Abstract
Pharmaceutical interventions play a vital role in managing various conditions, including weight-related issues such as obesity. In this context, lifestyle changes are often challenging to maintain, especially for individuals struggling with this condition. Obesity is strongly linked to serious health conditions like cardiovascular disease and insulin resistance, leading to a cascade of health risks. Importantly, the development of effective and safe weight loss medications has been challenging. Diabetes mellitus (DM), the incidence of which is also rising, is closely related to obesity. The annual rate of DM cases has increased significantly, mirroring trends in obesity. Pharmaceutical companies have made significant progress in developing drugs that address both diabetes and obesity. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have emerged as a promising class of medications with dual benefits in managing diabetes and aiding weight loss such as semaglutide, liraglutide, dulaglutide, exenatide, among others. However, despite their effectiveness, they can be expensive. The availability of various GLP-1RAs offers flexibility in diabetes management, but the surge in their prescription has led to a global shortage. Health authorities are working to address this issue, while pharmaceutical companies are exploring new paths to improve the quality of these drugs. In this context, tirzepatide stands out as a medication targeting key hormones involved in obesity and DM. Another potential breakthrough, retatrutide, is also being developed for these two conditions, but it requires further research. In this paper, the authors address all the GLP-1RA options developed to date, covering their mechanisms of action, efficacy, and chemical structures, among other aspects.
Keywords
Glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, obesity, diabetes mellitus, tirzepatide, semaglutide, liraglutide, peptidesIntroduction
Pharmaceutical interventions play a vital role in the treatment of various medical conditions. In the context of obesity, they can facilitate the management of the condition and also the physiological imbalances such as changes in weight. Such interventions are particularly pertinent since lifestyle modifications, which are a fundamental approach to addressing such conditions, can be challenging. Of note, individuals with obesity, a significant public health concern [1], often encounter difficulties in sustaining lifestyle changes.
Overweight and obesity are prevalent health issues affecting millions of individuals worldwide, including adults and children. The World Health Organization (WHO) defines these conditions as the accumulation of excess body fat, which can be defined using the body mass index (BMI). Several thresholds can be used to classify individuals as overweight or obese, and they vary for adults and children [2]. Recent estimates indicate a concerning trend and suggest that approximately half the global population may be affected by overweight and obesity within the next seven years. This trend is observed across both developed and developing countries, with the latter projected to experience a higher burden of these conditions [2–4].
An essential issue pertaining to obesity and overweight, supported by existing literature and meta-analyses, is their association with various conditions, notably cardiovascular disease and insulin resistance [5–8]. Obesity and overweight give rise to an obesity cascade, whereby having one of these conditions not only increases the incidence of dyslipidemia, which includes high low-density lipoprotein (LDL) cholesterol and triglyceride levels but also heightens the risk of developing diabetes mellitus (DM), which subsequently leads to an increased susceptibility to coronary heart disease and ultimately culminates in a heightened risk of stroke or myocardial infarction [6, 7].
In this context, the development of effective and safe pharmacotherapies for weight loss and obesity has been a complex and arduous process. Throughout the history of obesity pharmacotherapy, numerous instances of drug withdrawals have been documented as a result of adverse events [9, 10]. There are several categories of weight management drugs, including hormones, guanidines, hexose derivatives, amphetamine analogs, and others, each one with distinct mechanisms of action. Many of these drugs have multiple effects, and a notable one common to a number of them is the stimulation of energy expenditure, which is an effective mechanism for weight loss. In this regard, some drugs have been withdrawn from the market, for instance, GW501516 and 2,4-dinitrophenol [7].
Like obesity, the occurrence and prevalence of DM is also increasing. DM causes insulin resistance, vascular complications, and defective pancreatic B-cells, among other failures [11, 12]. Moreover, this condition poses a significant risk factor for disabilities and can lead to death. There is an intrinsic interrelation between diabetes and obesity—in such a way that they are referred to as twin diseases in the literature addressing these two conditions—not solely due to obesity being a predisposing factor for the development of type 2 DM (T2DM) [13]. Beyond this, the annual incidence and prevalence rates of T2DM are steadily rising [11, 13], thus mirroring the patterns observed in obesity. In accordance with a study by Nanda et al. [14] in 2022 the incidence cases jumped from 8 million to 21 million approximately in 1990 and 2019, respectively. In the same period studied, the prevalence figure jumped from 141 million to 437 million. The WHO also correlates the rise of T2DM to the increase in obesity and overweight worldwide. WHO has undertaken action to assist individuals in preventing DM, which also includes actions for type 1 DM (T1DM) patients [15].
