Affiliation:
1Department of Medicinal Chemistry, Federal State Budgetary Scientific Institution “Federal Research Center for Innovator and Emerging Biomedical and Pharmaceutical Technologies”, 125315 Moscow, Russia
Email: gudasheva_ta@academpharm.ru; tata-sosnovka@mail.ru
ORCID: https://orcid.org/0000-0002-5185-4474
Affiliation:
1Department of Medicinal Chemistry, Federal State Budgetary Scientific Institution “Federal Research Center for Innovator and Emerging Biomedical and Pharmaceutical Technologies”, 125315 Moscow, Russia
ORCID: https://orcid.org/0000-0003-3278-8915
Affiliation:
2Department of Pharmaceutical Chemistry, Federal State Budgetary Scientific Institution “Federal Research Center for Innovator and Emerging Biomedical and Pharmaceutical Technologies”, 125315 Moscow, Russia
ORCID: https://orcid.org/0009-0004-3584-3742
Explor Drug Sci. 2025;3:1008100 DOI: https://doi.org/10.37349/eds.2025.1008100
Received: January 17, 2025 Accepted: February 27, 2025 Published: March 21, 2025
Academic Editor: Alessandra Tolomelli, University of Bologna, Italy
Proteins from the neurotrophin family perform trophic and regulatory functions in the nervous and other body systems. Understanding the mechanisms of neurotrophin action is crucial not only for the evolution of fundamental scientific knowledge but also for developing new treatment strategies targeting neurotrophin signaling regulation. At our center, dimeric dipeptide mimetics of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3) have been obtained based on the structure of neurotrophins’ individual loops β-turns. These mimetics activated tyrosine kinase (Trk) receptors TrkA, TrkB, or TrkC specific to their respective neurotrophins, but exhibited varied activation patterns in the main post-receptor signaling cascades. Thus, some dipeptides activated all three main phosphoinositide 3-kinase (PI3K)/threonine-protein kinase (Akt), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and phospholipase C-gamma (PLC-γ) pathways, while others triggered only PI3K/Akt and PLC-γ or MAPK/ERK and PLC-γ. Herewith, dipeptides exhibited a specific set of effects (neuroprotective, differentiating, antidepressant-like, anxiolytic, memory-enhancing, analgesic, antidiabetic) within the spectrum of biological activities of their corresponding native neurotrophin. It was revealed that these effects are influenced by both the patterns of post-receptor signaling activation and the nature of progenitor neurotrophin, uncovering significant correlations. This article is dedicated to reviewing the data that has been collected.
Neurotrophins are a family of regulatory proteins that play a crucial role in supporting the development of the nervous system, ensuring its proper function throughout adulthood, and performing trophic and regulatory functions in other body systems. In mammals, four proteins from this family have been identified: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and NT-4 [1]. The main biological functions of neurotrophins are mediated through their interaction with tyrosine kinase (Trk) receptors (TrkA, TrkB, TrkC). NGF activates TrkA receptors; BDNF and NT-4 engage TrkB receptors; NT-3 interacts with all three types of Trk receptors, with the highest affinity for TrkC [2]. Binding of neurotrophins to Trk receptors leads to the activation of phosphoinositide 3-kinase (PI3K)/threonine-protein kinase (Akt), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), and phospholipase C-gamma (PLC-γ) post-receptor signaling cascades [1].
The PI3K/Akt signaling cascade is primarily involved in neuroprotection through the activation of various genes that regulate neuronal survival, stimulation of anti-apoptotic protein expression, and inhibition of pro-apoptotic proteins [3]. One of the components of the PI3K/Akt pathway is the mammalian target of rapamycin (mTOR) protein, a pivotal regulator of ribosome biogenesis and protein translation [4]. The involvement of the PI3K/Akt/mTOR signaling cascade in synaptogenesis has been established [5].
The MAPK/ERK signaling cascade, like the PI3K/Akt pathway, is involved in neuroprotection [6]. However, under pathological conditions, it may contribute to neuronal cell death [7]. Additionally, this cascade is implicated in cellular differentiation and proliferation [8]. A key downstream component of the MAPK/ERK pathway is the transcription factor cAMP response element-binding protein (CREB), which plays a critical role in synaptogenesis and synaptic plasticity [9, 10]. Furthermore, the MAPK/ERK cascade is a major regulator of pain sensitivity [11].
The PLC-γ1-mediated signaling pathway is involved in maintaining synaptic plasticity, regulating axon growth, and internalizing the neurotrophin/Trk receptor complex into signaling endosomes [12].
It has been established that the pathogenesis of many neurological and psychiatric disorders is associated with disruptions in neurotrophin signaling, which has prompted efforts to use neurotrophins as treatments for these conditions [13–17]. However, the clinical application of native neurotrophins has been largely unsuccessful, primarily due to low bioavailability and severe side effects, with hyperalgesia and weight loss being among the most common [15, 18]. To overcome the limitations of full-sized neurotrophins, several research groups are developing their low-molecular-weight mimetics, with some of these compounds currently advancing through preclinical or clinical stages [19–25]. A research group led by Burgess and Saragovi [24] created a small-molecule partial TrkA agonist, compound tavilermide (D3) (Figure 1), based on the pharmacophore of the 5C3 monoclonal antibody targeting the NGF-binding site of the human TrkA receptor. D3 is currently being developed by Mimetogen Pharmaceuticals (Canada) as a treatment for dry eye and has successfully completed phase 3 clinical trials (https://www.clinicaltrials.gov/study/NCT03925727?intr=Tavilermide%20ophthalmic%20solution&rank=2). D3 also demonstrated neuroprotective, anti-amyloidogenic, neuroregenerative, and cognitive-enhancing effects in a genetic mouse model of Alzheimer’s disease [19].
Longo’s research group [26, 27], using in silico screening based on similarity to the first loop of NGF, identified the compound LM11A-31 (Figure 2), which, in several genetic mouse models of Alzheimer’s disease, with chronic per os administration (50.0–100.0 mg/kg) counteracted the degeneration of cholinergic neurons, the accumulation of beta-amyloid and hyperphosphorylated tau protein, and reversed spatial memory deficits. Currently, LM11A-31 is undergoing phase 2 clinical trials as a treatment for Alzheimer’s disease (https://www.clinicaltrials.gov/study/NCT03069014?term=LM11A-31&rank=1).
