Affiliation:
1IRIDIA, Université Libre de Bruxelles, 1050 Bruxelles, Belgium
2Département de Cardiologie, Université de Mons, 7000 Mons, Belgium
Email: jean-marie.gregoire@ulb.be
ORCID: https://orcid.org/0000-0002-4636-848X
Affiliation:
1IRIDIA, Université Libre de Bruxelles, 1050 Bruxelles, Belgium
ORCID: https://orcid.org/0000-0002-0705-3514
Affiliation:
3ISIA Lab, Université de Mons, 7000 Mons, Belgium
ORCID: https://orcid.org/0000-0002-8261-238X
Affiliation:
1IRIDIA, Université Libre de Bruxelles, 1050 Bruxelles, Belgium
ORCID: https://orcid.org/0000-0001-5820-7360
Affiliation:
2Département de Cardiologie, Université de Mons, 7000 Mons, Belgium
4CHU Helora, site Kennedy, 7000 Mons, Belgium
ORCID: https://orcid.org/0000-0001-7787-1937
Explor Digit Health Technol. 2025;3:101141 DOI: https://doi.org/10.37349/edht.2025.101141
Received: September 17, 2024 Accepted: January 27, 2025 Published: February 24, 2025
Academic Editor: Shariful Islam, Deakin University, Australia; Atanas G. Atanasov, Medical University of Vienna, Austria
The article belongs to the special issue Wearable Technologies and Application of Machine Learning in Healthcare
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia and can lead to severe complications such as stroke. Artificial intelligence (AI) has emerged as a vital tool in predicting and detecting AF, with machine learning (ML) models trained on electrocardiogram (ECG) data now capable of identifying high-risk patients or predicting the imminent onset of AF. Precision medicine aims to tailor medical interventions for specific sub-populations of patients who are most likely to benefit, utilizing large genomic datasets. Genetic studies have identified numerous loci associated with AF, yet translating this knowledge into clinical practice remains challenging. This paper explores the potential of AI in precision medicine for AF and examines its advantages, particularly when integrated with or compared to genomics. AI-driven ECG analysis provides a practical and cost-effective method for early detection and personalized treatment, complementing genomic approaches. AI-based diagnosis of AF allows for near-certain prediction, effectively relieving cardiologists of this task. In the context of preventive identification, AI enhances the accuracy of predictive models from 75% to 85% when ML is employed. In predicting the exact onset of AF—where human capability is virtually nonexistent—AI achieves a 74% accuracy rate, offering significant added value. The primary advantage of utilizing ECGs over genomic data lies in their ability to capture lifetime variations in a patient’s cardiac activity. AI-driven analysis of ECGs enables dynamic risk assessment and personalized adaptation of therapeutic strategies, optimizing patient outcomes. Genomics, on the other hand, enables the personalization of care for each patient. By integrating AI with ECG and genomic data, truly individualized care becomes achievable, surpassing the limitations of the “average patient” model.
The aim of precision medicine is to optimize a specific preventive, diagnostic, or therapeutic intervention for a given sub-population of patients who are most likely to benefit from it [1]. The application of precision medicine to cardiovascular disease (CVD) has the potential to improve health and revolutionize prevention and treatment options, just as has happened in the field of oncology [2].
Artificial intelligence (AI) algorithms are emerging as a promising approach for predicting cardiovascular events, particularly atrial fibrillation (AF), facilitating enhanced prevention, detection, and treatment. These algorithms may prove to be at least as effective as genomic approaches.
The objective of this paper is to explore the potential of precision medicine in the context of AF, the most prevalent arrhythmia in cardiology through the integration of genomics and AI.
Predictive medicine seeks to determine an individual’s predisposition to certain diseases with the aim of delaying or even preventing their onset [3]. In this context, prediction primarily involves genome analysis, interpreting the information encoded in DNA. However, this concept can be broadened to include all techniques that enable the prediction of disease occurrence in an individual.
In computational terms, prediction refers to the output of a model—such as machine learning (ML)—generated from the data input into it. This output could be a diagnosis, like disease screening. For instance, using ML to screen for AF has become a well-established practice [4]. Another potential output is the identification of patients at high risk of developing a disease, enabling long-term prevention strategies. AI can also be tasked with providing short-term forecasts, such as predicting AF episodes just minutes before they occur, with the accuracy of these predictions increasing as the event approaches, like meteorology: the closer you are to the event, the higher the probability of accurate prediction (Table 1).