Over several decades, pharmaceutical companies have devoted efforts to interrelated diseases and conditions, such as overweight, obesity, and DM. An important breakthrough was the development of glucagon-like peptide-1 receptor agonists (GLP-1RAs), which bind the incretin hormone named glucagon-like peptide-1 (GLP-1) on their receptors, thereby contributing to the maintenance of glucose homeostasis in the body and the regulation of postprandial metabolism [16, 17]. Furthermore, the advancement of synthetic compounds as GLP-1RAs gained ground due to the incorporation of structural changes that made these compounds resistant to degradation by the enzyme dipeptidyl peptidase-4 (DPP-4), unlike native GLP-1 [18]. This class of drugs has been demonstrated to be highly effective but also more expensive than other anti-diabetes pharmacotherapies [insulin, sulfonylureas, pioglitazone, DPP-4 inhibitors, sodium-glucose co-transporter 2 (SGLT-2) inhibitors, and metformin] [19]. According to the European Medicines Agency, two of these medications, namely semaglutide and liraglutide, have been used for at least a year by an estimated 20 million patients [20].
Incretin hormones
GLP-1 and glucose-dependent insulinotropic polypeptide (GIP, also known as gastric inhibitory peptide) are two incretin hormones that play a pivotal role in modulating insulin release and regulating postprandial metabolism [21–23].
GLP-1 has a short action, with the first phase occurring 10 min to 15 min after a meal and the second 30 min to 60 min later. This hormone is secreted by cells expressed in the small intestine, namely in the ileum, immediately after food intake. It contributes to weight loss and glucose control, suppresses glucagon secretion, and decreases the rate of gastric emptying [18]. GLP-1 receptor (GLP-1R) is a class B family member of G-protein-coupled receptors [16–18, 23]. The satiety-inducing effect of GLP-1 exerted through its impact on the central nervous system (CNS) in the hypothalamus, has established a promising precedent for the use of GLP-1RAs in weight management. Importantly, this class of G-protein-coupled receptors is found not only within the pancreas and CNS but also throughout the peripheral nervous system, in pulmonary and renal tissues, among others [17, 24].
GLP-1 and GIP are secreted by enteroendocrine cells. However, they have distinct characteristics. The former elicits a satiety effect and promotes weight loss, whereas the latter alone may contribute to weight gain and promote cytokine expression. Unlike GLP-1, GIP is released mainly in cells located in the upper intestine and it exerts a dual function depending on the moment. It can stimulate glucagon secretion during episodes of hypoglycemia but can also release insulin and suppress glucagon release during episodes of hyperglycemia [23, 25, 26]. In addition to the recognized physiological functions and established significance of GIP, this incretin hormone plays a major role in obesity, as reflected by the observation that individuals with obesity show elevated levels of freely circulating GIP. Furthermore, an observable presence of K-cells, which are responsible for GIP production, has been documented in the intestines of patients with T2DM. Consequently, targeting GIP receptors (GIP-Rs) holds promise for mitigating fat accumulation and potentially offering therapeutic benefits for individuals with T2DM [27]. Therefore, GLP-1 and GIP have the potential to regulate the secretion of insulin, which is an extremely important hormone as it is also essential for good blood flow and promotes vessel relaxation [16, 27–31].
Despite their apparent divergent functions and features, these incretin hormones work synergistically to facilitate metabolic regulation. GIP-R agonists (GIP-RAs) do not interfere with the effect of GLP-1Rs in lowering glucose levels and weight [27]. Therefore, the activation of the receptors of these two incretin hormones brings significant benefits to patients.
There are currently nine GLP-1RAs with authorization from the US Food and Drug Administration (FDA) for the treatment of overweight and/or obesity and T2DM [31, 32].
In chronological order (Table 1), the initial incretin mimetic compound introduced was the GLP-1RA exenatide, marketed first as ByettaTM by AstraZeneca and then by Bristol-Myers Squibb Company, and as BydureonTM by AstraZeneca. This drug was designated for the enhancement of glycemic regulation in adults diagnosed with T2DM [18, 33–36]. For ByettaTM, which is administered twice-daily 60 min before the two main meals, the mean peak of plasma concentration (211 pg/mL) is achieved in 2 h [37]. In 2017, Bydureon BCiseTM and BydureonTM, new versions of exenatide, were approved. The former requires fewer steps for administration and shows a mean plasma concentration of 208 pg/mL on week 10 [38], while the latter shows a peak plasma concentration of around 300 pg/mL after 6–7 weeks [39]. Some of the traits of BydureonTM and Bydureon BCiseTM differ considerably. For example, BydureonTM showed a higher percentage of nausea in clinical trials while Bydureon BCiseTM showed more frequent adverse reactions related to the injection site. Despite these observations, the difference in plasma drug levels may indicate a potential advantage of higher weight loss for BydureonTM over Bydureon BCiseTM [40]. Importantly, the only exenatides on the market are ByettaTM, administered twice-daily, and Bydureon BCise TM, once-daily, the other was discontinued.