AlzeCure Pharma pharmaceutical company (Sweden), using high-throughput cellular screening of a compound library for the ability to potentiate NGF/TrkA and BDNF/TrkB signaling, identified novel positive allosteric modulators of Trk receptors [28].
The most potent compound, ACD856, demonstrated neuroprotective activity, increased BDNF levels in vitro and in vivo, and also enhanced cognitive functions, as well as exhibited antidepressant-like effects in various mouse tests (0.3–3.0 mg/kg, subcutaneous) [29]. ACD856 is currently in phase 1 clinical trials as a treatment for Alzheimer’s disease (https://www.clinicaltrials.gov/study/NCT05077631?term=ACD856&rank=1). The structure formula of ACD856 has not yet been disclosed. The structure of the closest analogue of ACD856, compound ACD855, is shown in Figure 3.
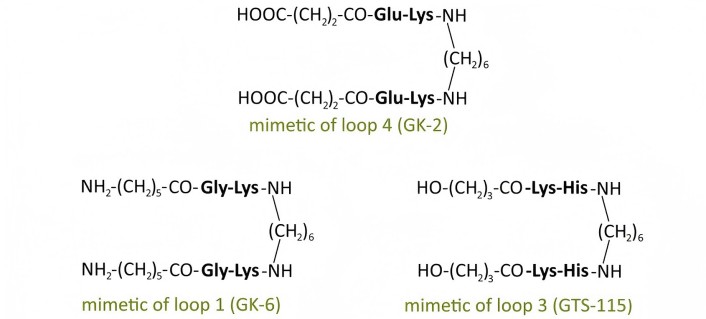
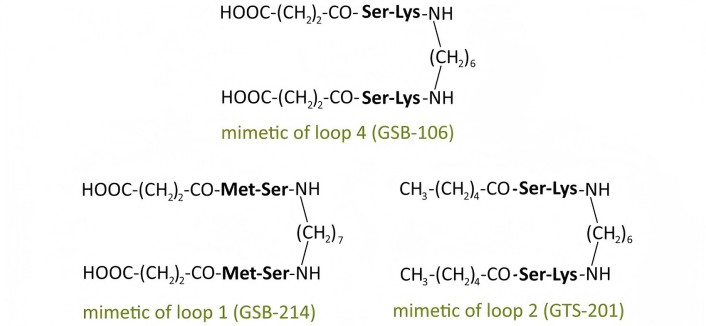
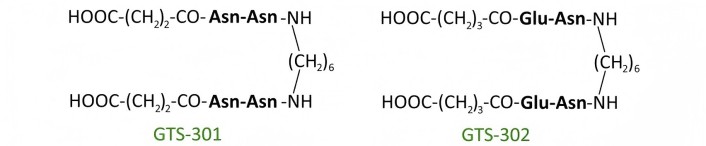
In our center, a hypothesis was proposed regarding the possibility of selective activation of Trk receptor signal transduction pathways using low-molecular-weight mimetics of individual neurotrophin loops [30–32]. Based on this assumption, as well as the hypothesis [30–32] regarding the key role of the most exposed, often central, dipeptide fragments of the β-turns in the loop-like structures of neurotrophins in receptor interaction, dimeric dipeptide mimetics of NGF, BDNF, and NT-3 were developed. It included mimetics of the NGF loops 1, 3 and 4: bis-(N-aminocaproyl-glycyl-L-lysine) hexamethylenediamide (GK-6), bis-(monosuccinyl-L-glutamyl-L-lysine) hexamethylenediamide (GK-2) and bis-(N-gamma-oxybutyryl-L-lysyl-L-histidine) hexamethylenediamide (GTS-115), respectively (Figure 4); mimetics of the BDNF loops 1, 2 and 4: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide (GSB-214), bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide (GTS-201), and bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide (GSB-106), respectively (Figure 5); and mimetics of the NT-3 loop 4: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide (GTS-301) and bis-(N-gamma-oxybutyryl-L-glutamyl-L-asparagine) hexamethylenediamide (GTS-302) (Figure 6) (RU Patent 2410392, 2011; CN Patent 102365294 B, 2016; US Patent 9683014 B2, 2017; EP Patent 2397488, 2019; Ru Patent 2022108924, 2023). The compounds were designed according to a unified plan: the most exposed dipeptide fragment of the β-turn was preserved, the preceding amino acid residue was replaced with its bioisostere, and dimerization was performed at the C-termini using a hexa- or heptamethylenediamine spacer [30, 31, 33].
As demonstrated in vitro on HT-22 mouse hippocampal neuronal cell line using Western blot analysis, the obtained mimetics in concentrations of 10–8–10–6 M activated Trk receptors specific to the full-length neurotrophin within 5–180 min of incubation: NGF mimetics activated TrkA, BDNF mimetics activated TrkB, and NT-3 mimetics activated TrkC [34–38]. For the loop 4 mimetics of NGF and BDNF, the selectivity of interaction with TrkA and TrkB receptors, respectively, was confirmed using HT-22 knockout cells lacking the trka or trkb genes: the neuroprotective activity of these compounds was observed only in the presence of receptors specific to the native neurotrophin [39, 40]. According to the proposed hypothesis [30–32], dipeptide mimetics of individual neurotrophin loops exhibited different patterns of activation of post-receptor signaling cascades. In HT-22 cells, Western blot analysis using antibodies to Akt (pan), ERK1/2, and PLC-γ1 showed that all dipeptides activated PLC-γ1, with some additionally triggering both the PI3K/Akt and MAPK/ERK pathways, while others targeted only one of these two pathways [33–38, 41, 42]. The activation of Trk receptors and their post-receptor signaling pathways by dipeptide mimetics of NGF, BDNF, and NT-3 is shown in Table 1.
