Abstract
Pancreatic cystic lesions are frequently found incidentally on cross-sectional imaging and are broadly classified into mucinous and non-mucinous cysts. While some exhibit benign behavior, others have a malignant potential and are considered noninvasive precursors of pancreatic ductal adenocarcinoma. Guidelines from various societies propose risk stratification based on morphologic features and cyst fluid analysis. Fluid analysis through EUS-guided fine needle aspiration contributes to improved classification and recently, targeted DNA or RNA-based next generation sequencing is emerging as a critical investigation tool for diagnostic confirmation and risk stratification of pancreatic cysts. Each of these modalities has specific strengths and limitations, highlighting the need for a multi-modal approach for comprehensive assessment to guide clinical decision making. In this perspective, we aim to provide a thorough clinicopathologic framework for diagnosing and risk stratifying pancreatic cysts encompassing imaging findings, cyst fluid analyses, and next generation sequencing.
Keywords
Pancreatic cystic lesions, mucinous cysts, non-mucinous cysts, pancreatic cancer, molecular analysis, next generation sequencingIntroduction
Pancreatic cystic lesions (PCL) are a common finding detected incidentally on imaging, performed for other reasons, with the majority being asymptomatic [1]. They exhibit a prevalence of 1.2–2.6% on computed tomography (CT) scans and 2–38% on magnetic resonance imaging (MRI) in the general population, and this increases with age [2]. Although the majority don’t harbor advanced neoplasia, others require long-term follow-up due to the risk of malignancy, whereas a small, but significant minority would require surgical intervention [3].
The ability to discern malignant from benign cysts is limited on cross-sectional imaging. Imaging modalities and endoscopic ultrasound (EUS) have limitations in both accurately distinguishing the different subtypes of PCL, as well as discerning the risk of advanced neoplasia.
Cyst fluid analysis has emerged as an investigation tool that can guide clinical decision making [4]. Several genetic aberrations in oncogenic hotspots have also been developed to aid in the classification of PCL including but not limited to: KRAS, GNAS, TP53, RNF43, SMAD4, CDKN2A, VHL, PIK3CA, and RNA-based biomarkers such as CEACAM5 and KRT7/20 [5, 6]. Taken together, these studies further help discern the malignant potential of PCL and guide clinical decision making.
PCL are broadly classified into mucinous and non-mucinous cysts, with the former having a higher risk of progression to advanced neoplasia [7]. Mucinous cysts include mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasms (IPMNs), while non-mucinous cystic lesions include pseudocysts, serous cystadenomas (SCAs), as well cystic degeneration of solid tumors such as solid pseudopapillary neoplasms (SPNs) and cystic neuroendocrine tumors.
The classification above is based on pathologic diagnosis of resected pancreatic specimens. However, accurately discerning the different subtypes of PCL as well as the degree of dysplasia and malignant potential can be a diagnostic challenge. This perspective highlights the investigative modalities used in the diagnosis and differentiation of the various types of PCL lesions, with an emphasis on the molecular markers as an avenue for early detection of advanced neoplasia.
Guidelines used in the management and risk stratification of patients diagnosed with mucinous cysts
In the last two decades, several societies have proposed guidelines to assist clinicians in the diagnosis, surveillance, and treatment of pancreatic cysts (Table 1). Approximately 80% of newly diagnosed incidental pancreatic cysts are branch duct intraductal papillary mucinous neoplasm (BD-IPMN) and the purpose of these guidelines is to risk stratify these lesions among a large cohort of patients. In 2006, the International Association of Pancreatology (IAP) put forth the Sendai guidelines for the management of BD-IPMNs and MCNs. These were revised and largely subsumed by the Fukuoka guidelines in 2012 and 2017 and most recently by the Kyoto guidelines [8, 9]. BD-IPMNs are defined as a cyst measuring > 5 mm that communicates with the main pancreatic duct (MPD) whereas main duct intraductal papillary mucinous neoplasm (MD-IPMN) is defined as diffuse or segmental main duct dilation > 5 mm without evidence of ductal obstruction. MPD dilation of 5–9 mm, enhancing mural nodule < 5 mm, cyst size ≥ 3 cm, presence of a thickened or enhanced cyst wall, abrupt change in pancreatic duct caliber with distal pancreatic atrophy, acute pancreatitis, elevated carbohydrate antigen 19-9 (CA19-9), lymphadenopathy and cyst growth rate of ≥ 2.5 mm/year were all considered “worrisome features” [10]. While an MPD diameter of ≥ 10 mm, presence of an enhancing solid component ≥ 5 mm, and obstructive jaundice, were considered “high-risk stigmata”.
