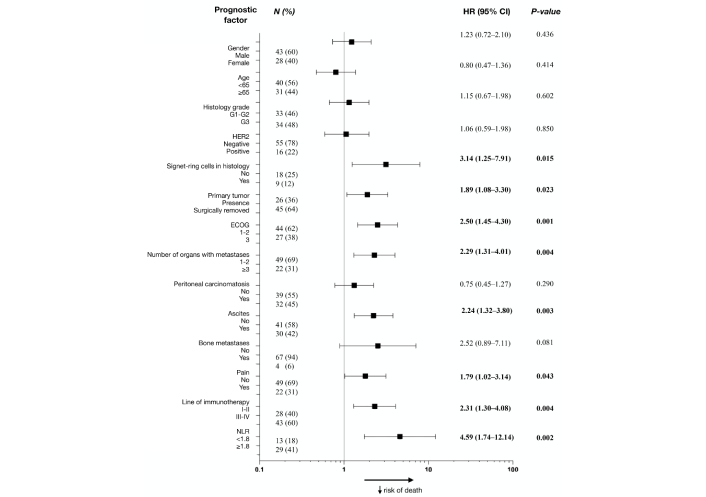
Forest plot for univariate analysis of overall survival in patients with MSS. HR: hazard ratio; HER2: human epidermal growth factor receptor 2; ECOG: Eastern Cooperative Oncology Group; NLR: neutrophil-lymphocyte ratio; MSS: microsatellite stable
Ascites (p < 0.001), administration of ICIs in III-IV lines (p = 0.004), and NLR ≥ 1.8 (p = 0.006) were independent unfavorable prognostic factors for OS (Table 7). Each factor was assigned a score from 1 to 2 depending on its statistical significance in multivariate analyses, thus, patients with ascites scored 2 points, patients with NLR ≥ 1.8, and those who received immunotherapy in III–IV lines scored 1 point. According to our score system, survival analyses of patients concluded that сombine patients were in favorable and unfavorable prognostic groups with 0 to 2 points and 3 to 4 points, respectively (Table 8). The median OS in each group was 13 and 2 months, respectively (p < 0.001), and mPFS was 8 months vs. 1 month (p < 0.001) (Figure 6).
Multivariate analyses for overall survival of patients with MSS
| Factor | P | Exp (B) | 95% CI min | 95% CI max |
|---|---|---|---|---|
| Primary tumor(yes vs. no) | 0.050 | 0.33 | 0.10 | 1.00 |
| Ascites(yes vs. no) | < 0.001 | 0.20 | 0.08 | 0.49 |
| Line of immunotherapy (I–II vs. III–IV) | 0.004 | 0.24 | 0.09 | 0.63 |
| NLR (< 1.8 vs. ≥ 1.8) | 0.006 | 0.23 | 0.08 | 0.65 |
MSS: microsatellite stable; NLR: neutrophil-lymphocyte ratio
Survival rates of patients with MSS according to the number of prognostic factors
| Prognostic group | Prognostic index, score | N, % | 12-month ОS, % | 6-month PFS, % |
|---|---|---|---|---|
| Favorable | 0–2 | 17 (43) | 50% | 51% |
| Unfavorable | 3–4 | 22 (57) | 9% | 5% |
MSS: microsatellite stable; OS: overall survival; PFS: progression-free survival
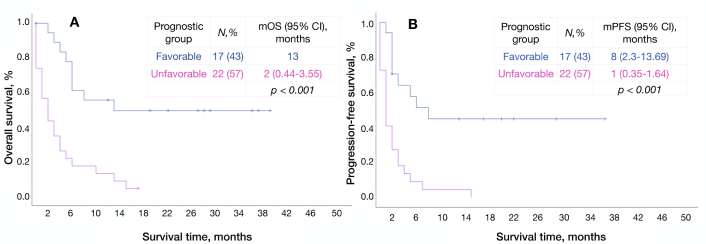
Survival curves of patients with MSS according to prognostic groups. A: Overall survival, B: progression-free survival. mOS: median overall survival; mPFS: median progression-free survival; MSS: microsatellite stable
Univariate analyses for OS in patients with MSI-H
ECOG 2–3 performance status and pain were unfavorable prognostic factors for OS in patients with MSI-H, however, no independent prognostic factors were observed (Table 9). There was a trend to better OS in patients with low NLR (HR 4.08; 95% CI 0.80–20.76; р = 0.09).