GLP-1RAs approved by the FDA
| API and trade name | Original approval date | Administration | Therapeutic indication | References |
|---|---|---|---|---|
| Exenatide (ByettaTM) | Apr-28-2005 | Twice-daily 250 mcg/mL | To improve glycemic control in adults with T2DM | [33, 36] |
| Liraglutide (VictozaTM) | Jan-25-2010 | Once-daily 18 mg/3 mL (6 mg/mL) | To reduce the risk of major adverse cardiovascular events in T2DM adult patients, and an adjunct to diet and exercise to improve glycemic control in T2DM patients 10 years and older | [34] |
| Liraglutide (SaxendaTM) | Dec-23-2010 | Once-daily 18 mg/3 mL (6 mg/mL) | For chronic weight management in adult patients as an adjunct to a reduced-caloric diet and increased physical activity | [61, 62] |
| Exenatide1 (BydureonTM) | Jan-27-2012 | Once-weekly 2 mg/vial (extended-release) | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [39] |
| Lixisenatide (LyxumiaTM) | Jan-31-2013 | Once-daily 10 mcg/0.2 mL, or 20 mcg/0.2 mL | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [12, 52, 104] |
| Exenatide1 (Bydureon PenTM) | Feb-28-2014 | Once-weekly 2 mg (extended-release) | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [35] |
| Dulaglutide (TrulicityTM) | Sep-18-2014 | Once-weekly 1.5 mg/0.5 mL | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [46, 70] |
| Albiglutide1 (TanzeumTM) | Apr-15-2014 | Once-weekly 30 mg or 50 mg in a single-dose pen | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [48, 49] |
| Lixisenatide1 (AdlyxinTM) | Jul-28-2016 | Once-daily 50 mcg/mL or 100 mcg/mL | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [51] |
| Exenatide (Bydureon BCise TM) | Oct-20-2017 | Once-weekly 2 mg/0.85 mL (auto-injector) | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [38, 40, 105] |
| Semaglutide (OzempicTM) | Dec-5-2017 | Once-weekly 2 mg/1.5 mL (1.34 mg/mL), 4 mg/3 mL (1.34 mg/mL), or 8 mg/3 mL (2.68 mg/mL) | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [54, 56] |
| Semaglutide (RybelsusTM) | Sep-20-2019 | Once-daily/oral tablets: 3 mg, 7 mg, or 14 mg | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [106] |
| Semaglutide (WegovyTM) | Jun-4-2021 | Once-weekly 0.25 mg/0.5 mL, 1 mg/0.5 mL, 1.7 mg/0.75 mL, or 2.4 mg/0.75 mL | For chronic weight management in patients 12 years and older as an adjunct to a reduced-caloric diet and increased physical activity | [8, 55, 107] |
| Tirzepatide (MounjaroTM) | May-13-2022 | Once-weekly 2.5 mg/0.5 mL, 5 mg/0.5 mL, 7.5 mg/0.5 mL, 10 mg/0.5 mL, 12.5 mg/0.5 mL, or 15 mg/0.5 mL in single-dose pen | To improve glycemic control in adults with T2DM as an adjunct to diet and exercise | [86, 108] |
Data found in Table 1 was retrieved from the FDA databases, including the Orange Book, and the European Medicines Agency website. The availability of the mentioned drugs varies across countries, and not all dosage forms and strengths are universally accessible [109, 110]. 1 Status discontinued. API: active pharmaceutical ingredient
As will be addressed throughout this review, antidiabetic drugs pose a great perspective in drug repurposing. In this matter, exenatide may carry an effect in preventing cognitive decline, where it is possible to link DM with an increased risk of developing dementia and other neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), as insulin can cross the blood-brain barrier (BBB) having important roles in the CNS, such as in learning and in long-term memory consolidation [41, 42]. In clinical trials investigating the impact of exenatide in the CNS, it is possible to find outcomes ranging from no cognitive benefit to brain health to improvement of motor and cognitive functions in PD [43–45]. In the laboratory, exenatide demonstrated a protective characteristic to the brain, reduced inflammation, and supported dopamine in the brain, among others [43–45]. All these findings did not provide enough support to state which exenatide indeed ceased the progression of PD. Further investigation is demanded in this field.
Subsequently, there followed the emergence of liraglutide, commercially known as VictozaTM, in 2010, manufactured by Novo Nordisk. This medication was intended to treat glycemic control in patients aged 10 years and above afflicted by T2DM [34]. Additionally, liraglutide was introduced as SaxendaTM also by Novo Nordisk. The series of advancements was concluded with the introduction of four more drugs for the treatment of T2DM in adults, including one that was discontinued: marketed by Eli Lilly dulaglutide in 2014 which, according to the FDA and the Australian Therapeutic Goods Administration (TGA) is undergoing a worldwide shortage currently due to increased demand, and the estimated availability of this drug was extended to Dec-31-2023 [46, 47]. By GlaxoSmithKline in 2014, albiglutide TanzeumTM was withdrawn from the market in 2017 due to business issues and not safety issues [48, 49]. Sanofi, lixisenatide in 2016 [12] as AdlyxinTM, but it has been discontinued in the U.S. market also due to a business decision, not due to efficacy and safety issues [50, 51]. Importantly, AdlyxinTM keeps being marketed as LyxumiaTM in many countries in Europe [52]. Semaglutide in 2017 by Novo Nordisk was marketed as OzempicTM which according to the FDA and TGA is also undergoing a shortage due to an unexpected increase in demand, the shortage was extended to last approximately until Dec-31-2023 [53], and by Novo Nordisk, WegovyTM which is also undergoing shortage [54–56]. Of all the GLP-1RAs, semaglutide has the widest range of product options in concentration mg/mL. The most recent approval, as of the year of writing this paper (2022, referred to as “this year”), is tirzepatide, marketed as MounjaroTM, for managing T2DM [57, 58]. Tirzepatide also has a wide range of product presentations, being available in 2.5 mg/0.5 mL, 5 mg/0.5 mL, 7.5 mg/0.5 mL, 10 mg/0.5 mL, 12.5 mg/0.5 mL, or 15 mg/0.5 mL formats in a single-dose pen.