Activation of Trk receptors and their main post-receptor signaling pathways by dipeptide mimetics of NGF, BDNF, and NT-3
| Parent neurotrophin | Code | Neurotrophin loop | Concentration (M) | Trk receptor activation | Activation of post-receptor signaling cascades | ||
|---|---|---|---|---|---|---|---|
| PI3K/Akt | MAPK/ERK | PLC-γ1 | |||||
| NGF | GK-2 [37, 41] | 4 | 10–8 | TrkA | ++ | ns | ++ |
| GK-6 [37, 41] | 1 | 10–6 | TrkA | + | + | + | |
| GTS-115 [34, 41] | 3 | 10–6 | TrkA | ++ | + | + | |
| BDNF | GSB-106 [38, 41] | 4 | 10–8 | TrkB | + | ++ | ++ |
| GSB-214 [38, 41] | 1 | 10–7 | TrkB | + | ns | ++ | |
| GTS-201 [35, 41] | 2 | 10–7 | TrkB | ns | + | ++ | |
| NT-3 | GTS-301 [33, 42] | 4 | 10–6 | TrkC | ns | ++ | ++ |
| GTS-302 [36, 42] | 10–6 | TrkC | ++ | ++ | + | ||
Full-length NGF, BDNF and NT-3 at concentrations of 10–9 M were used as positive controls. Trk: tyrosine kinase; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; MAPK/ERK: mitogen-activated protein kinase/extracellular signal-regulated kinase; PLC-γ1: phospholipase C-gamma; GK-2: bis-(monosuccinyl-L-glutamyl-L-lysine) hexamethylenediamide; GK-6: bis-(N-aminocaproyl-glycyl-L-lysine) hexamethylenediamide; GSB-106: bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide; GSB-214: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide; GTS-115: bis-(N-gamma-oxybutyryl-L-lysyl-L-histidine) hexamethylenediamide; GTS-201: bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide; GTS-301: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide; GTS-302: bis-(N-gamma-oxybutyryl-L-glutamyl-L-asparagine) hexamethylenediamide; +: the effect is inferior to the full-length neurotrophin in terms of intensity and/or duration; ++: the effect is comparable to the full-length neurotrophin in terms of intensity and duration; ns: non-significant
The dipeptides demonstrated pharmacological activity in vitro (10–9–10–5 M) and in vivo [0.01–10.0 mg/kg, intraperitoneally (ip), orally], exhibiting diverse sets of effects [32, 33, 43]. We investigated the potential links between these effects and the patterns of post-receptor signaling activation, as well as the nature of the parent neurotrophin.
One of the main biological functions of neurotrophins is to support the survival of neurons, which is achieved both by stimulating the expression of pro-survival genes and by inhibiting pro-apoptotic proteins [1].
It has been established in vitro, that all dipeptide mimetics of NGF, BDNF, and NT-3, regardless of the pattern of post-receptor signaling activation, improved the survival of HT-22 hippocampal neuronal cell line under oxidative stress conditions at micro-nanomolar concentrations [30, 31, 33–36].
The contribution of PI3K/Akt and MAPK/ERK pathways to the neuroprotective activity of the BDNF loop 4 mimetic (GSB-106) and the NGF loop 1 mimetic (GK-6), both of which activate all major post-receptor signaling cascades of Trk receptors, was examined through pharmacological inhibition analysis [37, 44]. Human SH-SY5Y neuroblastoma cells in serum-free medium were used for GSB-106 [44], while GK-6 was tested on HT-22 cells under oxidative stress conditions [37]. It was observed that specific inhibitors of PI3K or MEK1 (LY294002 and PD98059, respectively) diminished the protective effects of the dipeptides. Inhibition of PI3K/Akt nearly entirely eliminated the neuroprotective effects, whereas blocking MAPK/ERK reduced them by about 20%. The results of these studies are consistent with the literature, which indicates that both PI3K/Akt and MAPK/ERK pathways are associated with neuroprotection, at least in vitro, with PI3K/Akt playing a more significant role [1, 45].
The neuroprotective effects of NGF, BDNF, and NT-3 dipeptide mimetics in vivo were investigated using a rat model of ischemic stroke, induced by transient middle cerebral artery occlusion (MCAO). The dipeptides were administered ip for 7 days post-surgery at doses ranging from 0.1 mg/kg to 1.0 mg/kg. Their efficacy was assessed primarily through the reduction of brain infarct volume, determined by morphometric analysis of brain sections stained with 2,3,5-triphenyltetrazolium chloride. Notably, neuroprotection was observed only for compounds that engage the PI3K/Akt signaling cascade in their post-receptor activation patterns. These are mimetics of the NGF loops 3 and 4 (GTS-115 and GK-2) [46], mimetics of the BDNF loops 1 and 4 (GSB-214 and GSB-106) [38], and the NT-3 loop 4 mimetic (GTS-302) (Povarnina P, et al. unpublished data).
The most active compounds were the mimetics of the highly exposed 4th loops of NGF and BDNF, specifically dipeptides GK-2 and GSB-106. Dipeptide GK-2 activated PI3K/Akt and PLC-γ1, while GSB-106 activated PI3K/Akt, MAPK/ERK, and PLC-γ1. Both compounds reduced brain infarct volume by 60% [38, 46]. The NT-3 loop 4 mimetic GTS-302, which activates PI3K/Akt, MAPK/ERK, and PLC-γ1, showed lower activity, decreasing infarct volume by 40%. Mimetics of the NGF loop 3, which activates PI3K/Akt, MAPK/ERK, and PLC-γ1, and the BDNF loop 1, which activates PI3K/Akt and PLC-γ1, demonstrated relatively lower efficacy, reducing infarct volume by 25% and 30%, respectively [38, 46]. Dipeptides that activate only MAPK/ERK and PLC-γ1, specifically the BDNF loop 2 mimetic GTS-201 and the NT-3 loop 4 mimetic GTS-301, were found to be inactive (Povarnina P, et al. unpublished data). The in vivo neuroprotective activity was also absent in the NGF loop mimetic 1, the dipeptide GK-6, which activated PI3K/Akt, MAPK/ERK, and PLC-γ1. This could be attributed to the relatively weaker activation of PI3K/Akt by GK-6 compared to the in vivo active NGF mimetics, GK-2 and GTS-115. While GK-6 activated PI3K/Akt for only 1 h after being introduced into the culture medium [37], the latter two mimetics sustained activation for at least 3 h [34, 37]. The data on the neuroprotective activity of dipeptide neurotrophin mimetics in the ischemic stroke model are summarized in Table 2.
