Affiliation:
1U.O. di Medicina Interna Metabolica Nutrizionale, Azienda Ospedaliero Universitaria di Modena, 41124 Modena, Italy
2Dipartimento di Scienze Mediche e Chirurgiche Materno-Infantili e dell’Adulto, Università degli Studi di Modena e Reggio Emilia, 41121 Modena, Italy
ORCID: https://orcid.org/0000-0001-8590-6757
Affiliation:
1U.O. di Medicina Interna Metabolica Nutrizionale, Azienda Ospedaliero Universitaria di Modena, 41124 Modena, Italy
2Dipartimento di Scienze Mediche e Chirurgiche Materno-Infantili e dell’Adulto, Università degli Studi di Modena e Reggio Emilia, 41121 Modena, Italy
Affiliation:
1U.O. di Medicina Interna Metabolica Nutrizionale, Azienda Ospedaliero Universitaria di Modena, 41124 Modena, Italy
2Dipartimento di Scienze Mediche e Chirurgiche Materno-Infantili e dell’Adulto, Università degli Studi di Modena e Reggio Emilia, 41121 Modena, Italy
ORCID: https://orcid.org/0000-0002-3842-4701
Affiliation:
1U.O. di Medicina Interna Metabolica Nutrizionale, Azienda Ospedaliero Universitaria di Modena, 41124 Modena, Italy
2Dipartimento di Scienze Mediche e Chirurgiche Materno-Infantili e dell’Adulto, Università degli Studi di Modena e Reggio Emilia, 41121 Modena, Italy
Affiliation:
1U.O. di Medicina Interna Metabolica Nutrizionale, Azienda Ospedaliero Universitaria di Modena, 41124 Modena, Italy
2Dipartimento di Scienze Mediche e Chirurgiche Materno-Infantili e dell’Adulto, Università degli Studi di Modena e Reggio Emilia, 41121 Modena, Italy
Email: pietro.andreone@unimore.it
ORCID: https://orcid.org/0000-0002-4794-9809
Explor Drug Sci. 2023;1:292–298 DOI: https://doi.org/10.37349/eds.2023.00020
Received: March 29, 2023 Accepted: May 28, 2023 Published: August 30, 2023
Academic Editor: Fernando Albericio, Universities of KwaZulu-Natal, South Africa; Universidad de Barcelona, Spain
The article belongs to the special issue Innovative Therapeutics in Hepato-Gastroenterology
Thrombocytopenia is one of the most frequent implications of liver cirrhosis. This condition, when present in the severe form [platelet count (PLT) less than 50 × 109/L] correlates, with an increased risk of bleeding during the main diagnostic-therapeutic procedures which cirrhotic patients usually undergone. In these cases, generally, an infusion of platelets is performed, albeit in recent years has been replaced by a cycle of second generation thrombopoietin receptor (TpoR) agonists. This article reports two different cases concerning respectively an 83-year-old female patient suffering from arterial hypertension, aneurysm of the sub-renal aorta, hepatitis C virus (HCV)-positive liver cirrhosis responsive to treatment with antiviral drugs, and a 2.0 cm diameter hepatocellular carcinoma (HCC) nodule localized in the hepatic segment III and a 53-year-old female patient with HCV-positive liver cirrhosis complicated by portal hypertension with splenomegaly, thrombocytopenia, and F3 esophageal varices at high risk of bleeding. Both of them, eligible for invasive procedures such as HCC transarterial chemoembolization (TACE) and for esophageal variceal band ligation, were prescribed prophylaxis with TpoR agonists due to their severe and persistent thrombocytopenia. These two cases show how a short course of lusutrombopag allows to safely perform one or more invasive procedures and how the administration of the drug can be repeated without losing efficacy. Furthermore, this drug shows an excellent safety profile and avoids the risks of platelet transfusion. In conclusion, second generation TpoR agonists can be considered the prophylactic treatment of choice to reduce the risk of bleeding in patients with liver cirrhosis and severe thrombocytopenia.