Chemical structure of GLP-1RA peptides
To the effect of this discussion and although exenatide (found in the venom of the Gila monster) binds to the GLP-1R similarly to the GLP-1 peptides, an artificial classification of peptides was conducted (Figure 1).
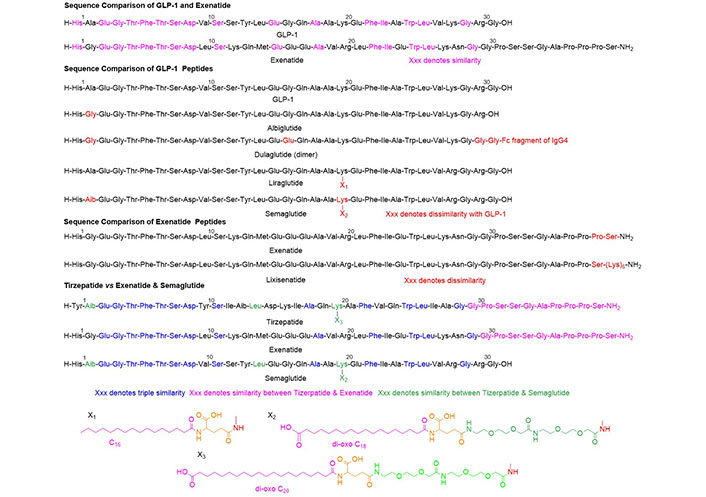
Chemical structure of the peptides discussed herein. Aib: alpha-aminoisobutyric acid; Ala: alanine; Arg: arginine; Asp: aspartic acid; Gln: glutamine; Glu: glutamic acid; Gly: glycine; His: histidine; Leu: leucine; Lys: lysine; Phe: phenylalanine; Ser: serine; Thr: threonine; Trp: tryptophan; Tyr: tyrosine; Val: valine; IgG4: Immunoglobulin G4
GLP-1 and exenatide show approximately 50% similarity and include highly conservative changes such as Ala, Val, and Ala in the former vs. Gly, Leu, and Val in the latter. The carboxy (C)-terminal is almost identical.
Albiglutide, liraglutide, and semaglutide basically share the sequence of GLP-1. In liraglutide and semaglutide, the presence of the pending chain in the Lys20 ending in a fatty acid is responsible for the albumin binding of these compounds, which ensures enhanced stability. The Aib (α-methyl-Ala or α, α-dimethyl-Gly) in position 2 increases stability to the action of enzyme DPP-4 and therefore prolongs their half-life. Dulaglutide is a dimer formed by a GLP-1 covalently linked to a fragment crystallizable (Fc) of human IgG4.
Lixisenatide is exenatide with a chain of five Lys residues at the C-terminus to increase stability.
Tizerpatide can be defined as a hybrid of exenatide and semaglutide. These three compounds share the C-terminal part while tizerpatide and exenatide share the N-terminal region (N stands for the nitrogen in the amino group; the N-terminal refers to the end of a protein of the peptide chain where the amino group is located). On the other hand, tizerpatide and semaglutide share the pending moiety at Lys20, which is responsible for higher stability.
Liraglutide
A prominent example of GLP-1RA on the market, manufactured by Novo Nordisk, is the broadly used liraglutide, and was approved in 2010 (SaxendaTM) [59] for weight management. This medication is to be used in addition to lifestyle changes regarding diet and exercise (not indicated for pediatric use) [60], and not indicated to treat T2DM, given in a higher dose than the first liraglutide [61–63]. The first liraglutide by Novo Nordisk was VictozaTM (original approval date 2010), which is indicated for pediatric and adult use (> 10 years) and is also used as an adjunct to lifestyle changes. However, it is also indicated to help blood glucose management in T2DM and to reduce cardiovascular complications in adults [64].
GLP-1RAs are used as an additional therapy to accompany lifestyle changes in diet and exercise. Liraglutide decreases body weight (BM), delays gastric emptying, stimulates insulin release, reduces glucagon release, and lowers BM by allowing the proper management of calorie intake, thereby leading to improved control of blood glucose and enhanced weight management. In contrast to endogenous GLP-1, analogs of GLP-1 are protected from metabolic degradation and thus have a half-life of approximately 13 h. The same 13 h half-life duration applies to both liraglutides [16, 60, 65].