Neuroprotective efficacy of NGF, BDNF, and NT-3 dipeptide mimetics in a rat model of ischemic stroke induced by transient middle cerebral artery occlusion
| Parent neurotrophin | Code | Neurotrophin loop | Threshold dose (mg/kg, ip) | Activation of post-receptor signaling cascades | Reduction in brain infarct volume (%) | ||
|---|---|---|---|---|---|---|---|
| PI3K/Akt | MAPK/ERK | PLC-γ1 | |||||
| NGF | GK-2 [46] | 4 | 0.5 | ++ | ns | ++ | 60 |
| GK-6 [46] | 1 | Not active | + | + | + | ns | |
| GTS-115 [46] | 3 | 1.0 | ++ | + | + | 25 | |
| BDNF | GSB-106 [38] | 4 | 0.1 | + | ++ | ++ | 60 |
| GSB-214 [38] | 1 | 0.1 | + | ns | ++ | 30 | |
| GTS-201 [46] | 2 | Not active | ns | + | ++ | ns | |
| NT-3 | GTS-301 | 4 | Not active | ns | ++ | ++ | ns |
| GTS-302 | 1.0 | ++ | ++ | + | 40 | ||
ip: intraperitoneally; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; MAPK/ERK: mitogen-activated protein kinase/extracellular signal-regulated kinase; PLC-γ1: phospholipase C-gamma; GK-2: bis-(monosuccinyl-L-glutamyl-L-lysine) hexamethylenediamide; GK-6: bis-(N-aminocaproyl-glycyl-L-lysine) hexamethylenediamide; GTS-115: bis-(N-gamma-oxybutyryl-L-lysyl-L-histidine) hexamethylenediamide; GSB-106: bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide; GSB-214: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide; GTS-201: bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide; GTS-301: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide; GTS-302: bis-(N-gamma-oxybutyryl-L-glutamyl-L-asparagine) hexamethylenediamide; +: the effect is inferior to the full-length neurotrophin in terms of intensity and/or duration; ++: the effect is comparable to the full-length neurotrophin in terms of intensity and duration; ns: non-significant. All effects not labeled as “ns” are statistically significant. The data on the reduction in brain infarct volume are presented as means. The reference drug Mexidol (100.0 mg/kg, ip), reduced brain infarct volume by 40% in the same experimental protocol
Overall, the obtained results are consistent with the literature, which indicates that the neuroprotective effects of neurotrophins are primarily mediated through PI3K/Akt signaling [47–49]. It is important to note that the MAPK/ERK pathway, under pathological conditions such as ischemia, may exacerbate neurodegeneration [50, 51]. Unlike in vitro models, where MAPK/ERK contributes to neuroprotection by activating anti-apoptotic mechanisms [44, 52], in experimental ischemic stroke, this signaling cascade can also play a pro-pathological role by contributing to processes such as inflammation and disruption of the blood-brain barrier [50].
Antidiabetic effects were observed with NGF, BDNF, and NT-3 dipeptide mimetics following chronic intraperitoneal and oral administration (0.1–10.0 mg/kg) in streptozotocin-induced diabetes models in rats and mice [53–56]. Consistent with the in vivo neuroprotective activity, our findings indicate that activation of PI3K/Akt is essential for the antidiabetic effects of NGF and BDNF mimetics. This requirement may be related to the role of this signaling cascade in protecting pancreatic β-cells. According to the literature, the PI3K/Akt pathway is involved in maintaining the viability and phenotypic stability of pancreatic β-cells, and the involvement of PI3K/Akt signaling disruption in the pathogenesis of diabetes is confirmed by experimental and clinical studies [57, 58].
Among the BDNF mimetics, the most effective was GSB-214, a dipeptide based on the 1st loop, which activates PI3K/Akt and PLC-γ. This compound reduced hyperglycemia in diabetic mice with the greatest effect reaching 84% of the maximum possible and maintained its efficacy for up to 44 days after discontinuation [53]. The loop 4 mimetic, GSB-106, which activates PI3K/Akt, MAPK/ERK and PLC-γ, showed less pronounced activity, achieving a maximum antihyperglycemic effect of 42% with a duration of up to 4 days post-treatment. The loop 2 mimetic, GTS-201, which activates MAPK/ERK and PLC-γ, was inactive [53].
The NGF loop 4 mimetic, dipeptide GK-2, also demonstrated high antidiabetic activity. Similar to GSB-214, it activated the PI3K/Akt and PLC-γ signaling cascades. The dipeptide exhibited antihyperglycemic effects in diabetic mice, reaching up to 90% of the maximum possible effect from day 4 to day 60 post-diabetes induction (44 days after the end of compound administration) [56]. It also showed cytoprotective properties for β-cells and was effective in reducing diabetes-related polydipsia, polyphagia, weight changes, and diabetic neuropathy in mice and rats [55, 56, 59]. The involvement of PI3K/Akt activation in the antidiabetic activity of GSB-214 and GK-2 was confirmed through inhibitor analysis—administration of the specific PI3K inhibitor LY294002 completely abolished effects of these dipeptides in the streptozotocin-induced diabetes models [60, 61].
It is interesting to note that the NT-3 loop 4 mimetic, dipeptide GTS-301, also demonstrated activity in experimental diabetes [54], despite only activating the MAPK/ERK and PLC-γ pathways in vitro, with no influence on PI3K/Akt. This dipeptide exhibited antihyperglycemic effects, achieving up to 53%. This suggests that the antidiabetic activity mediated by NT-3 mimetics may be realized through mechanisms independent of PI3K/Akt signaling.
The data on the antidiabetic activity of dipeptide mimetics of NGF, BDNF, and NT-3 are summarized in Table 3.