Thrombocytopenia, defined as platelet counts (PLTs) below 150 × 109/L, is one of the most common complications of liver cirrhosis. This condition is present in almost 80% of patients with advanced chronic liver disease and is conventionally classified into mild (< 150 × 109/L), moderate (< 100 × 109/L), and severe (< 50 × 109/L). Thrombocytopenia, from a pathophysiological perspective, is the result of: a) increased splenic sequestration due to portal hypertension, b) decrease in thrombopoietin production because of reduced liver synthesis capacity, and c) suppression of megakaryocytopoiesis [1, 2]. Thrombocytopenia, together with changes in platelet function and the coagulative/fibrinolytic system, is the basis of a complex hemostasis disorder frequently seen in subjects suffering from advanced liver disease. Unlike the healthy subject, in the cirrhotic patient, the hemostasis system presents a labile balance. This equilibrium can be destabilized, both in the hemorrhagic and thrombotic outcome, by various triggering factors (e.g., infections, bleeding of esophageal varices, refractory ascites, and invasive diagnostic-therapeutic procedures) [3].
There is a limited number of tests available to study the coagulative status in liver cirrhosis. Those used in clinical practice are: international normalized ratio (INR) which, if lengthened, correlates with a reduction in the plasma concentration of coagulative factors synthesized by the liver; PLT which gives us information on the number of platelets, but not in their function, and a reduction of the count is considered an indicator of increased bleeding risk [3, 4]. In clinical practice, subjects with advanced liver disease have increased INR values (between 1.5–2.0) and a PLT below 50 × 109/L. These subjects, not infrequently, have to undergo repeated invasive procedures such as esophageal variceal band ligation (EVBL), loco-regional treatments of hepatocellular carcinoma (HCC), liver biopsies, and others [5]. In the literature, there aren’t prospective studies, in patients with liver cirrhosis, showing the minimum platelets’ cut-off to reduce the risk of bleeding when the most common diagnostic and therapeutic procedures need to be performed [6]. Since there are only retrospective studies on the minimum cut-off PLT to reduce the risk of bleeding, guidelines from various scientific societies state that platelet transfusion can be considered in patients with low PLTs prior to an invasive procedure [4, 6]. Consequently, the indication for prophylactic platelet transfusion in patients with counts below 50 × 109/L is personalized and based both on the bleeding risk of the procedure and the patient’s clinical condition [6].
In recent years, platelet transfusion can be avoided by treating patients with the second generation thrombopoietin receptor (TpoR) agonists (TpoR, lusutrombopag, and avatrombopag) [1]. These drugs have the ability to stimulate the bone marrow to produce platelets, resulting in a transient increase in PLT [4]. Specifically, lusutrombopag is a drug to be taken orally at a dosage of 3 mg/day for 7 days [3]. The pivotal studies of this drug show that the median peak PLT and maintenance, above the threshold, is on day 12 and 19 from the start of administration, respectively [3]. Therefore, in patients undergoing elective invasive procedures, the need for platelet transfusion is unnecessary. Furthermore, the drug showed no safety concerns on thrombotic events, in particular with regard to the development of portal vein thrombosis. These studies evaluated the efficacy in reducing the need for platelet transfusions in subjects with advanced liver disease and severe thrombocytopenia undergoing invasive procedures [7]. Unfortunately, there is no information, even in real life, on the efficacy and tolerability of TpoR drugs in repeated use or in the case of multiple procedures following a single therapeutic cycle [7, 8].
In this paper, the cases of two patients undergoing repeated invasive procedures after single or multiple courses of lusutrombopag therapy are described.
This is the case of an 83-year-old female patient suffering from arterial hypertension, aneurysm of the sub-renal aorta, and hepatitis C virus (HCV)-positive liver cirrhosis responsive to treatment with antiviral drugs. Liver cirrhosis was complicated by portal hypertension with splenomegaly, severe thrombocytopenia, F3 esophageal varices, and moderate congestive gastropathy. A further complication was the finding of a 2.0 cm diameter HCC nodule localized in the hepatic segment III diagnosed by computed tomography and magnetic resonance imaging. In addition, at the age of 79, she developed a prior HCC at hepatic segment VII that was treated with radiofrequency ablation. In the past, the patient had episodes of porto-systemic encephalopathy.