Guidelines for the management and risk stratification of pancreatic cystic lesions
| Association/Society | Scope | Risk stratification/Indications for resection |
|---|---|---|
| IAP, 2024 (Kyoto) [9] | IPMNs | High risk stigmataMPD dilation ≥ 10 mmEnhancing mural nodule ≥ 5 mm or solid componentObstructive jaundiceCytology positive or suspiciousWorrisome featuresCyst ≥ 3 cmMPD dilation > 5 mm and < 10 mmEnhancing mural nodule < 5 mmNew onset or worsening diabetesThickened/Enhancing cyst wallsLymphadenopathyAbrupt changed in duct caliber with distal atrophyGrowth rate ≥ 5 mm/yearAcute pancreatitisElevated CA19-9 |
| ACR, 2017 [11] | Incidentally detected cysts | High risk stigmataEnhancing solid componentMPD > 10 mm absence of obstructionHGD cytologyWorrisome featuresCyst > 3 cmEnhancing cyst wallNon-enhancing mural noduleMPD > 7 mm |
| ACG, 2018 [12] | Newly diagnosed lesions w/o family history or genetic predispositions | Concerning featuresCyst growth > 3 mm/yearMural noduleMPD dilation > 5 mmIPMN/MCN > 3 cm |
| AGA, 2015 [7] | Asymptomatic non-neoplastic PCL | Concerning featuresCyst > 3 cmDilated MPDSolid componentHGD cytology |
| European, 2018 [13] | All PCLMCN resection if > 4 cm (all other regardless of size) | Absolute indicationHGD cytologySolid massEnhancing mural nodule > 5 mmMPD dilation > 10 mmRelative indicationsGrowth rate > 5 mm/yearCA19-9 > 37 U/mLEnhancing mural nodule < 5 mmCyst diameter > 4 cmMPD dilation 5–9.9 mm |
IAP: International Association of Pancreatology; IPMNs: intraductal papillary mucinous neoplasms; MPD: main pancreatic duct; ACR: American College of Radiology; HGD: high-grade dysplasia; MCN: mucinous cystic neoplasm; ACG: American College of Gastroenterology; AGA: American Gastrointestinal Association; PCL: pancreatic cystic lesions
Surgical resection is generally recommended in all patients with MCN, MD-IPMN, mixed duct-IPMN, and BD-IPMN with high-risk stigmata or several worrisome features in surgically fit patients [6]. Patients who are found to have suspicious or positive results on fluid cytology or molecular testing indicative of advanced neoplasia should also be considered for surgical intervention. These guidelines note that while individual BD-IPMN has a lower potential for malignancy than MD-IPMN, the cumulative incidence of malignant transformation increases on a year-by-year basis. Furthermore, a “field defect phenomenon” and dual carcinogenesis in the remnant pancreas highlight the need for long-term surveillance even in the absence of the aforementioned worrisome features [9].
The 2015 guidelines of American Gastrointestinal Association (AGA) are largely restricted to asymptomatic lesions and specifically exclude solid neoplastic lesions including MD-IPMN without side branch involvement. Risk stratifications dictate surveillance rather than surgical resection and suggest that patients with pancreatic cysts containing at least two high-risk features such as size equal to or greater than 3 cm, a dilated MPD, or the presence of an associated solid component, be examined with EUS-fine needle aspiration (FNA) [14].
American College of Gastroenterology (ACG) 2018 guidelines focused on newly diagnosed lesions in patients without a strong family history or genetic variants known to predispose them to pancreatic cancer. They include MCN, MD-IPMN, BD-IPMN, and SCA. Worrisome features that would mandate EUS-FNA include cyst size of ≥ 3 cm, main duct diameter > 5 mm, or a change in main duct caliber with upstream atrophy. As with IAP guidelines, the presence of mural nodules or solid components, as well as associated jaundice or secondary pancreatitis are also indications for FNA, and cytology positive or suspicious for advanced neoplasia would warrant surgery [12].
Finally, in 2017, the Pancreas Subcommittee of the American College of Radiology (ACR) developed its own set of guidelines for managing PCL that were detected incidentally on CT or MRI. These recommendations implemented the same risk stratification system of “high-risk stigmata” and “worrisome features” as the 2017 IAP guidelines but added a main duct caliber of 7 mm or greater as a high-risk stigma [15]. Outside of the U.S., guidelines developed by the European Study on Cystic Tumors of Pancreas in 2018 were the most comprehensive with four distinct categories of cysts; IPMN and MCN fell into the category of epithelial-neoplastic. Like the risk stratification system used in ACR and IAP guidelines, indications for surgery were sorted into absolute criteria and relative criteria. Absolute indications included enhancing mural nodules greater than 5 mm, main duct size ≥ 10 mm, positive cytology for advanced neoplasia and solid components, while relative indications included main duct diameter between 5–9.9 mm, cystic growth rate of > 5 mm/year, increased CA19-9 in the absence of jaundice, enhancing mural nodules < 5 mm, and cyst diameter > 4 cm [11]. Notably, while all other guidelines recommend resection of all MCN, European guidelines suggest resection only for those with diameter > 4 cm [10, 13].
Imaging evaluation
MRI provides a detailed morphologic assessment of PCL including the presence of mural nodularity, internal septation, ductal features, communication with the pancreatic duct, and the presence of enhancement. MRI has high soft tissue resolution which on T2-weighted image sequences, permits better characterization of PCL than CT [16]. However, MRI also has its disadvantages as it is suboptimal in identifying calcifications, occasionally produces motion artifacts due to its high sensitivity to movement, and its higher cost [17, 18]. CT scans are commonly used to evaluate PCL based on various morphological features including the presence of calcifications, thick septations, and wall thickening [19–21]. It also provides better evaluation for pancreatic changes and adjacent lymphadenopathy. However, CT is suboptimal in evaluating small cystic lesions, and more importantly, it is limited in the characterization of PCL that have overlapping morphological features.