Antidiabetic activity of dipeptide mimetics of NGF, BDNF, and NT-3 in a streptozotocin-induced diabetes model in mice and rats
| Parent neurotrophin | Code | Neurotrophin loop | Threshold dose (mg/kg, ip) | Activation of post-receptor signaling cascades | Antihyperglycemic activity, percent of the maximum possible effect (%) | ||
|---|---|---|---|---|---|---|---|
| PI3K/Akt | MAPK/ERK | PLC-γ1 | |||||
| NGF | GK-2 [56] | 4 | 0.5 | ++ | ns | ++ | 90 |
| BDNF | GSB-106 [53] | 4 | 0.5 | + | ++ | ++ | 42 |
| GSB-214 [53] | 1 | 0.5 | + | ns | ++ | 84 | |
| GTS-201 [53] | 2 | Not active | ns | + | ++ | ns | |
| NT-3 | GTS-301 [54] | 4 | 0.1 | ns | ++ | ++ | 53 |
ip: intraperitoneally; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; MAPK/ERK: mitogen-activated protein kinase/extracellular signal-regulated kinase; PLC-γ1: phospholipase C-gamma; GK-2: bis-(monosuccinyl-L-glutamyl-L-lysine) hexamethylenediamide; GSB-106: bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide; GSB-214: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide; GTS-201: bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide; GTS-301: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide; +: the effect is inferior to the full-length neurotrophin in terms of intensity and/or duration; ++: the effect is comparable to the full-length neurotrophin in terms of intensity and duration; ns: non-significant. All effects not labeled as “ns” are statistically significant. The table shows the most pronounced antihyperglycemic effects of the compounds, achieved during treatment or its discontinuation. These effects are presented as percentages of the maximum possible effect and are provided as average values. The antihyperglycemic effect of the reference drug Metformin (300.0 mg/kg, orally) in the same experimental design reached up to 85% of the maximum possible
It is well established that BDNF, NGF, and NT-3 are involved in the pathogenesis of depression, with the association between this disorder and dysregulation of BDNF signaling being the most well-supported [62–66].
Comparative studies on the antidepressant-like activity of dipeptide mimetics from various loops of NGF, BDNF, and NT-3 in the forced swim test in mice revealed that only the loop 4 mimetics of BDNF and NT-3 (GSB-106 and GTS-302, respectively) displayed this activity following acute administration (0.1–10.0 mg/kg, ip) [36, 67]. These mimetics, similar to full-length neurotrophins, activated the PI3K/Akt, MAPK/ERK, and PLC-γ pathways. This highlights the necessity of engaging all three signaling cascades of Trk receptors to achieve antidepressant-like effects with acute administration of dipeptide neurotrophin mimetics. It also suggests that such activity is more characteristic of BDNF and NT-3 than NGF. According to the literature, BDNF and NT-3 exhibit antidepressant-like activity following acute intracerebral administration, whereas NGF demonstrates such effects only with prolonged treatment [68, 69]. The requirement for activating the PI3K/Akt, MAPK/ERK, and PLC-γ pathways of TrkB receptors for the antidepressant-like effects of the BDNF mimetic dipeptide GSB-106 was confirmed through pharmacological inhibitor analysis. Specific inhibitors for these pathways (LY294002, PD98059, and U73122, respectively) and the Trk receptor blocker K252A each completely abolished the dipeptide’s activity in the forced swim test [67, 70].
In subchronic ip administration, all studied dipeptides exhibited antidepressant-like activity, except for the BDNF loop 2 mimetic GTS-201, which activates MAPK/ERK and PLC-γ without affecting PI3K/Akt [33, 36, 67]. However, the NT-3 loop 4 mimetic GTS-301, which shares the same post-receptor signaling pattern as GTS-201, was active in this test [33]. The difference in in vivo activity between GTS-201 and GTS-301 likely stems from their distinct MAPK/ERK signaling activation kinetics. GTS-201 triggered MAPK/ERK activation for only up to 5 min [35], while GTS-301 induced sustained activation lasting up to 180 min [42]. The involvement of MAPK/ERK activation in the realization of antidepressant-like effects during subchronic administration of compounds may be due to this cascade leading to the activation of the transcription factor CREB, one of the key regulators of synaptic plasticity [71].
The antidepressant-like properties of the most active dipeptides, GSB-106 and GTS-302, were confirmed in a chronic social stress model in mice: both compounds counteracted the manifestation of anhedonia and deterioration of hippocampal neuroplasticity [70, 72].
Data on the antidepressant-like activity of NGF, BDNF, and NT-3 dipeptide mimetics are summarized in Table 4.
Antidepressant-like activity of NGF, BDNF and NT-3 dipeptide mimetics in the forced swim test in mice
| Parent neurotrophin | Code | Neurotrophin loop | Threshold dose (mg/kg, ip) | Activation of post-receptor signaling cascades | Decrease in immobility time compared to the control group (%) | |||
|---|---|---|---|---|---|---|---|---|
| PI3K/Akt | MAPK/ERK | PLC-γ1 | Acute administration | Subchronic administration | ||||
| NGF | GK-2 [67] | 4 | 1.0 | ++ | ns | ++ | ns | 14 |
| GK-6 [67] | 1 | 2.0 | + | + | + | ns | 15 | |
| BDNF | GSB-106 [67] | 4 | 1.0 | + | ++ | ++ | 20 | 16 |
| GSB-214 [67] | 1 | 1.0 | + | ns | ++ | ns | 20 | |
| GTS-201 [67] | 2 | Not active | ns | + | ++ | ns | ns | |
| NT-3 | GTS-301 [33] | 4 | 10.0 | ns | ++ | ++ | ns | 27 |
| GTS-302 [36, 43] | 0.5 | ++ | ++ | + | 31 | 34 | ||
ip: intraperitoneally; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; MAPK/ERK: mitogen-activated protein kinase/extracellular signal-regulated kinase; PLC-γ1: phospholipase C-gamma; GK-2: bis-(monosuccinyl-L-glutamyl-L-lysine) hexamethylenediamide; GK-6: bis-(N-aminocaproyl-glycyl-L-lysine) hexamethylenediamide; GSB-106: bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide; GSB-214: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide; GTS-201: bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide; GTS-301: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide; GTS-302: bis-(N-gamma-oxybutyryl-L-glutamyl-L-asparagine) hexamethylenediamide; +: the effect is inferior to the full-length neurotrophin in terms of intensity and/or duration; ++: the effect is comparable to the full-length neurotrophin in terms of intensity and duration; ns: non-significant. All effects not labeled as “ns” are statistically significant. The data on the reduction in immobility time compared to the control group (%) are presented as means. The reference drug Amitriptyline (10.0 mg/kg, ip) reduced immobility time under the same conditions in various experiments up to 40%
A range of clinical and experimental data indicates the involvement of BDNF and NT-3 in the pathogenesis of anxiety disorders [17, 73, 74]. We evaluated the potential anxiolytic activity of dipeptide mimetics of BDNF and NT-3 through their acute administration (0.1–10.0 mg/kg, ip) in the elevated plus maze test in mice.