The three prediction tasks for AI and clinical and therapeutic matches
| ML task | Medical task | Therapeutic strategy |
|---|---|---|
| AF detection | AF diagnosis | Anticoagulant |
| Identifying patients who will develop AF | AF prevention | Lifestyle changes |
| Predicting the onset of AF | AF avoidance | PITP |
AI: artificial intelligence; ML: machine learning; AF: atrial fibrillation; PITP: pill in the pocket
It’s crucial to acknowledge that the prediction tasks where AI brings the most significant value are often the most challenging. Accurately predicting the onset of inherently unpredictable events like AF is particularly difficult. After all, we are dealing with cardiac “accidents” or cerebrovascular “accidents”. While preventing such incidents is possible, precisely forecasting their occurrence remains a far more complex and demanding task.
AI predictions are based on the constitution of a model, an algorithm, into which data are placed as input. The model is generally given the results we know and want to obtain as output. Learning the model involves adjusting the importance given to the parameters (the weights) to obtain the best predictions (Figure 1).
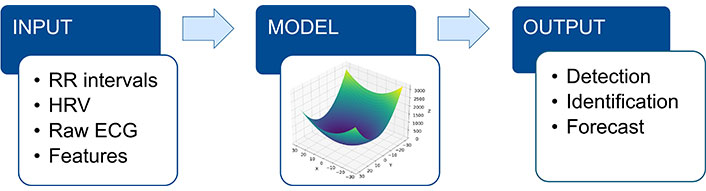
The AI model uses input data (RR intervals; HRV; raw ECG; ECG features) to make a prediction (output). RR intervals: intervals between successive heartbeats; AI: artificial intelligence; HRV: heart rate variability; ECG: electrocardiogram
AF can be entirely asymptomatic, which makes early detection critical to preventing its most common and serious complication: stroke. One of the primary objectives of AF screening is to reduce the risk of ischemic stroke by detecting AF before it leads to severe outcomes. Screening can be performed using various methods, such as simple pulse or blood pressure measurements, but the diagnosis must ultimately be confirmed by an electrocardiogram (ECG) [5]. This is a task well-suited for AI. The effectiveness of AF screening is closely tied to its prevalence within the population being screened, as outlined by Bayes’ theorem. For instance, the Apple Watch study demonstrated the feasibility of large-scale screening through a connected device, but it also highlighted the low cost-effectiveness of such screening in populations with a low prevalence of the disease; only 0.52% of participants received notifications for irregular pulse [6]. This emphasizes the critical need to target specific groups for screening to maximize efficiency. The detection stage is the most straightforward and, consequently, the most widely discussed. This is because it occurs at the latest stage of disease progression, often when AF is at its most advanced, just before complications like stroke develop (Figure 2). A PubMed search using the terms “atrial fibrillation prediction” yielded more than 15,000 articles, many of which report impressive accuracy rates exceeding 99% for AF diagnosis using AI. Prediction is often perceived differently depending on the context, and it is important to remember that these 15,000 articles are almost exclusively about AF screening, not about identifying patients who will develop AF but are still in sinus rhythm. The number of articles on prediction in the latter category can be counted on the fingers of two hands. For obvious economic reasons, AF patients should be detected preferentially in high-risk groups. Clinical risk scores have been developed and validated for this purpose [7]. Groups to be targeted include all patients with diagnosed/established coronary artery disease as it remains one of the strongest risk factors for AF [8], elderly people > 65 years of age [9, 10], those with heart failure [11] and those with dementia as AF is very closely associated with dementia [12]. Genetic analysis could also be used to select at-risk patients [13].