Univariate analysis of overall survival in patients with MSI-H
| Risk factors and category | N, % | HR, (95% CI) | P | |
|---|---|---|---|---|
| All | 27 | |||
| Age | < 65≥ 65 | 1512 | 0.78 (0.27–2.21) | 0.649 |
| Gender | MaleFemale | 1314 | 0.93 (0.33–2.59) | 0.900 |
| ECOG | 0–12–3 | 207 | 4.34 (1.50–13.49) | 0.007 |
| Histology grade | G1–G2G3NA | 8181 | 2.00 (0.62–6.45) | 0.243 |
| Signet-ring cells in histology | NoYesNA | 61110 | 1.65 (0.41–6.65) | 0.475 |
| Presence of primary tumor | NoYes | 1611 | 0.94 (0.33–2.66) | 0.910 |
| Number of organs with metastases | 1–2≥ 3 | 225 | 1.99 (0.70–5.63) | 0.195 |
| Ascites | NoYes | 1710 | 1.32 (0.47–3.74) | 0.592 |
| Peritoneal carcinomatosis | NoYes | 1710 | 0.84 (0.28–2.49) | 0.765 |
| Bone metastases | NoYes | 243 | 1.30 (0.29–5.79) | 0.727 |
| Pain | NoYes | 207 | 3.93 (1.34–11.49) | 0.012 |
| Line of immunotherapy | I–IIIII–IV | 1311 | 2.16 (0.67–6.99) | 0.196 |
| NLR | < 1.8≥ 1.8 | 68 | 4.08 (0.80–20.76) | 0.090 |
MSI-H: high microsatellite instability; ECOG: Eastern Cooperative Oncology Group; NLR: neutrophil-lymphocyte ratio
Discussions
We have analyzed the outcomes of 122 patients who received ICIs for mGC in Russia. Research has shown higher efficacy of anti-PD1 inhibitors in Asian patients compared to European ones [18]. However, real-world data suggested comparable survival outcomes in the Asian population: mOS and mPFS were 5.8 months (95% CI 5.29–7.00) and 1.8 months (95% CI 1.71–1.97), respectively [39]. DCR was 39.4%. Similarly, mOS and mPFS of patients who received nivolumab in the European study were 6.3 months (95% CI 3.3–9.3) and 2.1 months (95% CI 1.4–2.8), respectively [38]. In our study, most patients had PD-L1 positive mGC, so we can not support the benefit of anti-PD-L antibodies in patients with positive PD-L1 CPS. A trend toward better OS was observed in patients with PD-L1 CPS ≥ 5: mOS was 15 months compared to 6 months in patients with PD-L1 CPS СPS < 5. Our study is probably underpowered to detect statistically significant differences in survival rates according to PD-L1 CPS expression. The results of phase III randomized controlled trials strongly suggest the benefit of combining ICIs with standard first-line chemotherapy in patients with PD-L1 CPS ≥ 5–10 mGC [6, 14].