Anxiolytic effects were observed in mimetics of the BDNF and NT-3 loops 4, which activate PI3K/Akt, MAPK/ERK, and PLC-γ [43, 75], as well as in the BDNF loop 2 mimetic (GTS-201), which targets MAPK/ERK and PLC-γ [75]. The BDNF loop 1 mimetic (GSB-214), which activates PI3K/Akt and PLC-γ, and the NT-3 loop 4 mimetic (GTS-301), which affects MAPK/ERK and PLC-γ, were ineffective [75].
Data on the anxiolytic activity of dipeptide mimetics of BDNF and NT-3 are summarized in Table 5.
Anxiolytic activity of BDNF and NT-3 dipeptide mimetics in the elevated plus maze test in mice
| Parent neurotrophin | Code | Neurotrophin loop | Threshold dose (mg/kg, ip) | Activation of post-receptor signaling cascades | Increase in the time spent in open arms compared to the control group (times) | ||
|---|---|---|---|---|---|---|---|
| PI3K/Akt | MAPK/ERK | PLC-γ1 | |||||
| BDNF | GSB-106 [75] | 4 | 1.0 | + | ++ | ++ | 6.2 |
| GSB-214 [75] | 1 | Not active | + | ns | ++ | ns | |
| GTS-201 [75] | 2 | 1.0 | ns | + | ++ | 3.6 | |
| NT-3 | GTS-301 | 4 | Not active | ns | ++ | ++ | ns |
| GTS-302 [43] | 1.0 | ++ | ++ | + | 1.9 | ||
ip: intraperitoneally; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; MAPK/ERK: mitogen-activated protein kinase/extracellular signal-regulated kinase; PLC-γ1: phospholipase C-gamma; GSB-106: bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide; GSB-214: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide; GTS-201: bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide; GTS-301: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide; GTS-302: bis-(N-gamma-oxybutyryl-L-glutamyl-L-asparagine) hexamethylenediamide; +: the effect is inferior to the full-length neurotrophin in terms of intensity and/or duration; ++: the effect is comparable to the full-length neurotrophin in terms of intensity and duration; ns: non-significant. All effects not labeled as “ns” are statistically significant. The data on the increase in the time spent in open arms compared to the control group are presented as means. The reference drug Diazepam (1.0 mg/kg, ip) increased the time spent in open arms under the same conditions by 3.2 times
A large body of evidence has accumulated regarding the roles of NGF, BDNF, and NT-3 as regulators of plastic changes in the brain that underlie memory processes [76–78]. We investigated the memory-enhancing activity of dipeptide mimetics of NGF, BDNF and NT-3 following acute administration (0.1–10.0 mg/kg, ip) in the novel object recognition test in rats. Testing was performed 1 h and 24 h after the rats were familiarized with the objects to assess short-term and long-term memory, respectively. It was found that the NGF loop 4 mimetic (GK-2) and the BDNF loop 1 mimetic (GSB-214), which activate PI3K/Akt and PLC-γ without affecting MAPK/ERK, improved long-term memory in this test without influencing short-term memory [79]. The NT-3 loop 4 mimetics GTS-301, which activates MAPK/ERK and PLC-γ, and GTS-302, which activates PI3K/Akt, MAPK/ERK, and PLC-γ, improved both short-term and long-term memory [43].
The NGF loop 1 mimetic (GK-6) and the BDNF loop 4 mimetic (GSB-106), which activate all three major signaling cascades, as well as the BDNF loop 2 mimetic (GTS-201), which activates MAPK/ERK and PLC-γ, were found to be inactive [79].
The data on the memory-enhancing activity of dipeptide mimetics of NGF, BDNF, and NT-3 are summarized in Table 6.
Memory-enhancing activity of dipeptide mimetics of BDNF and NT-3 in the novel object recognition test in rats
| Parent neurotrophin | Code | Neurotrophin loop | Threshold dose (mg/kg, ip) | Activation of post-receptor signaling cascades | Memory-enhancing activity | ||
|---|---|---|---|---|---|---|---|
| PI3K/Akt | MAPK/ERK | PLC-γ1 | |||||
| NGF | GK-2 [79] | 4 | 0.5 | ++ | ns | ++ | Improves long-term memory |
| GK-6 [79] | 1 | Not active | + | + | + | ns | |
| BDNF | GSB-106 [79] | 4 | Not active | + | ++ | ++ | ns |
| GSB-214 [79] | 1 | 0.1 | + | ns | ++ | Improves long-term memory | |
| GTS-201 [79] | 2 | Not active | ns | + | ++ | ns | |
| NT-3 | GTS-301 | 4 | 1.0 | ns | ++ | ++ | Improves short- and long-term memory |
| GTS-302 [43] | 1.0 | ++ | ++ | + | Improves short- and long-term memory | ||
ip: intraperitoneally; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; MAPK/ERK: mitogen-activated protein kinase/extracellular signal-regulated kinase; PLC-γ1: phospholipase C-gamma; GK-2: bis-(monosuccinyl-L-glutamyl-L-lysine) hexamethylenediamide; GK-6: bis-(N-aminocaproyl-glycyl-L-lysine) hexamethylenediamide; GSB-106: bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide; GSB-214: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide; GTS-201: bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide; GTS-301: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide; GTS-302: bis-(N-gamma-oxybutyryl-L-glutamyl-L-asparagine) hexamethylenediamide; +: the effect is inferior to the full-length neurotrophin in terms of intensity and/or duration; ++: the effect is comparable to the full-length neurotrophin in terms of intensity and duration; ns: non-significant. All effects not labeled as “ns” are statistically significant. The reference drug Piracetam (200.0 mg/kg, ip), under the same conditions, improved only short-term memory
The observed improvement in only long-term memory following acute administration of NGF and BDNF mimetics aligns with the literature on the effects of full-length neurotrophins. Thus, full-length NGF and BDNF enhance long-term memory in rats in the novel object recognition test and the inhibitory avoidance task, without affecting short-term memory [80–82]. Moreover, blocking NGF or BDNF with antibodies in the novel object recognition test results in impaired long-term memory while leaving short-term memory unaffected [83]. In our study, a similar trend was observed with NGF and BDNF mimetics: active dipeptides improved only long-term memory. Interestingly, activity was noted exclusively for mimetics that activated the PI3K/Akt and PLC-γ pathways without affecting the MAPK/ERK. The PI3K/Akt cascade is known to play a crucial role in long-term memory formation through its downstream component, mTOR, a key factor in memory consolidation [84]. Meanwhile, the MAPK/ERK pathway is also well-established in long-term memory formation by activating transcription factors such as CREB, which are associated with the expression of proteins involved in neuroplasticity [85]. The absence of memory enhancement in NGF and BDNF dipeptide mimetics that activate both PI3K/Akt and MAPK/ERK signaling warrants further investigation to elucidate the underlying reasons.