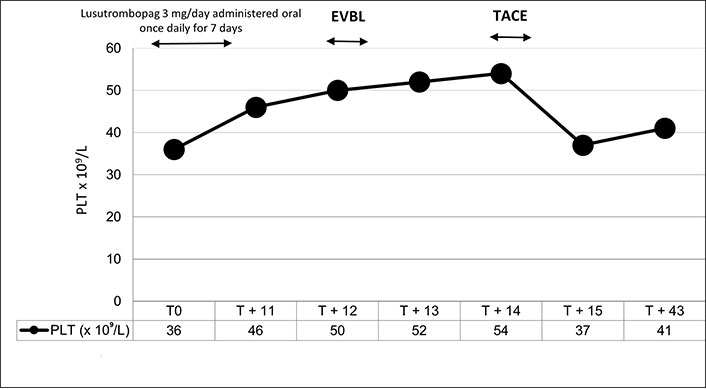
The patient was deemed eligible for HCC transarterial chemoembolization (TACE) and for EVBL due to their high risk of bleeding and the failure of propranolol therapy. Considering the severe thrombocytopenia, the patient has had a PLT consistently lower than 40 × 109/L in the last 6 months, a prophylaxis with TpoR was prescribed. Specifically, the patient took lusutrombopag 3 mg/day for 7 days and on day 12 the PLT was 50 × 109/L (Figure 1). On that date, EVBL was performed in the absence of peri- and post-procedural complications. On day 13 the PLT was 52 × 109/L and TACE was performed on day 14 without platelet transfusion. In addition, this procedure was without complications. On day 43, on the occasion of clinical re-evaluation, the PLT was 41 × 109/L and, therefore, in line with the values before lusutrombopag treatment.
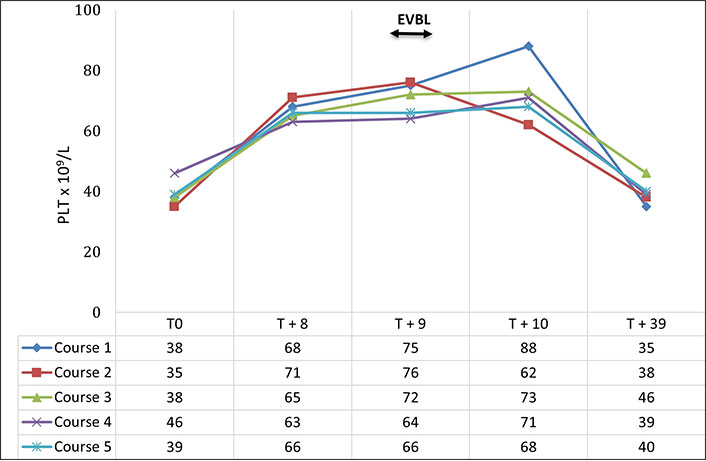
A 53-year-old female patient was evaluated in the outpatient clinic because of HCV-positive liver cirrhosis complicated by portal hypertension with splenomegaly, thrombocytopenia, and F3 esophageal varices at high risk of bleeding. Given the failure of non-cardioselective beta-blocker treatments (propranolol and carvedilol), the patient was considered eligible for EVBL, before which prophylaxis with TpoR agonists was prescribed due to the severe thrombocytopenia. Due to the difficulty in eradicating the esophageal varices, 5 band ligation sessions were scheduled preceded by 5 courses of lusutrombopag administration (3 mg/day for 7 days) in 10 months (Figure 2). The patient had a baseline PLT consistently below 50 × 109/L (from 35 × 109/L to 46 × 109/L). The first day after stopping each course of lusutrombopag treatment (day 8), the PLT was always greater than 60 × 109/L (from 63 × 109/L to 71 × 109/L). After all 5 treatments, the EVBL was performed on day 9 without platelet transfusion. On day 10 a further PLT was performed showing a value greater than 60 × 109/L (from 62 × 109/L to 88 × 109/L). There were no peri- and post-procedural complications. The PLT performed 30 days after the procedures was always less than 50 × 109/L (from 35 × 109/L to 46 × 109/L). Finally, the patient underwent serial abdominal ultrasound scans and none of these showed the presence of portal thrombosis.