EUS is an important diagnostic modality which in experienced hands, provides images with high spatial resolution, helping to accurately characterize cystic neoplasms. EUS offers the opportunity for FNA-guided fluid aspiration, allowing for cytopathologic examination, cyst fluid analysis, and molecular analysis [17, 18]. Nevertheless, it is an invasive procedure associated with rare complications including hemorrhage, and pancreatitis [22, 23].
Solid pseudopapillary neoplasms
SPNs commonly occur in young females in their 2nd and 3rd decades of life [24]. SPNs are usually large in size at the time of diagnosis with a mean long-axis tumor diameter of 6.8 cm. Despite its larger size, there is usually no biliary or pancreatic duct obstruction [25].
SPNs are characterized as a well-encapsulated thick-walled mass with varying solid, cystic, necrotic, and hemorrhagic components giving it a heterogeneous appearance. Hemorrhagic components of the lesion demonstrate higher than water attenuation on unenhanced CT and appear hyperintense on T1-weighted imaging. Solid components of the lesion show little enhancement on post-contrast imaging [26–28]. Peripheral/rim or septal calcifications may also be seen on CT scans, especially in larger lesions [29]. On MRI, the cystic components are T2 hyperintense while the characteristic thick capsule is T2 hypointense and demonstrates progressive delayed enhancement on post-contrast images (Figure 1) [30]. These features can help differentiate SPN from other PCL, such as SCA and MCN.
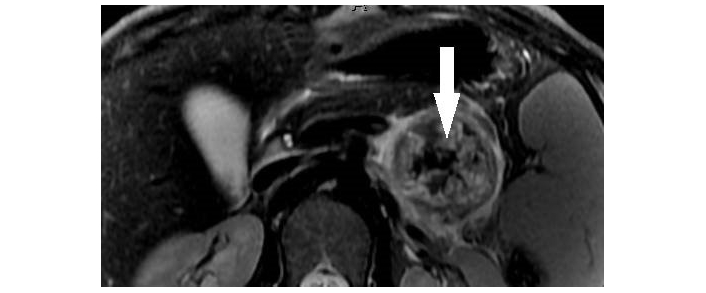
Solid pseudopapillary neoplasm of pancreas. Axial T2-weighted MR image demonstrates a partially cystic mass in the tail of pancreas with internal T2 hypointense hemorrhagic/solid components (arrowhead) and a hypointense capsule
On EUS, SPN may appear solid or may have mixed solid-cystic appearance. Though EUS may not add much to cross-sectional imaging in terms of morphological characterization of SPN, it is vital in obtaining histological diagnosis via EUS-guided FNA [31]. The most common findings seen on histopathology are an increase in cellularity, with neoplastic cells developing loose papillary clusters with fibrovascular central cores. Foamy macrophages, multinucleated giant cells, and hemorrhagic remains are also seen in the background [32, 33].
The recommended treatment for all SPN is surgical resection [34, 35]. Although they are low-grade neoplasms and generally follow an indolent course, they have a low but significant risk of metastatic spread in 5–15% mainly involving the liver and/or peritoneum [36, 37]. Therefore, upfront surgical treatment is generally recommended, even for asymptomatic lesions.
Serous cystadenomas
SCAs are commonly seen in females in their 5th to 6th decade of life and characteristically have microcystic morphology with a central area of calcification [38]. The classic “honeycomb” pattern consists of numerous sub-centimeter micro-cysts that cannot be differentiated on imaging. The oligocystic pattern exhibits cysts of > 2 cm and occasionally overlaps with MCN and IPMN in morphological features [39]. Hence, cyst fluid analysis further aids in differentiating between SCA and IPMN/MCN [40].
On MRI, SCAs appear as a cluster of small cysts grouped together within the pancreas (Figure 2A). Signal characteristics are as follows: Cystic foci demonstrate hyperintensity on T2 (Figure 2B), hypointensity on T1 (Figure 2C) and the central fibrous scar is hypointense on T2, T1 C+ (Gd): on delayed contrast enhanced imaging, the fibrous septa between the cysts appear enhanced [41].
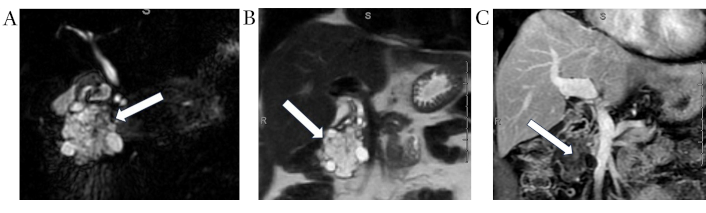
Serous cystadenoma. Coronal MRCP (A) and coronal T2 weighted (B) images demonstrate a large lobulated multilocular cystic lesion (white arrows) with honey-combed appearance in the pancreatic head. Corresponding post-contrast coronal T1 weighted image (C) demonstrates numerous internal thin enhancing septations (white arrow). MRCP: magnetic resonance cholangiopancreatography
On EUS, the SCA commonly appears as a cystic lesion with multiple anechoic cystic spaces with septations and an occasional central hyperechoic calcification [42]. The key findings on EUS-FNA of SCA include cytologically bland, non-mucinous epithelium that tests positive for melan-A, inhibin, CDK19, and negative for chromogranin and synaptophysin [42].