NGF, BDNF, and NT-3 play significant roles in the regulation of pain sensitivity [86–88]. Experimental and clinical research has shown that NGF enhances pain sensitivity across various routes of administration, presenting a challenge for its therapeutic use [86]. BDNF, on the other hand, exhibits dual effects depending on the dose and method of delivery, acting as either pronociceptive or antinociceptive. Specifically, BDNF demonstrates analgesic properties when administered directly into the midbrain, near the periaqueductal gray and dorsal raphe nuclei, reducing responses to thermal and chemical (formalin) stimuli [88, 89]. This analgesic effect is linked to elevated levels of neurotransmitters, including endogenous opioids, in both the brain and spinal cord. However, intrathecal administration of BDNF has been shown to produce pronociceptive effects [90, 91]. NT-3 predominantly exhibits antinociceptive activity [92–94], although a few studies have reported increased pain sensitivity following exogenous NT-3 administration [95].
The potential impact of neurotrophin dipeptide mimetics on pain sensitivity was examined following their acute administration (0.01–10.0 mg/kg, ip) using the tail-flick test in rats. Among the NGF mimetics, the dipeptide GK-2, which activates PI3K/Akt and PLC-γ, exhibited analgesic effect, which was observed 0.5 h after administration and persisted for 24 h [37]. This effect may be related to partial agonistic activity of GK-2 towards NGF. In contrast, the dipeptide GK-6, which activates PI3K/Akt, MAPK/ERK, and PLC-γ, had the opposite effect, lowering pain thresholds as noted at 1 h and 24 h post-administration [37]. These findings are consistent with literature describing the predominant role of the MAPK/ERK cascade in the development of hyperalgesia upon TrkA receptor activation [96]. Interestingly, the mutant NGF, which lacks hyperalgesic properties, activates PI3K/Akt and, compared to the native neurotrophin, induces only weak activation of MAPK/ERK [97].
Among the BDNF mimetics, the dipeptide GSB-106, which activates PI3K/Akt, MAPK/ERK, and PLC-γ, exhibited the most pronounced analgesic activity [98]. This dipeptide significantly increased pain thresholds from 0.5 h to 48 h after administration. The dipeptide GSB-214, which activates PI3K/Akt and PLC-γ, had no effect on pain sensitivity. These results suggest the involvement of the MAPK/ERK signaling pathway in the analgesic effects of BDNF mimetics, a hypothesis confirmed through inhibitory pharmacological analysis. Inhibition of the MAPK/ERK pathway abolished the analgesic activity of GSB-106, whereas blockade of the PI3K/Akt pathway had no impact on this effect (Kolik L, et al. unpublished data).
The results for NT-3 mimetics resembled those observed with BDNF mimetics. The dipeptides GTS-301 and GTS-302—the former activating MAPK/ERK and PLC-γ, and the latter activating all three major signaling cascades—exhibited analgesic effects that became evident 30 min after administration and persisted for 24 h. Notably, the effect of GTS-301 was more pronounced [99].
The data on the effects of dipeptide mimetics of NGF, BDNF, and NT-3 on pain sensitivity are summarized in Table 7.
The effects of dipeptide mimetics of NGF, BDNF, and NT-3 on pain sensitivity in the tail flick and hot plate tests in mice and rats
| Parent neurotrophin | Code | Neurotrophin loop | Threshold dose, (mg/kg, ip) | Activation of post-receptor signaling cascades | Pain threshold compared to the control group 24 h after administration of the compound (%) | ||
|---|---|---|---|---|---|---|---|
| PI3K/Akt | MAPK/ERK | PLC-γ1 | |||||
| NGF | GK-2 [37] | 4 | 1.0 | ++ | ns | ++ | 144(analgesia) |
| GK-6 [37] | 1 | 2.0 | + | + | + | 62(algesia) | |
| BDNF | GSB-106 [98] | 4 | 0.1 | + | ++ | ++ | 144(analgesia) |
| GSB-214 | 1 | Not active | + | ns | ++ | ns | |
| NT-3 | GTS-301 [99] | 4 | 0.01 | ns | ++ | ++ | 120(analgesia) |
| GTS-302 | 0.1 | ++ | ++ | + | 110(analgesia) | ||
ip: intraperitoneally; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; MAPK/ERK: mitogen-activated protein kinase/extracellular signal-regulated kinase; PLC-γ1: phospholipase C-gamma; GK-2: bis-(monosuccinyl-L-glutamyl-L-lysine) hexamethylenediamide; GK-6: bis-(N-aminocaproyl-glycyl-L-lysine) hexamethylenediamide; GSB-106: bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide; GSB-214: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide; GTS-301: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide; GTS-302: bis-(N-gamma-oxybutyryl-L-glutamyl-L-asparagine) hexamethylenediamide; +: the effect is inferior to the full-length neurotrophin in terms of intensity and/or duration; ++: the effect is comparable to the full-length neurotrophin in terms of intensity and duration; ns: non-significant. All effects not labeled as “ns” are statistically significant. Effects of mimetics on pain thresholds are presented as average values. Morphine, used as a reference drug, increased pain thresholds by 200% in the same experimental setup, and this effect persisted for 1 h after its administration
Thus, a preliminary conclusion can be drawn regarding the predominant role of the MAPK/ERK signaling cascade in the pronociceptive activity of NGF mimetics and the antinociceptive activity of BDNF and NT-3 mimetics.
Thus, we have developed dimeric dipeptide mimetics of the neurotrophins NGF, BDNF, and NT-3. These compounds exhibit distinct patterns of activation of the post-receptor signaling cascades of Trk receptors, specific to their parent neurotrophins. All mimetics activated the PLC-γ cascade, with some also activating the PI3K/Akt and MAPK/ERK pathways, while others activated only one of them. The mimetics demonstrated diverse sets of pharmacological activities in vivo following systemic administration (0.1–10 mg/kg). Using these mimetics, we identified correlations between different pharmacological activities and the specific patterns of post-receptor signaling, as well as the nature of the parent neurotrophin.