Pembrolizumab is currently the optimal treatment for mGC with PD-L1 CPS ≥ 10: the mOS of patients who received pembrolizumab monotherapy was seven months higher (17.4 months) compared to those who received chemotherapy [13], while the mOS of patients, who received a combination of ramucirumab with chemotherapy for second-line mGC, usually does not exceed 8–9 months [7, 8]. Concerning patients who failed the second line of treatment, in the ATTRACTION-2 trial, no differences in survival outcomes were observed according to PD-L1 TPS [18], due to lower predictive significance for ICIs compared to PD-L1 CPS [17, 40]. As is demonstrated in published data, we revealed statistically significant differences in OS in patients according to MSI status, and a trend towards better PFS in patients with MSI-H compared to MSS was observed: 10 months versus three months, respectively. Almost half of patients with MSI-H were still alive after two years of treatment: the survival curve of PFS reaches a plateau at around 40%, as in several studies of anti-PD-L1 inhibitors [16]. The administration of ICIs in patients with MSI-H alone, with chemotherapy, or via dual blockade in first-line treatment of mGC is still controversial.
In 2021, subgroup analyses of CheckMate-649 revealed that mOS in patients with MSI-H who received nivolumab and ipilimumab was not reached [5]. In updated results of the three-year follow-up of patients with MSI-H, mOS in patients who received nivolumab and oxaliplatin-containing chemotherapy were 38.7 months [14]. Currently, there are no randomized trials assessing the efficacy of anti-PD-L1 inhibitors in combination with chemotherapy compared to chemotherapy alone. However, the mPFS of patients with MSI-H in subgroup analyses of the Keynote-062 study was not reached compared to 11.2 months in patients who received paclitaxel alone [13]. 1-year and 2-year OS were also higher in the pembrolizumab group. In our study, five patients with MSI-H who received ICIs were treatment-naïve, and one of them was alive at the data cut-off. The mean number of immunotherapy cycles was 31. In our study, 18.2% of patients who failed immunotherapy received at least one line of treatment. That is slightly higher than in real-world data [38]. Obtained results indicate acceptable tolerability of ICIs despite issues with data collection.
Our analyses of 71 patients revealed ascites, NLR ≥ 1.8, and administration of anti-PD-L1 inhibitors in III and subsequent lines were prognostic factors for poor OS in patients with MSS mGC.
Peritoneal carcinomatosis is identified in 15%–25% of mGC cases, of which half of patients presented with ascites [41]. Ascitic tumor cells have an extremely immunosuppressive microenvironment [42, 43], resulting in lower survival rates in such patients [43]. Supporting data was obtained in analyses of 59 patients with MSI-H GC: mOS in patients with ascites was six months compared to 29 months in patients with peritoneal carcinomatosis only (p < 0.006) [43]. We did not find any differences in OS in patients with MSI-H according to the presence of ascites (p = 0.592), probably due to a small number of patients (n = 27). The prognostic role of NLR in patients with mGC was first demonstrated in 2019: in this study mOS of patients with low NLR (< 2.83) was 17.1 months compared to 9 months in patients with high NLR (p < 0.001) [44]. Currently, there is no standardized cut-off value of NLR: in existing literature, NLR cut-off levels ranged from 2.5 to 4 [45–47]. Our NLR cut-off (1.8) was detected using ROC analysis for predicting death with the largest square under the ROC-curve. mOS and mPFS in patients with low NLR were not reached compared to four months and two months in patients with high NLR (p < 0.001). However, patients with low NLR have ECOG 0–1 in 75% of cases, and 72% have 1–2 metastatic sites. Those factors were associated with favorable OS in univariate analyses of patients with MSS, which resulted in high outcomes in patients with low NLR. Similar to available data, ORR and DCR in such patients were significantly higher (p < 0.002 and p < 0.027, respectively) compared to those with high NLR [37, 48]. In one study, NLR was not correlated with survival, however, ORR was numerically higher in patients with low NLR [47]. In patients with mGC who received immunotherapy, low TLR is also associated with better survival rates and depths of response [35, 37].