The management of SCAs is primarily driven by the presence of symptoms as they have no malignant potential. If the diagnosis is definitive and the lesion is asymptomatic, observation with consideration for serial imaging can be pursued. Surgery is warranted if malignancy cannot be excluded or if the lesion is symptomatic, regardless of size. Currently, offering surgical resection to all SCAs > 4 cm regardless of the presence of symptoms is anecdotal [43, 44].
Mucinous cystic neoplasms
MCNs are predominantly diagnosed in women in their 4th–6th decade of life and are often located in the body or tail of the pancreas. All MCNs are associated with a significant risk of advanced neoplasia with a rate of high-grade dysplasia (HGD) (6–13%) and invasive carcinoma (IC) (4–12%) [45–49].
On CT, MCNs of the pancreas may appear as unilocular macrocysts that do not communicate with the pancreatic duct (Figure 3A). They are occasionally seen with peripheral eggshell calcification and mostly have heterogeneous cyst contents. The presence of thick, enhancing septations or mural nodules increases the likelihood of malignant transformation [50, 51].
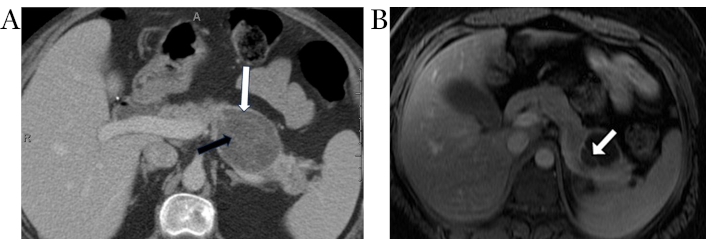
Pancreatic mucinous cystic neoplasm. Axial contrast-enhanced CT image (A) demonstrates an ovoid hypodense cystic lesion in the pancreatic body/tail (white arrow) with thin internal enhancing septations (black arrow). Note the lack of upstream pancreatic ductal dilation. Axial post contrast T1-weighted MR image (B) in a different patient shows a cystic lesion in the pancreatic tail with a thin enhancing internal septation (white arrow)
Typical MRI features of MCN include a well-defined, unilocular, or oligolocular cyst. On T1-weighted images, MCNs may exhibit variable signal intensity, depending on the proteinaceous content of the cyst fluid (Figure 3B). On T2-weighted images, MCNs are hyperintense. Unlike IPMNs, the lesions do not show communication with pancreatic duct. MRI is superior to CT in identifying internal thick septations, mural nodularity, and any enhancing components. After gadolinium injection, enhancement of thick cyst wall is seen best on delayed phases of imaging owing to its fibrous nature [52].
Intraductal papillary mucinous neoplasms
IPMNs occur in both sexes, with a slight predominance in older males in their 5th to 6th decade of life. They are considered precursor lesion that can potentially progress to pancreatic ductal adenocarcinoma (PDAC). A distinctive feature that helps differentiate IPMN from mucinous cystadenoma/cystadenocarcinoma is that these tumors communicate with the MPD or its branches, and thus are classified into three types based on ductal involvement: MD-IPMN, BD-IPMN, and mixed-type IPMN [2, 53].
CT shows IPMNs as single or multiple cystic hypodense lesions. MD-IPMNs directly involve the MPD associated mucin hypersecretion resulting in ductal dilation over 5 mm often with progressive atrophy of overlying pancreatic parenchyma (Figure 4A and 4B). BD-IPMNs may be unilocular, multicystic, or may have a tubular morphology (Figure 5A). Mixed-type IPMNs have features of both the BD-IPMNs and MD-IPMNs (Figure 5B) [18]. On MRI, IPMNs are hyperintense on T2-weighted imaging and demonstrate low signal on T1-weighted imaging due to its mucinous content. Communication with pancreatic duct is commonly noticeable. Enhancing mural nodules and pancreatic ductal dilation are better identified on MRI when compared to CT imaging [54].