Clinical trials have demonstrated that liraglutide achieves enhanced control of glycated hemoglobin levels [64]. This medication was found to show superior performance in all aspects than the placebo, but it was less effective than semaglutide [66]. However, in randomized trials involving children and adolescents with obesity and Prader-Willi syndrome, liraglutide did not show a significant difference compared to the placebo [67]. On the other hand, in obese women, liraglutide was reported to bring about greater weight loss than the placebo [68]. According to the FDA, liraglutide resulted in an average weight reduction of 2.37% and a reduction of 0.23 in the BMI standard deviation score (SDS) when compared to the placebo [69]. Of note, like the other drugs belonging to the same class, liraglutide carries a risk of thyroid C-cell tumor development, and the most commonly reported adverse reactions in clinical trials were gastrointestinal effects. Other common adverse reactions related to the gastrointestinal tract, but reported in the prescribing information of all GLP-1RAs on the market, are decreased appetite, abdominal pain, increased lipase, constipation, diarrhea, vomiting, and dyspepsia [38, 55, 56, 61, 70–72]. Hypoglycemia is also a common adverse reaction shared by these drugs.
Semaglutide
Another GLP-1 analog, namely semaglutide, was developed using protein acylation technology (protein acylation is a vital process for the structure that grants stability to proteins and enhances enzyme activity, among other functions) and received FDA approval in 2017 (OzempicTM) [73, 74].
Aimed at patients with T2DM, this medication also helps in weight management and lowers blood glucose levels by stimulating insulin release, with a decrease even in fasting glucose levels, lowering hemoglobin A1c (HbA1c), and inhibiting glucagon release. Semaglutide can be used in combination with sulfonylureas, insulin, and metformin (the latter being one of the most prescribed as the first DM medicine) or it can be used alone as an adjunct to diet and exercise.
The structural modifications, which were seen earlier herein, and the strong albumin binding of semaglutide protect it from enzyme degradation and prolong its half-life, which lasts 1 week [54, 56]. Regarding its pharmacokinetic and pharmacodynamic profile, semaglutide lowers fat mass and stimulates both phases of insulin release. Moreover, it has a high affinity to plasma albumin, a bioavailability of 89%, a clearance of 0.05 L/h, and a low potential to induce or inhibit the cytochrome P450 (CYP) enzymes.
In primary clinical trials, semaglutide demonstrated a similar performance and a similar pattern of side effects across various population groups, including White, Black, African, and Asian individuals, both men and women, as well as men and women aged 65 years and older [75]. According to the FDA, OzempicTM was approved based on seven clinical trial outcomes involving over 4,000 subjects [76]. In comparison with placebo and other drugs, including anti-diabetes drugs, semaglutide has been demonstrated to be superior or non-inferior in primary and secondary endpoints, such as lowering HbA1c levels, reducing BM, fasting plasma glucose (FPG) levels, and improving glycemic control. Even compared to other GLP-1RAs such as exenatide and liraglutide, semaglutide has achieved better results in HbA1c, waist circumference, BMI, and FPG [54]. When comparing semaglutide to liraglutide in terms of adverse events, the former tends to show a higher occurrence of such events [66].
Of note, medical practitioners are prescribing semaglutide for weight loss in an off-label use [20]. This trend is contributing to the current global shortage of this medication. According to Han et al. [77], OzempicTM continues to be the most popular and searched GLP-1RA on the internet [20]. Furthermore, the effects extend beyond this point. In clinical trials involving overweight and obese patients, changes in weight from the baseline were more pronounced with semaglutide when compared to liraglutide. These two medications are both developed by the same pharmaceutical company as mentioned earlier. Semaglutide, administered via once-weekly injections, demonstrated significantly superior outcomes. Patients using semaglutide at varying doses experienced weight loss ranging from 10% to over 20%, surpassing the results seen with liraglutide administered through daily injections. Additionally, when comparing the mean BM reduction, semaglutide at a dose of 0.2 mg outperformed liraglutide. Specifically, the former achieved a weight reduction of 13.8%, while the latter only achieved a 7.8% reduction. A similar superiority of semaglutide is seen in other clinical studies [8, 78, 79].
Comparing semaglutide to dulaglutide, another GLP-1RA also administered once-weekly and also indicated to T2DM, their steady state of maximum plasma concentration is reached after four to five weeks for semaglutide and absolute bioavailability of 89%, and for dulaglutide, the steady state is reached in one to three days with a mean absolute bioavailability of approximately 47% for 1.5 mg and over. The half-life of semaglutide is also longer than dulaglutide with one week and 5 days, respectively [56, 70].
In general, this drug’s summary indicates that it exhibits a more significant deviation from baseline compared to other drugs in its class, and better performances in key endpoints.
It was found that the main GLP-1RAs addressed in this review, semaglutide, and liraglutide, may be present in different locations in brain regions which is important to functions such as regulating appetite and metabolism.
Semaglutide reduces appetite and BW directly in the brain region responsible for controlling appetite, the hypothalamus, with some important differences between liraglutide which was not found to be present in some key spots in the brain. Semaglutide was also found in another specific brain region, the hindbrain, increasing the synthesis of peptides and enzymes such as prolactin-releasing peptide (PrRP) and tyrosine hydroxylase.
It was demonstrated that semaglutide has some difficulty in crossing the BBB and does not interact with endothelial cells of the BBB but works on circumventricular organs, acting in selected regions, thus regulating BW. Liraglutide was also found to have limited access to the brain through the BBB, like semaglutide, it interacts with circumventricular organs [80].