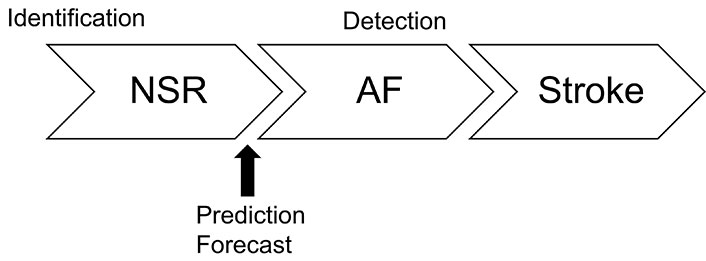
Timeline of the three AI tasks: identification when patients are still in NSR; forecast a few minutes or hours before the AF event; detection for AF screening to prevent stroke events in patients already in AF. AI: artificial intelligence; NSR: normal sinus rhythm; AF: atrial fibrillation
The CHA2DS2-VASc and CHARGE-AF scores, used to initiate anticoagulant therapy, can also be applied to predict the risk of developing AF [7]. The CHARGE-AF score shows an area under the receiver operating characteristic curve (AUC) of around 70% in the groups studied (internal validation: 75%, external validation: 65%) for developing predictive models of AF based on clinical variables. More recently, the C2HEST score, which is based on clinical factors, has been proposed to assess the risk of AF within one year [14]. Therefore, it is reasonable to estimate that a prediction based solely on clinical criteria in at-risk groups can achieve a maximum prediction accuracy of 75%.
The ECG database from the Prediction Challenge 2001 [AF Prediction Database (AFPDB)] is the most widely used AF prediction dataset [15, 16]. It includes 200 ECG recordings from 100 patients, with each patient contributing 2 recordings (only 53 recordings in total related to AF). Each recording lasts 30 min. The first part of the challenge was to identify which patients would develop AF based on sinus rhythm tracings, while the second part aimed to determine which recording in each pair immediately preceded an AF episode. The AFPDB, however, represents a small sample of selected ECGs, making it statistically unlikely that this dataset is truly representative of the broader population of AF patients. Since then, several teams have used the AFPDB to predict the onset of AF episodes, but unfortunately, these results have not been consistently reproducible [17].
A small number of teams have focused on predicting AF while patients are still in sinus rhythm. Their results are broadly comparable, although the methods and the level of patient risks differ. Table 2 summarizes the main characteristics of these studies.
Main features of AF prediction studies based on ECGs in sinus rhythm
| Input data | Model | N ECGs | N AF ECGs | Time to onset of AF | AUC (%) |
|---|---|---|---|---|---|
| 12 lead ECG [18] | CNN | 80,125 | 13,824 | < 31 days | 87 |
| 12 lead ECG [19] | DNN | 1,632,487 | 9,377 | < 1 year | 85 |
| 12 lead ECG [20] | RNN | 1,057 | 1,355 | < 1 day | 79 |
| 12 lead ECG [21] | CNN | 45,770 | 2,171 | < 5 years | 82 |
| 12 lead ECG [22] | CNN | 907,858 | 28,117 | > 31 days | 86 |
| 12 lead ECG [13] | ResNet | 669,782 | 22,695 | < 5 years | 78 |
| 2 lead ECG [23] | CNN | 29,884 | 3,307 | < 15 days; > 1 day | 79 |
| 1 lead ECG [24] | CNN | 169,013 | 13,553 | NA | 62 |
| 1 lead ECG [24] | CNN | 166,447 | 15,052 | NA | 62 |
| 1 lead ECG [24] | CNN | 143,503 | 10,951 | NA | 80 |
AF: atrial fibrillation; ECGs: electrocardiograms; N ECGs: number of ECGs; AUC: area under the receiver operating characteristic curve; CNN: convolutional neural network; DNN: deep neural network; RNN: recurrent neural network; ResNet: residual network; NA: not available
This is the most challenging for AI. The smaller the forecasting window for an event, the more difficult the prediction task becomes. Successive results from different teams using AFPDB data have shown improvements in accuracy, ranging from 79% to nearly 100%. However, it is important to note that the dataset was derived from selected data, which may limit its generalizability. A deep neural network (DNN) model was able to identify patients at risk of developing AF within two weeks following a 24-hour Holter monitoring in sinus rhythm [23]. The model successfully predicted future AF episodes with an AUC of 79%. A gradient-boosted decision trees model achieved an AUC of 73% and an accuracy of 74% in forecasting the onset of AF events from Holter monitoring data (Table 3) [25].