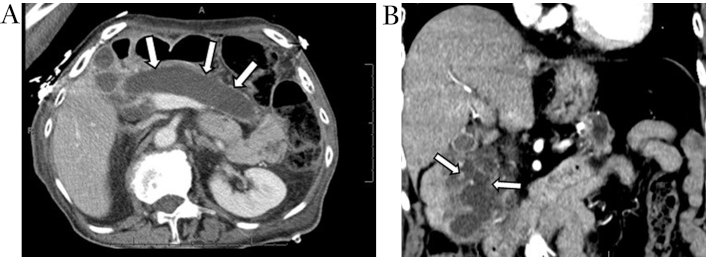
Main duct IPMN. Axial contrast-enhanced CT image (A) demonstrates diffuse marked dilation of the main pancreatic duct (white arrows). Coronal contrast-enhanced CT image (B) obtained 1-year later shows scattered enhancing mural nodules, a worry-some feature (white arrows). IPMN: intraductal papillary mucinous neoplasm
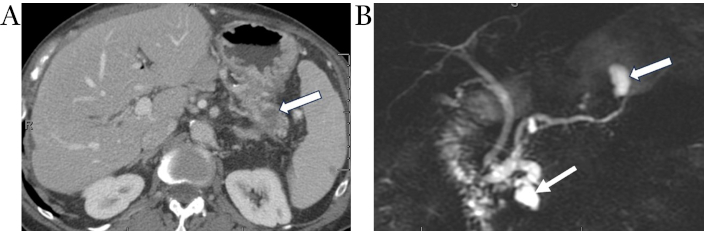
Metachronous pancreatic adenocarcinoma consistent with field-defect. Coronal MRCP image (A) demonstrates mixed IPMN with segmental dilation of main duct in the pancreatic head (arrowhead) and cystically dilated branch ducts in pancreatic head and tail. Axial contrast-enhanced CT image (B) obtained 3 years after Whipple procedure for adenocarcinoma of pancreatic head shows new focus of hypo-enhancing mass in the tail (white arrow) away from the previously documented main duct and branch duct IPMNs (arrowhead), consistent with the “field defect” phenomenon in the remnant pancreas. IPMN: intraductal papillary mucinous neoplasm
MD-IPMNs and mixed-typed IPMN demonstrate malignant transformation in approximately 45% of resected samples [6, 8]. In comparison with BD-IPMN, which exhibits a rate of advanced neoplasia in around 10–20% of resected specimens [6, 8]. Guidelines aimed at identifying worrisome and high-risk features for BD-IPMN have been set fourth, including the Sendai criteria followed by the Fukuoka guidelines which have been revised in 2017 and most recently the Kyoto guidelines [9, 55]. A recent study hypothesized that dilation of the uncinate duct is a radiographic indicator of high-risk disease, specifically IPMN associated with HGD or IC [56, 57]. IC may develop at a site distant from the primary location of the IPMN at a later stage, supporting the concept of field defect and dual carcinogenesis. This emphasizes the importance of radiological evaluation of the entire pancreas and further surveillance of the residual pancreas following resection of the cystic lesion [58].
Cystic fluid analysis of pancreatic cyst lesions
Although CT and MRI are able to detect PCL at high frequencies, these modalities have limitations when it comes to distinguishing the different types based on morphologic features alone [5]. While EUS has been shown to have a superior image resolution of the pancreas, studies have shown that morphologic features alone remain suboptimal in delineating the different subtypes or accurately predicting the risk of advanced neoplasia.
The effectiveness of EUS is highlighted when combined with FNA of pancreatic cyst fluid. EUS-guided-FNA for cyst fluid analysis including fluid cytology and different biochemical markers help bridge the void of inaccurate diagnosis as well as providing a reliable tool to accurately predict the risk of advanced neoplasia among the different PCL types [59–61].
Fluid cytology is a valuable diagnostic tool that helps differentiate benign versus malignant cystic lesions albeit the diagnostic sensitivity of fluid cytology obtained by EUS-FNA is low (approximately 28.7%). This is due inadequate sample retrieval, several overlapping pathologic features and false negative results. It does, however, have a relatively high specificity for confirming malignancy when other radiologic or clinical findings are present. According to the World Health Organization (WHO) definition, the cytologic grade demonstrates that the absolute risk of HGD/IC of “suspicious” and “positive” results are 91–100% and 100%, respectively [62–65].
Indications for pursuing EUS-guided FNA are typically driven by the presence of the aforementioned worrisome features to confirm the diagnosis, especially if surgery is being contemplated. Further, ambiguity on imaging would also mandate cytologic and fluid evaluation for diagnostic confirmation [8, 12, 66]. The approach to aspirate and analyze the cyst fluid is variable and is based on several factors including cyst location, size, and accessibility. EUS-guided aspiration is typically the preferred approach due to its high accuracy, yield and low risks. In the cases where EUS-FNA is not feasible, alternative approaches such as percutaneous aspiration or even surgical aspiration may be required. Percutaneous drainage is pursued in the setting of prior surgical bypass or altered anatomy [67]. On the other hand, surgical drainage is mostly reserved for drainage procedures in the setting of chronic pancreatitis and walled off pancreatic necromass [68].
Even though EUS-FNA is generally considered a safe procedure, however for cystic lesions there is some degree of risk involved with a complication rate 13.6% [69]. Factors that may increase the risk of adverse events include cyst location, size, wall thickness, operator experience, vascular involvement, needle size and protease inhibitor use [70]. Some of the more common complications acute pancreatitis (0.44%), bleeding (0.13%), and infection (0.05%) [69].