Semaglutide binds to GLP-1Rs on tanycytes, cells that may play a role in delivering this GLP-1 into the arcuate nucleus of the hypothalamus (ARH), a brain region whose function is to control food intake and energy expenditure [81]. On the other hand, liraglutide was not found in the posterior ARH, which may involve a decreased effect in inhibiting appetite and energy expenditure by this GLP-1.
While there are distinctions between semaglutide and liraglutide, they exhibit similarities in activating proteins associated with the inhibition of feeding and the promotion of energy expenditure, namely pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) neurons.
Semaglutide has been shown to access specific regions in the brain related to feeding and emotional processes, such as the lateral septal complex (LSc), an area abundant in GLP-1Rs. Additionally, it reaches areas associated with the sense of disgust, including the posterior Ventrum Pallidum (pVP), among others. In contrast, liraglutide, unlike semaglutide, does not exhibit a presence in the LSc region [80, 82, 83].
Tirzepatide is the first dual GIP and GLP-1RAs for DM
Tirzepatide (MounjaroTM), a novel drug manufactured by Eli Lilly and authorized by the FDA in 2022, was approved for the treatment of T2DM. Administered subcutaneously on a weekly basis, this pharmacotherapy represents a new addition to the therapeutic arsenal, and it is intended to complement lifestyle modifications such as changes in exercise and diet [57, 58, 84]. Tirzepatide can be used in combination with other anti-diabetes drugs, such as SGLT-2 inhibitors, insulin, and metformin, among others. Notably, tirzepatide stands out as the first of its kind in the treatment of T2DM due to its unique dual agonistic activity on the GLP-1 and GIP-Rs present in the pancreas and other regions in the body. By engaging these receptors, tirzepatide effectively reduces postprandial glucose levels through heightened insulin secretion and diminished glucagon release [22, 57, 85]. Its selective binding affinity to these two receptors is characterized by a higher affinity for GIP-Rs over GLP-1Rs [9, 10]. Furthermore, tirzepatide also elicits a sensation of satiety and contributes to weight loss.
The success observed with GLP-1RAs in promoting weight loss and glycemic control in T2DM has inspired optimism regarding the development of novel T2DM treatments, such as albiglutide, exenatide, and liraglutide, among others mentioned throughout this paper [9, 10, 17], and anti-obesity medications. In this regard, in clinical trials enrolling patients with T2DM and also obese patients without T2DM, when compared to selective GLP-1RAs, tirzepatide showed superiority in the key aspects: weight loss, improvement in LDL and triglycerides, and improvement in cardiovascular risk, glucose control, and HbA1c reduction [24, 84–86]. When compared to GLP-1RAs, insulin degludec and glargine, and placebo, tirzepatide also showed superiority in the same aspects. In this matter, insulin caused fewer gastrointestinal events than the dual agonistic tirzepatide and GLP-1RAs [22, 85, 87]. The cardiovascular benefits of tirzepatide are not well established to include in this review and more research is required in this respect.
Ongoing clinical trials for tirzepatide are being conducted to determine its effectiveness in treating a wide range of diseases and conditions. The significant interest in these trials is being driven by the global rise in obesity and overweight issues, which are closely linked to T2DM.
According to the FDA, tirzepatide received approval on the basis of the outcomes of nine clinical trials involving over 7,000 subjects with T2DM. On the clinicaltrials.gov website, it is possible to find many studies related to tirzepatide, from phase 1 to phase 3 studies, with estimated completion dates ranging from May-2024 to Dec-2029. However, no results have yet been posted for most of them. In the context of overweight and obesity, there is a phase 3 clinical trial underway studying the effect of tirzepatide in adults, and the reduction of morbidity and mortality, this study can be found by study identification (ID) NCT05556512 [88]. Another study (NCT04844918), with no results reported to date, seeks to investigate more about how tirzepatide plus a low-calorie diet and physical activity impacts BM in patients with obesity [89, 90]. In contrast, a similar study (NCT04184622) has shown promising results in favor of tirzepatide over a placebo. This study demonstrated a significant change in BM at week 72, with reductions of 15%, 19%, and 20.9% for the doses of 5 mg, 10 mg, and 15 mg administered once-weekly, respectively [91]. Importantly, adverse events had a minimal impact on treatment discontinuation. Tirzepatide has already been approved for use in adults with T2DM, and with these ongoing clinical trials, there is potential for it to be made available to a younger population. The study with ID NCT05696847 is currently conducting tests in pediatric patients aged 6 years to 11 years who are dealing with obesity. This is a phase 1 study set to conclude in May-2024 [89].
Other studies that are not yet recruited address a wide range of conditions, such as changes in coronary atheroma volume between tirzepatide and standard of care (SOC) compared to placebo and SOC [92], a study to investigate the hypothesis that tirzepatide benefits patients not only by its known mechanism of action but also via cellular changes in adipose tissue, leading possibly as well to improvement in metabolic health [93]. Another study is underway on the use of tirzepatide in a rare disease named Wolfram syndrome with onset in childhood [94]. This condition has no selective standard treatment so far, and it can lead to diseases such as T2DM, loss of vision through optic atrophy, deafness, and diabetes insipidus [92, 94, 95].