It was found that all dipeptides exhibited neuroprotective activity in vitro, indicating that activation of either PI3K/Akt and PLC-γ, or MAPK/ERK and PLC-γ, mediated by any type of Trk receptor, is sufficient. In in vivo experiments on a rat model of ischemic stroke, PI3K/Akt activation was shown to be essential for the neuroprotective effects of NGF, BDNF, and NT-3 mimetics—compounds that did not activate this pathway had no impact on infarct size. This is consistent with literature data on the neuroprotective roles of PI3K/Akt [1].
The PI3K/Akt signaling pathway was also found to be essential for the antidiabetic activity of NGF and BDNF mimetics, which, in our studies, was likely associated with the increased viability of pancreatic β-cells. The involvement of PI3K/Akt in the antidiabetic effects was confirmed through pharmacological inhibitor analysis.
Antidepressant-like activity was notably stronger in BDNF and NT-3 mimetics than in NGF mimetics, with the most significant effects seen in compounds that simultaneously activated all three major post-receptor signaling cascades. These compounds exhibited robust antidepressant-like effects even after a single administration. Pharmacological inhibitor analysis highlighted the necessity of activating all three pathways—PI3K/Akt, MAPK/ERK, and PLC-γ—for their efficacy.
A clear association between the MAPK/ERK signaling cascade and pain sensitivity was demonstrated. In the case of NGF mimetics, activation of MAPK/ERK led to increased pain sensitivity, while a mimetic that selectively activated only PI3K/Akt and PLC-γ exhibited analgesic effects. This aligns with literature reports indicating that a mutant form of NGF, which does not induce hyperalgesia, activates PI3K/Akt and PLC-γ but triggers MAPK/ERK signaling only weakly compared to the native protein [96]. For BDNF and NT-3 mimetics, the presence of MAPK/ERK in the pattern of post-receptor signaling activation correlated with analgesic activity, whereas its absence was associated with a lack of effect on pain sensitivity. The contribution of MAPK/ERK activation to the analgesic activity of BDNF mimetics was confirmed through pharmacological inhibition analysis.
The data on the identified correlations are summarized in Table 8.
The relationships between the diverse pharmacological activities of NGF, BDNF, and NT-3 dipeptide mimetics, their patterns of post-receptor signaling activation, and the nature of the parent neurotrophins
| Activation patterns of post-receptor signaling pathways of Trk receptors by dipeptide mimetics of neurotrophins | 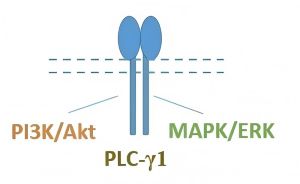 |  |  | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parent neurotrophins | NGF | BDNF | NT-3 | NGF | BDNF | BDNF | NT-3 | ||
| Dipeptide mimetic | GTS-115 | GK-61 | GSB-106 | GTS-302 | GK-2 | GSB-214 | GTS-201 | GTS-301 | |
| Neuroprotective activity | In vitro | + | + | + | + | + | + | + | + |
| In vivo | + | ns | ++ | + | ++ | + | ns | ns | |
| Antidiabetic activity | Not assessed | Not assessed | + | Not assessed | ++ | ++ | ns | + | |
| Memory-enhancing activity | Not assessed | ns | ns | + | + | + | ns | + | |
| Antidepressant-like | Not assessed | + | ++ | ++ | + | + | ns | + | |
| Anxiolytic activity | Not assessed | not assessed | + | + | Not assessed | ns | + | ns | |
| Effect on pain sensitivity | Not assessed | ↑ | ↓ | ↓ | ↓ | ns | Not assessed | ↓ | |
NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; ns: non significant; GK-2: bis-(monosuccinyl-L-glutamyl-L-lysine) hexamethylenediamide; GK-6: bis-(N-aminocaproyl-glycyl-L-lysine) hexamethylenediamide; GTS-115: bis-(N-gamma-oxybutyryl-L-lysyl-L-histidine) hexamethylenediamide; GSB-106: bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide; GSB-214: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide; GTS-201: bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide; GTS-301: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide; GTS-302: bis-(N-gamma-oxybutyryl-L-glutamyl-L-asparagine) hexamethylenediamide. All effects not labeled as “ns” are statistically significant. ↑: increase in pain thresholds; ↓: decrease in pain thresholds; 1 Although NGF mimetics GTS-115 and GK-6 both activated all major post-receptor signaling cascades of Trk receptors, GK-6, in contrast to GTS-115, induced only short-term activation of the PI3K/Akt pathway. This presumably explains the lack of neuroprotective activity of GK-6 in vivo
Currently, peptide drugs have become a focal point of global interest due to their distinct advantages over non-peptide compounds, such as high specificity, safety, low possibility of generating resistance [100]. Dipeptide mimetics of neurotrophins hold significant promise as potential therapeutics for post-stroke conditions, neurodegenerative diseases, diabetes, depression, anxiety disorders, and more.
Akt: threonine-protein kinase
BDNF: brain-derived neurotrophic factor
CREB: cAMP response element-binding protein
D3: tavilermide
ERK: extracellular signal-regulated kinase
GK-2: bis-(monosuccinyl-L-glutamyl-L-lysine) hexamethylenediamide
GK-6: bis-(N-aminocaproyl-glycyl-L-lysine) hexamethylenediamide
GSB-106: bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide
GSB-214: bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide
GTS-115: bis-(N-gamma-oxybutyryl-L-lysyl-L-histidine) hexamethylenediamide
GTS-201: bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide
GTS-301: bis-(N-monosuccinyl-L-asparaginyl-L-asparagine) hexamethylenediamide
GTS-302: bis-(N-gamma-oxybutyryl-L-glutamyl-L-asparagine) hexamethylenediamide
ip: intraperitoneally
MAPK: mitogen-activated protein kinase
mTOR: mammalian target of rapamycin
NGF: nerve growth factor
NT-3: neurotrophin-3
PI3K: phosphoinositide 3-kinase
PLC-γ: phospholipase C-gamma
Trk: tyrosine kinase
TG: Conceptualization, Writing—review & editing. PP: Writing—original draft. VD: Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This work was conducted under the government contracts of the Ministry of Science and Higher Education of the Russian Federation [FGFG-2025-0006 and FGFG-2025-0008]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2238
Download: 52
Times Cited: 0