Biochemical markers
Carcinoembryonic antigen
Carcinoembryonic antigen (CEA) plays a valuable role in the diagnosis of PCL, particularly in distinguishing between mucinous and non-mucinous cysts [41, 71]. The validated cut-off used in clinical practice to lean the diagnosis towards a mucinous cyst is 192 ng/mL, and it’s associated with a specificity and sensitivity of 84% and 75%, respectively. Comparably, CEA levels below 5 ng/mL demonstrate a high sensitivity of 95% and a specificity of 50% for diagnosis of non-mucinous cysts, such as pseudocysts and SCA [72].
Despite being considered one of the most accurate biomarkers for differentiating mucinous from non-mucinous cysts, CEA is not effective in reliably distinguishing benign, premalignant, or malignant mucinous cysts, which is supported by several studies that found no correlation between the CEA level and the presence of advanced neoplasia [41, 73–75].
Amylase
Amylase is another biochemical marker that has a role in the diagnosis of PCL, particularly pseudocysts and IPMNs. Several studies have proven that higher amylase levels have been consistently found in pseudocysts, and a comprehensive meta-analysis revealed that cyst fluid amylase level below 250 IU/L had a remarkably high specificity of 98% for ruling out pseudocysts [72, 76–78].
Apart from its role in ruling out pseudocysts, high amylase levels cannot reliably distinguish between IPMN and MCN. One would theoretically expect that IPMN would have higher cyst fluid amylase levels when compared to MCN, since IPMN communicate with the pancreatic duct [79–81]. While amylase levels can provide useful information, the level should be interpreted in conjunction with other cyst fluid markers, clinical and radiological features [82, 83].
Glucose
Glucose levels in pancreatic cyst fluid has also been identified as a valuable diagnostic marker, especially in differentiating mucinous from non-mucinous cysts. Several studies have consistently shown that cyst fluid glucose levels are significantly lower in mucinous cysts [84]. A study by Carr et al. [71], reported that with a glucose of ≤ 50 mg/dL, the sensitivity was 92%, specificity was 87%, and overall diagnostic accuracy was 90% in differentiating mucinous from non-mucinous pancreatic cysts [84]. Another study [85] suggested an even lower cutoff ≤ 40 mg/dL, which had 84.78% sensitivity and 73.5% specificity for detecting MCN.
Compared to the standard CEA cutoff of > 192 ng/mL, a glucose cutoff of ≤ 50 mg/dL has been shown to have better sensitivity, though slightly lower specificity, in diagnosing mucinous pancreatic cysts [84, 86]. Hence, the typical cutoff value for cyst fluid glucose used to differentiate mucinous from non-mucinous pancreatic cysts is around 50 mg/dL (2.8 mmol/L), as this threshold demonstrates high diagnostic criteria [86].
Molecular markers
Despite advancements in imaging, endoscopic, cytopathologic and biomarker analysis, identifying precisely which PCL harbors malignancy or is at risk of progression remains a challenge. Given that most precancerous lesions have a low risk of malignant transformation, molecular and genetic profiling including next generation sequencing (NGS) of cell-free DNA or mi-RNA further aids in proposing treatment and surveillance that can guide clinical decision making [5]. For instance, mutations in the mitogen-activated protein kinase genes such as KRAS and GNAS are specific for mucinous cysts, whereas mutations in TP53, SMAD4 are linked with more advanced lesions [87]. Alterations in KRAS, GNAS, and/or BRAF are present in 89% mucinous cysts. Among mucinous cysts with advanced neoplasia, mutations in TP53, SMAD4, and the mammalian target of rapamycin (mTOR) genes were identified in 86% of cases [87].
KRAS is a proto-oncogene that plays a crucial role in various cellular pathways. Several studies have identified links between pancreatic neoplasms and KRAS mutation. In a study by Nikiforova et al. [88], 618 pancreatic cyst specimens were obtained and evaluated for molecular mutations, of which 38% KRAS mutations. However, KRAS mutations had an overall 100% specificity and a sensitivity of 89% for mucinous differentiation [88]. Thus, the utility of probing for KRAS mutations have been shown to be a valuable marker to be able to differentiate between mucinous cysts such as IPMN and MCN, and non-mucinous cysts [89].
GNAS is a gene that encodes for a subunit of the G-protein that produced cAMP. While KRAS and GNAS are classically evaluated in tandem, they do play different roles in tumor development. When mutated it can lead to abnormal and overactive cells growth [90]. In a study by Wu et al. [91], 66% of IPMN cyst fluid showed a mutation in GNAS, while 96% of cysts exhibited either a KRAS or a GNAS mutation. In another investigation, researchers analyzed EUS-FNA samples of in both IPMN and non-IPMN cysts. GNAS mutation was detected in 47.2% of IPMN samples [92]. In another study by Paniccia et al. [87], GNAS mutations were present in 30% of cystic fluid samples, and occurred with either KRAS (78%), or BRAF (10%). As such, GNAS mutation are highly specific to IPMN and coupling it with a KRAS mutation or elevated CEA improves diagnostic accuracy as they are not found in MCNs.