An initial clinical trial (phase 1) comparing tirzepatide with other selective GLP-1RAs showed that it led to significantly greater weight loss compared to both dulaglutide and placebo [96, 97]. Furthermore, when considering adverse events like hypersensitivity, hypoglycemia, and injection-related events, no significant differences were observed between tirzepatide and dulaglutide [24, 98]. This observation suggests that tirzepatide may potentially be a more favorable choice for T2DM patients. Of note, there is an ongoing phase 3 clinical trial, with an estimated completion date in Oct-2024, comparing the effects of tirzepatide and dulaglutide on cardiovascular events in T2DM patients [96]. With health authorities announcing a shortage of dulaglutide, tirzepatide emerges as a potentially suitable alternative to address this worldwide shortfall. The same was seen with tirzepatide vs. semaglutide, where a higher weight loss and fat mass loss, improvement in insulin resistance, and lowering glucagon secretion on the meal tolerance test was observed in the group treated with tirzepatide than with semaglutide or placebo. According to Azuri et al. [99] in 2023, they found tirzepatide’s cost-effectiveness was better than semaglutide’s. The price difference for each 1% reduction in BM was notable, with tirzepatide costing $985, whereas semaglutide was priced at $1,845. Additionally, the annual drug cost for semaglutide was significantly higher at $17,485 compared to tirzepatide, which costs $12,568. It is worth noting that the weight loss percentage compared to placebo was 17% for tirzepatide and 12% for semaglutide. Adverse events were similar between the two drugs [97, 99, 100]. Importantly, the possibility of tirzepatide supplying the current shortage of these drug classes also applies to the current worldwide shortage of semaglutide.
Retatrutide, the latest newcomer to the field
Eli Lilly is pursuing another breakthrough in the field of diabetes and obesity. Trials are currently ongoing for their new drug, retatrutide (LY3437943), which functions as a peptide agonist targeting GIP, GLP-1, and glucagon receptors (GCGRs). Unlike tirzepatide which is a dual hormone RA [57], retatrutide is a triple hormone RA [101]. Up to this point, this novel proposed drug has shown promising results in terms of reducing BM.
Retatrutide is administered weekly, and it has a half-life that is similar to that of semaglutide (OzempicTM). Semaglutide is primarily indicated for the treatment of T2DM. However, it has also been prescribed off-label for weight loss due to its demonstrated effectiveness in promoting weight reduction, particularly when administered at higher doses. Similarly, retatrutide is more efficient at reducing BM especially when administered at higher doses. Notably, this reduction seems to be more pronounced among obese patients and in women compared to men. The 12 mg dose has been particularly successful, resulting in a peak weight loss of 30% from the patient’s baseline. Furthermore, positive changes from the baseline have been observed at important endpoints: fasting glucose levels, glycated hemoglobin, lipid profiles, and insulin levels. The side effect profile risk analysis was very similar to that of all the drugs discussed herein [101].
While tirzepatide and retatrutide utilize the advantages of incretin hormone RAs outlined in this review, the new antibody AMG-133, currently undergoing phase 2 clinical trials, is a GLP-1RA and a GIP-RA. It is expected to complement the therapeutic arsenal in the field of diabetes, overweight, and obesity.
Despite its antagonistic effect on GIP-Rs, AMG-133 is expected to induce a reduction in BM, as evidenced by clinical trials so far, wherein it manifested a 14% decrease in participants’ BM at the highest administered dose [102]. As mentioned in this review, GIP may play a role in both weight loss and gain. Prolonged antagonism of GIP-Rs, observed in preclinical models, results in a reduction in BM. The mechanism of this phenomenon is still under discussion, with a potential compensatory mechanism hypothesized. Given the demonstrated synergistic interplay between GLP-1R and GIP-R, akin to tirzepatide, the antagonism of GIP-R might enhance GLP-1R function [103].
Conclusions
In conclusion, this review has highlighted the extensive variety of GLP-1RAs on the market. With important differences between them, for instance after administration, semaglutide and liraglutide hold limited access to brain areas which may involve the selectivity of BBB or not, with evidence demonstrating that GLP-1RAs may not regulate food intake through specific brain regions containing GLP-1Rs, but rather acting in different regions in the CNS [65]. This diversity is beneficial as it provides medical practitioners with a wide array of alternative medications for their patients, particularly in the context of the current shortage of semaglutide and dulaglutide. However, it is important to emphasize that the choice of treatment, whether for initial therapy or as a replacement, requires careful consideration of various factors, including potential side effects, patient choices, current clinical status, past treatments, cost considerations, and others.
Despite being among the most expensive options for diabetes treatment, GLP-1RAs hold significant importance due to their dual benefits in managing diabetes and aiding weight loss. This dual action is particularly valuable as DM and obesity often coexist, placing a burden on patients, especially those using insulin in conjunction with other types of drug classes.
Over the decades the world has seen a connection between DM and obesity. What is important is that there is also a link between the medications designed to combat obesity and those meant to treat diabetes. In some cases, anti-obesity drugs can offer benefits to T2DM patients, while anti-diabetes medications like semaglutide might also be beneficial for obese patients. A medication that addresses both conditions can be immensely advantageous for these groups of patients.