Trend in the number of platelets after treatment with lusutrombopag 3 mg/day for 7 days
The second generation TpoR provides a tool that allows to avoid platelet transfusion in patients with chronic liver disease who require invasive diagnostic or therapeutic procedures [4]. Platelet transfusion, indeed, is an expensive and complex operation as it must be performed a few hours before the procedure and, above all, not without complications for the patient [9]. Specifically, the main adverse reactions are represented by transfusion-related reactions, such as hemolysis in case of incompatibility of the AB0 blood groups between the donor and recipient [10], anaphylactic shock or allergic reactions [11], and infectious risk given by the presence of viruses or bacteria in the transfused platelet pool which, in rare cases, can even lead to death [11]. Furthermore, the transfused platelets have both a limited temporal action, as they are degraded more rapidly than those produced by the subject, and a reduced function, as the non-self platelets lose their ability to form the platelet plug [12, 13]. Platelet transfusion, however, remains the gold standard in patients who need urgent procedures [4].
In this paper, two emblematic case reports are described where a subject was treated with a single course of lusutrombopag and subjected to two different invasive procedures, while another received repeated courses of the drug before 5 rounds of EVBL.
In the first case, there is evidence that after a single treatment with lusutrombopag, the number of platelets remains above 50 × 109/L for several days before returning to baseline values, allowing to carry out more procedures with a single treatment. This opportunity has a positive impact on planning hospital admissions as well as preventing the risk of adverse events and/or reduced efficacy of close platelet transfusions. Furthermore, the efficacy and tolerability of lusutrombopag in 83-year-old patient are reported. About this topic in the registration studies, the mean age of enrolled subjects was lower and respectively 55.2, 55.7, and 68.9-year-old [7, 8]. Considering that many of the cirrhotic subjects in the world are of advanced age, we believe that data on the safety and efficacy of lusutrombopag in older populations are welcome for a generalized use of this drug.
In the second case, it was demonstrated that repeated treatments with lusutrombopag continue to maintain an excellent efficacy, in terms of PLT, and are associated with a high safety profile. In this latter regard, thrombotic events in the abdominal venous system have never occurred.
To date, literature presents few data related to the use of second generation TpoR. However, there is a general consensus about the excellent response in terms of PLT increase, with a high safety profile both using single and multiple treatments [14–16]. There is, however, no agreement from scientific societies on the use of platelet transfusions or TpoRs in subjects with chronic liver disease and severe thrombocytopenia that are candidates for invasive procedures [4, 6, 17–20]. This disagreement is due to the fact that to date we do not have: a) randomized controlled trials on the risk of bleeding among patients treated with platelet transfusion or TpoR compared with an untreated control group, b) available tests that allow us to stratify the bleeding risk during and after invasive procedures. In the latter, viscoelastic tests are being studied, but at the moment there are insufficient data to demonstrate their effectiveness. Due to the lack of these data, the recent indications of the European Association for the Study of the Liver (EASL) postpone the decision based on the evaluation of clinical conditions and haemocoagulation profile of the individual patient [6]. The results of this experience are limited, retrospective, and not adequate to make up for the discordant guidelines of scientific societies but they can be a useful reference in the management of patients for whom it may be difficult to identify the most appropriate treatment.
In conclusion, given the absence of tools that define the risk of bleeding, TpoR can represent the treatment of choice for the prophylaxis of cirrhotic patients with severe thrombocytopenia, who must electively undergo invasive procedures at a high risk of bleeding. This approach permits to avoid platelet transfusion and overall related risks [21].
EVBL: esophageal variceal band ligation
HCC: hepatocellular carcinoma
HCV: hepatitis C virus
PLT: platelet count
TACE: transarterial chemoembolization
TpoR: thrombopoietin receptor
The authors want to thank Silvia Anichini, PhD, freelance medical writer and Sanitanova for editorial assistance.
DS and PS: Conceptualization, Data curation, Formal analysis, Validation, Writing—original draft. CF and AR: Resources. PA: Conceptualization, Supervision, Funding acquisition, Project administration, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
In accordance with the constitutional regulation of the Ethics Council of the University of Modena and Reggio Emilia, ethical approval is not required for cases of less than 5 persons. The study was conducted in accordance with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Data can be obtained from the corresponding author upon reasonable request (Pietro Andreone, pietro.andreone@unimore.it).
An unconditional grant for this study was provided by
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2758
Download: 24
Times Cited: 0
Elena Franchi ... Pietro Andreone
Matteo Biagi ... Pietro Andreone