RNF43 is a tumor suppressor gene, which encodes a protein that is involved in several biological processes such as cell division and differentiation. RNF43 mutations are associated with different types of malignancies such as PDAC [93]. However, RNF43 has been shown to be a potential marker to predict malignant transformation. In a study by Sakihama et al. [94], 106 MCN with varying grades of dysplasia, high grade dysplasia and IC had a higher frequency of RNF43 mutations in comparison to lower grade lesions [94]. Another study by Zhou et al. [95], used mice that had a RNF43 knockout and an activated KRAS, like in IPMN, these mice showed increased cystic papillary lesions as well as PDAC [95]. When these mice were treated with a growth inhibitor that selectively inhibited mutant RNF43 cell, increased survival as well as decreased malignant transformation were observed in these mice. As such loss of RNF43 is an important prognostic tool to monitor PDAC development in precancerous pancreatic cysts [95].
SPN
Sequencing of the whole-exome has revealed a relative scarcity of genetic alterations in SPNs. The only recurrent mutations identified were those located in the oncogene CTNNB1 [96]. Mutations in TP53 and PIK3CA have been described in SPNs, but these are rare findings. Additionally, SPNs do not harbor mutations in KRAS, GNAS, RNF43, PTEN, CDK2A, SMAD4, and VHL [96, 97].
It is important to note that E-cadherin and β-catenin are the most useful immunohistochemical markers for differentiating SPNs from pancreatic neuroendocrine tumors. While SPNs show loss of E-cadherin and nuclear accumulation of β-catenin, neuroendocrine tumors generally express E-cadherin and lack nuclear β-catenin [96–98].
SCA
The most common molecular alteration found in SCA is the loss of heterozygosity of the chromosome 3p region, where the VHL gene is located [96, 97]. Epithelial markers typically expressed in SCAs include cytokeratin’s, EMA, and MUC1 [99, 100]. To note, SCAs do not express the aberrations that are usually found in mucinous cysts, such as GNAS, KRAS, RNF43, TP53, PIK3CA, CDKN2A, SMAD4, and PTEN [96, 97, 101, 102].
MCN
The most common genetic alteration found in MCN is KRAS mutations, where the prevalence increases with the degree of dysplasia [103]. Similar to IPMNs, 8–35% of MCNs have somatic mutations in RNF43, which might suggest that this gene plays a role in the formation of mucin-producing cysts in the pancreas [96, 97]. Additionally, mutations and/or deletions in PIK3CA, PTEN, CDK2A, TP53, and SMAD4 also appear in MCNs and are associated with advanced neoplasia. GNAS mutations, however, are distinctly absent in MCNs.
IPMN
The genetic alteration most frequently found in IPMN is the oncogenic KRAS mutation, with a prevalence of > 80% [91, 94]. Although KRAS mutations are found in all subtypes of IPMNs, they tend to be more closely associated with branch duct locations. Additionally, 65% of IPMNs have somatic mutations in the oncogene GNAS [91, 104]. Together, KRAS and/or GNAS activating mutations are harbored in > 96% of IPMNs and are deemed early events during tumorigenesis [91]. Genetic alterations in the tumor suppressor gene RNF43 are also commonly observed in IPMNs, with inactivating mutations occurring in 14–38% of cases [96, 97]. In addition, other genes are frequently mutated in IPMNs, including TP53, PIK3CA, PTEN, CDKN2A, and SMAD4. TP53 mutations tend to arise later in the progression of IPMNs and are more commonly seen in advanced neoplastic lesions [105]. Similarly, it has been reported that PIK3CA mutations and deletions of the PTEN gene are strongly associated with high-grade IPMNs and PDAC [106]. While losses in the CDKN2A gene are less common overall, they are more prevalent in IPMNs exhibiting HGD compared to those with low-grade dysplasia. Lastly, SMAD4 is rarely inactivated in low-grade IPMNs, but mutations in this gene, accompanied by a corresponding loss of heterozygosity, are typically observed in advanced neoplastic stages of the disease [107, 108].
Proteomic analysis and pancreatic cyst fluid
Proteomic analysis is starting to play an equally crucial role in profiling lesions. Through proteomic examination, Chen et al. [109] found nine differently expressed proteins in mucinous compared with non-mucinous cystic fluids. Four of these identify mucinous lesions with a sensitivity of 81%, specificity of 90%, and accuracy of 86% [109]. Other studies have looked at the various mucin proteins found in PCL such as thymosin-beta-4, ubiquitin, and VEGF-A. High levels of VEGF-A (over 5,000 pg/mL) as being significantly associated with benign serous cystic neoplasms [110].
A variety of other molecular markers in pancreatic cyst fluid have also been examined and found to hold prognostic value. Hao et al. [111] found that higher concentrations of cytokines, including interleukins (IL)-1b, 5, and 8, are associated with cysts containing HGD or malignancy [111]. IL-1b alone was found to be an independent predictor of high-risk vs low risk cysts, with a positive predictive value of 71%. Prostaglandin E2 (PGE2) was also found to be elevated in pancreatic cancer tissue and hypothesized to differentiate IPMNs and MCNs with the former showing higher levels [112]. Finally, telomere fusion status has been assessed as a prognostic marker of malignancy, with evidence of shortened chromosomal telomeres in dysplastic IPMNs [111].