While most GLP-1RAs share similar adverse events, primarily gastrointestinal ones, other classes of anti-diabetes medicines may also include a range of side effects, such as anxiety, nervousness, and depression. Furthermore, the unexpected surge in the prescription and sales of dulaglutide and semaglutide has resulted in a global shortage of these drugs. This situation raises concerns about its potential impact on tirzepatide in the future, given its superior and unique mechanism of action. It is important to note that health authorities lack the power to prevent clinical decisions made by doctors in order to lower the amount of new prescriptions, but rather they provide guidance. Fortunately, these authorities are taking action to address the shortage of these GLP-1RAs, for example, by advising practitioners not to prescribe these medications to new patients during the shortage.
The availability of various GLP-1RAs offers flexibility in diabetes management, and their dual benefits make them valuable tools in addressing diabetes and obesity. The shortage of specific medications underscores the need for careful consideration when choosing treatments, taking into account patient-specific factors. Additionally, health authorities are actively working to mitigate the challenges posed by this drug shortage. Pharmaceutical companies, in collaboration with health authorities, are conducting clinical trials that could potentially bring about additional options to address the shortages of existing medications.
Of note, as seen in Table 1, there are not as many GLP-1 mimetics intended for obesity or overweight as there are specifically for T2DM. This situation, plus the shortages faced, might drive an increase in the off-label prescription rates of these drugs.
Medical practitioners should seek the best patient outcomes when prescribing GLP-1RAs for aesthetic purposes as they must be aware that it is not only aesthetics that matters, but health is also an issue, physical and mental, as the interest and prescription of GLP-1RAs increased exponentially over the past years [77]. This heightened attention to patient health is underscored by the rise in adverse events possibly associated with the use of GLP-1RAs, including thoughts of self-harm and suicidal ideation. This is becoming a growing concern in Europe [20], and more in-depth studies are being carried out to explore these issues.
In summary, GLP-1RAs can provide sustained and efficient outcomes in the context of glucose regulation and weight loss in T2DM, overweight, and/or obese patients. However, healthcare professionals should be aware of potential new adverse events that may emerge due to the heightened demand for these medications. Furthermore, it is becoming increasingly evident that pharmaceutical companies may struggle with production challenges as they face a rising prescription rate of their anti-diabetes and anti-obesity products. In this regard, they will need to explore new paths to improve the quality of these medications or develop new ones related to incretin hormones. One such example is tirzepatide, whose full potential in the market is yet to be fully understood.
To this point, tirzepatide stands as the only medication targeting the two significant incretin hormone receptors discussed in this review. In clinical trials, it has successfully achieved the desired outcomes for a novel drug, including innovation, safety, effectiveness, superiority, and non-inferiority when compared to placebo or other medications. An important aspect of this novel drug is its pricing. This paper has shown that GLP-1RAs can be one of the most expensive drug classes for treating T2DM and obese patients. In this context, tirzepatide was significantly more affordable than one of the best-selling GLP-1RAs currently available. Moreover, it demonstrated superior efficacy compared to the latter [101].
Retatrutide, also developed by the same pharmaceutical giant (Eli Lilly), holds promise in expanding the arsenal of medications for diabetes and obesity treatment. However, there are still studies and research that need to be conducted, particularly in the context of obesity treatment. Considerable expectation also surrounds AMG-133 for the forthcoming outcomes.
Abbreviations
| Aib: | alpha-aminoisobutyric acid |
| Ala: | alanine |
| BBB: | blood-brain barrier |
| BM: | body weight |
| BMI: | body mass index |
| CNS: | central nervous system |
| DM: | diabetes mellitus |
| DPP-4: | dipeptidyl peptidase-4 |
| FDA: | Food and Drug Administration |
| GIP: | glucose-dependent insulinotropic polypeptide |
| GIP-Rs: | glucose-dependent insulinotropic polypeptide receptors |
| GLP-1: | glucagon-like peptide-1 |
| GLP-1R: | glucagon-like peptide-1 receptor |
| GLP-1RAs: | glucagon-like peptide-1 receptor agonists |
| Gly: | glycine |
| HbA1c: | hemoglobin A1c |
| Leu: | leucine |
| Lys: | lysine |
| PD: | Parkinson’s disease |
| T2DM: | type 2 diabetes mellitus |
| Val: | valine |
| WHO: | World Health Organization |
Declarations
Author contributions
ACM: Conceptualization, Formal analysis, Investigation, Writing—original draft, Writing—review & editing. BGdlT and FA: Conceptualization, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
Fernando Albericio is the Editor-in-Chief of Exploration of Drug Science, but he had no involvement in the journal review process of this manuscript. The rest of the authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
The datasets analyzed for this study can be found in Orange Book (https://www.accessdata.fda.gov/scripts/cder/ob/results_exclusivity.cfm) and European Medicines Agency (https://www.ema.europa.eu/en/homepage).
Funding
Not applicable.
Copyright
© The Author(s) 2024.