Appropriate selection of pretreated patients with mGC who will benefit from ICIs is needed, considering the increasing data on clinical and biological predictive and prognostic factors. The novel prognostic index was developed in Russian patients with mGC with three prognostic groups based on three risk factors: ECOG 0–1/≥ 2, Hgb level < 10/≥ 10, and progression-free interval of < 5/≥ 5 months after the completion of the first-line chemotherapy [12]. mOS were 13.5 months, 6 months, and 2.9 months according to prognostic groups (p < 0.001). In the modified JCOG prognostic index, which included 608 Asian patients with mGC, risk factors for poor OS were ECOG ≥ 1, presence of primary tumor, high serum alkaline phosphatase level, diffuse Lauren type, and NLR ≥ 4 [36]. However, this study included patients who have failed only first-line treatment of mGC, with low tumor volume (0 to 1 metastatic site in 72% of patients), and with good performance status (ECOG 0–1 in 97% of patients) [36]. The mOS of patients in favorable, intermediate, and unfavorable risk groups were 20.5 months, 13.5 months, and 10.2 months, respectively (p < 0.001) [36].
Limitations of our study are the heterogeneous sample and its small size, inappropriate response assessment, insufficient data on lab tests, and retrospective trial design, resulting in difficulties in distinguishing prognostic and predictive factors. However, we determined an unfavorable prognostic group with mPFS of 1 month, which might help in patient selection for the treatment of mGC with ICIs.
The results of our retrospective real-world analyses of ICIs for mGC are similar to existing data. The low efficacy of immunotherapy was observed in heavily pretreated patients with ascites and NLR ≥ 1.8. More patients need to be included in further analyses with those who did not receive ICIs in the latter lines to determine the prognostic significance of the developed prognostic index.
Abbreviations
| AEs: | adverse events |
| anti-PD-L1: | anti-programmed death-ligand 1 |
| CLDN18.2: | claudin-18 isoform 2 |
| CPS: | combined positive score |
| CR: | complete response |
| DCR: | disease control rate |
| EBV: | Epstein-Barr virus |
| ECOG: | Eastern Cooperative Oncology Group |
| HER2: | human epidermal growth factor receptor 2 |
| ICIs: | immune checkpoint inhibitors |
| irAE: | immune-related adverse event |
| mGC: | metastatic gastric cancer |
| mOS: | median overall survival |
| mPFS: | median progression-free survival |
| MSI: | microsatellite instability |
| MSI-H: | high microsatellite instability |
| MSS: | microsatellite stable |
| NLR: | neutrophil-lymphocyte ratio |
| ORR: | objective response rate |
| OS: | overall survival |
| PD-L1: | programmed death-ligand 1 |
| PFS: | progression-free survival |
| PLR: | platelet-lymphocyte ratio |
| PR: | partial response |
| TCGA: | The Cancer Genome Atlas |
| TMB: | tumor mutational burden |
Declarations
Acknowledgments
Authors express their appreciation to Evdokimov V. I., Fedorinov D. S., and Tsaryova A. S. for their help in collecting data. Participating centers: N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia; Moscow Multidisciplinary Clinical Center “Kommunarka”, Moscow Healthcare Department; Branch “Oncology Center No. 1 of the City Clinical Hospital named after S. S. Yudin of the Moscow Department of Health”; A.S. Loginov Moscow Clinical Scientific Center, Moscow Healthcare Department; Moscow City Oncology Hospital No. 62, Moscow Healthcare Department.
Author contributions
AR: Investigation, Methodology, Writing—original draft. MF: Investigation, Methodology, Writing—review & editing, Formal analysis. DP, ML, LZ, DS, MV, IP, and NB: Writing—review & editing. AT: Conceptualization, Supervision, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The research proposal was submitted at the academic meeting and approved by the local ethics committee on 23.01.2021. The Local Committee of Scientific Research Ethics at N.N. Blokhin National Medical Research Center of Oncology was registered with the Federal Service for Supervision of Health and Social Development on 10.02.2006 (license number 01-3775/06).
Consent to participate
The Local Committee of Scientific Research Ethics at N.N. Blokhin National Medical Research Center of Oncology exempted informed consent to participate in this manuscript.
Consent to publication
Not applicable.
Availability of data and materials
Requests for accessing the datasets should be directed to [Alexey Tryakin, atryakin@gmail.com].
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.