Molecular analysis of the malignant potential of pancreatic cysts is not limited to pancreatic cyst fluid. Several authors have suggested using pancreatic fluid or plasma as a source of molecular biomarkers. The use of pancreatic juice collected from the duodenum avoids the risk of direct sampling of the pancreas and could be more representative of lesions throughout the pancreas, as opposed to a single cyst [5]. Similarly, plasma analysis presents an avenue for less invasive bio-specimen collection and could be used for the surveillance of at-risk patients. Levink et al. [113], compared mutation detection rate in plasma versus pancreatic fluid. They hypothesized that due to the proximity that pancreatic fluid analysis would show higher mutation detection rates. Despite finding higher concentrations of cell free DNA, there was no difference in mutation dection rate, supporting the theory that plasma analysis could be used for genomic analysis [113]. In another study by Visser et al. [114], they showed that the presence of DNA mutation in pancreatic juice—particularly of key oncogenic drivers such as TP53, SMAD4, or CDKN2A, as well as hypermethylation of NPTX2—had a near 100% specificity for the presence of HGD [114]. Similarly, Yang et al. [115] analyzed 25 different protein biomarkers in plasma extracellular vesicles and found they had high discriminatory power in detecting atypical SCA that usually missed on typical imaging findings.
With multiple systematic reviews and meta-analyses confirming the prognostic power of molecular analyses of cyst fluid (as well as pancreatic juice and plasma), future challenges will largely center around integrating these new biomarkers into existing diagnostic and surveillance frameworks. Different sequencing strategies, gene coverage, and thresholds for mutation calling can impact the consistency of results among institutions [116]. Techniques for identifying circulating tumor cells vary and target different characteristics of tumor cells [5]. It will be essential to integrate these analyses with currently available imaging modalities.
Conclusions
In conclusion, while the overall potential for malignant transformation of PCL remains low, there is a clear subset of lesions that are at much higher risk for advanced neoplasia. Despite recent advances in CT and MRI, high-resolution cross-sectional imaging is not sufficient in identifying mucinous vs non-mucinous precancerous lesions with the additional challenge stemming from overlapping morphologic features. Although EUS gives better resolution, morphologic features alone are still poor predictors of pancreatic cyst type and the presence of advanced neoplasia, with an accuracy that ranges from 40%–90% [7]. Utility of EUS is enhanced when coupled with FNA of the cyst fluid and brushing of the epithelial lining for CEA, cytopathological studies, and other such diagnostic studies [15].
Due to the low prevalence of pancreatic cancer in the general population, early detection efforts must be targeted to patients with precancerous PCL, those with a family history of pancreatic cancer or risk factors for malignancy. A comprehensive clinicopathologic effort starting with clinical presentation, radiology, EUS, cytopathological studies along with NGS would be required to effectively identify patients with precancerous lesions who would benefit from surgical intervention. Even with the mentioned approaches, one must consider the prevalence of multifocal neoplasia and the genetic heterogeneity within precancerous lesions [15].
Molecular and genetic approaches which involve the integration of genomic markers, is starting to show promise in achieving a more specific and sensitive classification of precancerous cysts. When combined with standard clinical practices and parameters, the clinical utility of key oncogenic driver genes such as KRAS, PTEN, GNAS, and VHL, have been shown to enhance sensitivity and specificity in characterization and detecting early precancerous lesions. There is a need to study and understand the different types of precancerous PCL, while taking into consideration the heterogeneity and the presence of the different types at the same time in the pancreas.
Abbreviations
| ACG: | American College of Gastroenterology |
| ACR: | American College of Radiology |
| AGA: | American Gastrointestinal Association |
| BD-IPMN: | branch duct intraductal papillary neoplasm |
| CEA: | carcinoembryonic antigen |
| CT: | computed tomography |
| EUS: | endoscopic ultrasound |
| FNA: | fine needle aspiration |
| HGD: | high-grade dysplasia |
| IAP: | International Association of Pancreatology |
| IC: | invasive carcinoma |
| IPMNs: | intraductal papillary neoplasms |
| MCN: | mucinous cystic neoplasm |
| MD-IPMN: | main duct intraductal papillary neoplasm |
| MPD: | main pancreatic duct |
| MRI: | magnetic resonance imaging |
| NGS: | next generation sequencing |
| PCL: | pancreatic cystic lesions |
| PDAC: | pancreatic ductal adenocarcinoma |
| SCAs: | serous cystadenomas |
| SPNs: | solid pseudopapillary neoplasms |
Declarations
Author contributions
REA: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. NB and NVT: Investigation, Writing—original draft, Writing—review & editing. AT and ND: Investigation, Writing—original draft. SA: Conceptualization, Investigation, Writing—review & editing, Supervision.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All the images in the article were collected anonymously and there are no identifiable personal data, following Chart #245 CFR 46.104(d), therefore the Human Research Protection Office at UPMC exempted the ethical approval of this manuscript.
Consent to participate
Human Research Protection Office at UPMC exempted informed consent to participate in this manuscript.
Consent to publication
All the images in the article were collected anonymously, and there is no identifiable personal data; therefore, consent to publication is not required.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.