Approximations of the performances obtained by using the classical statistics used by humans compared to those obtained by AI for the three different predictions
Note that the score attributed to the human for detection is necessarily 100% since it is the human who sets the reference value (the ground truth). AI: artificial intelligence
The growing awareness of the significant role of genetics in the development of AF has led to the characterization of its genetic architecture, particularly through genome-wide association studies (GWAS) [26–28]. In 2003, the first AF gene (KCNQ1), which encodes the cardiac potassium channel, was identified [29]. The identification of KCNQ1 as responsible for AF led to the screening of other potassium and cardiac ion channels as candidate genes. Over 100 loci in the genome are now known to be associated with AF [30]. Nevertheless, in the general population, genetic variations in AF that have a significant impact are very rare. Translating genetic risk into clinical practice remains a significant challenge and may not be the best approach for achieving precision medicine. Nevertheless, medical advancements in genetics and AI offer promising prospects for more personalized approaches to treatment, paving the way for truly individualized care [28].
The genetic code contains the blueprint that makes each individual unique. Similarly, an individual can be uniquely identified by their ECG, which, like the genetic code, carries distinct information specific to that person. A growing body of research supports this analogy. Conducting an ECG for screening purposes is significantly more feasible than performing genetic analysis for several reasons. ECGs are widely available, inexpensive, non-invasive, and can be performed quickly, even in primary care settings. In contrast, genetic analysis is more costly, time-consuming, and may require specialized equipment and expertise. This makes AI-enhanced ECGs a preferable option for large-scale or routine screening efforts, especially in settings where access to genetic testing is limited.
This applies to AF. Notably, AI has the ability to predict the onset of AF more than a year before the first episode in patients who are still in normal sinus rhythm (NSR), offering significant potential for early intervention and preventive care [19]. Moreover, AI can also identify several other frequent CVDs from a simple ECG [31]. AI thus enables individualized predictive cardiology. It is only a step away from being integrated into precision cardiology. It should be noted that in the case of AF, AI allows for the optimization of treatment in accordance with the desired outcomes, which may include the avoidance of specific disease consequences (Table 1).
ECGs have several advantages over DNA: they can be performed repeatedly as an individual ages, and changes that may increase the risk of developing heart disease can be detected and prevented at the most appropriate time. In addition, performing ECGs in at-risk sub-populations increases the performance of AI according to Bayes’ theorem [24, 32]. Moreover, ECGs are less complex and costly to perform than genomic analyses. A recent study showed that deep learning applied to a resting 12-lead ECG during sinus rhythm can effectively predict the risk of new-onset AF with high performance in a tertiary cardiac care center population, outperforming existing clinical and polygenic scores [13].
AI-based ECG analysis is therefore perfectly suited to personalization, as all the recommendation algorithms at work in cultural sites and social networks clearly demonstrate, since the implicit principle of AI techniques is to adapt the processing of each person’s data, whether biometric or not, based on the actual processing applied to all those who resemble them. Each person is considered individually but based on what has worked for those who resemble them. There is no reason that this principle should not find its way into the practice of medicine.
Moving from the gene to diagnosis or treatment remains challenging, especially for common CVDs where multiple loci or genes are involved. It may be easier to go the other way, i.e., to use large amounts of data from groups of individuals with the same traits and eventually focus on one. It would be interesting to bring the two approaches together as if they were at different ends of the road leading to the patient. AI could be used to identify monogenic heart diseases from the genome and polygenic ones from the ECG. AI functions like a powerful lens, uncovering detailed insights from an ECG that even the most skilled cardiologist might overlook. This brings us to the equation: one genome = one individual = one ECG. In other words, applying AI-driven ECG analysis could enable precision medicine based on ECGs, much like how it is currently achieved through genetic analysis. This approach holds the potential to deliver individualized care tailored to a person’s unique heart profile, just as genomics informs treatment decisions today (Figure 3).
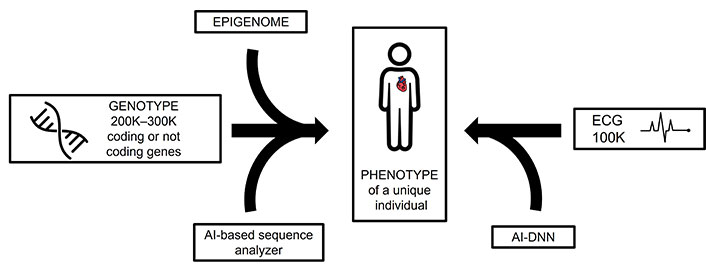
Discrimination of an individual using two complementary AI methods: on the left, a sequencer based on deep learning, and, on the right, a DNN based on an ECG. AI: artificial intelligence; ECG: electrocardiogram; DNN: deep neural network
Traditionally, advancements in patient care have been achieved by focusing on the average patient, as represented by the center of the Gaussian curve. However, targeting the average can ultimately lead to mediocrity: while we may avoid the worst outcomes, we also miss the chance to achieve the best. This represents a significant missed opportunity.
While the use of clinical scores to guide treatment decisions represents an undeniable advancement in the field of medicine, the true challenge for AI in medicine is to enable the widespread adoption of precision medicine. This will require a shift from group medicine to a more individualized approach, reminiscent of what AI generally allows in other domains: such as personalized marketing or consumer profiling and recommendation. The true potential of AI lies in its ability to tailor treatments to the individual patient, utilizing simple techniques like ECG rather than relying on costly technological interventions. However, although AI has been a part of medicine since its inception, experiencing various “winters” and “springs”, it will still take time before AI fully realizes its potential and finds its rightful place in personalized healthcare.
Most studies on AF prediction are retrospective. Prospective studies are essential to validate the superiority of AI-optimized patient selection for screening over traditional clinical scoring methods. Furthermore, prospective research should confirm the feasibility of short-term AF prediction to justify therapeutic interventions, such as a pill in the pocket (PITP)-like strategy. A potential application could involve the use of a connected watch or wearable device that can alert the patient when a signal indicating an incoming AF episode is received. In such cases, the patient can be prompted to take appropriate medication to prevent an AF episode. In the current context, there is no clinical proof of this concept, and this justifies the need for prospective studies. In this case, the goal is to reduce the AF burden in patients who are already receiving anticoagulant therapy in the hope of slowing the progression to long-term permanent AF. In patients with pacemakers, integrating an ML prediction algorithm before the atrial overdrive pacing algorithm could significantly reduce the burden of AF in those at high risk of developing a persistent form, particularly when ablation is either undesired or unsuccessful [33].
Ensuring data quality is critical for obtaining reliable predictions. Therefore, it is essential to verify input ECGs for potential artifacts. Caution is advised when using DNNs, as they often operate as black boxes, limiting their justification to statistical results obtained by cross-validation. Whenever possible, interpretable ML models should be prioritized [34]. These models provide insights into input features, enhancing reliability and trustworthiness. Exceptionally high performance may indicate overfitting—a phenomenon where a model learns not only the underlying patterns but also the noise and specific details of the training data. This over-specialization compromises the model’s ability to generalize effectively to unseen data, reducing its predictive accuracy in real-world scenarios.
In addition to technical challenges, numerous ethical concerns may arise. One critical question is: who is held accountable in the event of an error in diagnosis or treatment based on an AI prediction? Should the responsibility lie with the algorithm’s developer, the medical institution, or the clinician? ECGs and other medical data are highly sensitive, and their use in training AI models requires strict protocols to ensure confidentiality and safeguard against breaches or leaks.
Over-reliance on AI could diminish the role of clinicians in the decision-making process, potentially leading to the dehumanization of care. Furthermore, AI tools often operate with inherent levels of uncertainty, which are not always clearly communicated to physicians. This lack of transparency can result in decisions based on incomplete or misinterpreted information. Additionally, excessive reliance on automated systems may erode clinicians’ skills over time, further complicating their ability to provide high-quality, independent care.
Precision medicine through AI offers transformative possibilities for AF diagnosis and treatment, moving beyond the limitations of traditional approaches. By leveraging ML and ECG data, AI enhances predictive accuracy and enables early intervention, paving the way for more personalized, effective care. While challenges remain, particularly in predicting complex events like AF onset, the integration of AI with genomic and clinical data promises a future where individualized care becomes the standard, optimizing patient outcomes and revolutionizing cardiovascular healthcare.
AF: atrial fibrillation
AFPDB: Atrial Fibrillation Prediction Database
AI: artificial intelligence
AUC: area under the receiver operating characteristic curve
CVD: cardiovascular disease
ECG: electrocardiogram
ML: machine learning
JMG: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. CG and FM: Software, Validation, Funding acquisition. HB and SC: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported in part by the French Community of Belgium [FRIA funding: FC 038733]; this project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska Curie grant agreement [No 101034383]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Vasile Denis Manolescu ... Emanuele Lindo Secco
Daniela Gawehns ... Matthijs van Leeuwen
