Affiliation:
1School of Medicine, Eastern Virginia Medical School, Norfolk, VA 23451, USA
†These authors contributed equally to this work.
Affiliation:
2School of Medicine, University of Utah, Salt Lake City, UT 84132, USA
†These authors contributed equally to this work.
Affiliation:
3Department of Neurosurgery, University of Florida, Gainesville, FL 32608, USA
Email: Brandon.Lucke-Wold@neurosurgery.ufl.edu
ORCID: https://orcid.org/0000-0001-6577-4080
Explor Drug Sci. 2024;2:268–276 DOI: https://doi.org/10.37349/eds.2024.00046
Received: August 22, 2023 Accepted: January 22, 2024 Published: May 21, 2024
Academic Editor: Cheorl Ho Kim, Sungkyunkwan University, Samsung Advances Institute of Health Science and Technology (SAIHST), Republic of Korea
The potent pain-relieving properties of opioids come at a steep price. Their addictive nature and side effects raise critical concerns in managing pain after surgical spine procedures. Postoperatively, spinal surgeries often accompany acute intense pain, which presents a significant challenge in optimal recovery. This paper reviews the historical approach to pain management in spine surgeries and expands on the use of alternatives and novel agents with reduced addictive potential. Additionally showcasing individualized multimodal strategies for postoperative pain management beyond pharmacological approaches such as cognitive behavioral therapy (CBT), physical therapy, and transcutaneous electrical nerve stimulation (TENS). Given the global opioid addiction crisis, there is a growing need for a fundamental shift towards safer and effective alternatives. Transitioning beyond opioid-centric practices in spinal surgery can optimize pain relief while improving patient outcomes and minimizing risk.
Opiates have historically been used to treat post-neurosurgical pain. Physicians have utilized different modalities to optimize maximal pain control and minimize opiate use [1]. This paper aims to address pain management in spinal procedures and the historically used strategies, currently employed methods, and methods that are undergoing FDA approval. There are current pursuits in which pain medications are evolving to become more targeted and specific in hopes of lessening adverse side effects [2].
Multiple non-pharmaceutical methods have been used for patients who have undergone spinal surgery and patients who qualify to receive spinal surgery. One of these non-pharmaceutical approaches is physical therapy. Physical therapy is used to strengthen muscles that can assist in relieving pain from these spinal cord injury (SCI) patients [3]. Physical therapy also aids in preventing muscular atrophy from surgery, which in turn helps stabilize the spine while in recovery and reduce spine pain [4]. Another method that has assisted SCI patients is spinal cord stimulators. Spinal cord stimulators are used to address nerve pain in hopes of preventing surgery or to help with post-surgical pain [5–7]. The neurostimulator mechanism releases specific frequencies that interfere with neuronal signaling of the spinal cord; this has shown improvements in SCI patients and has been proven to help with failed spinal surgery patients [5]. Using non-pharmaceutical methods helps prevent unnecessary pain medication prescriptions, aiming to decrease medication dependency.
Cognitive behavioral therapy (CBT) is a well-applied form of psychotherapy in pain management, which focuses on reducing pain and distress by modifying physical sensations, challenging negative thinking, and helping patients adopt healthier coping mechanisms (Table 1) [8]. CBT applied perioperatively and continued postoperatively has proven to significantly reduce maladaptive coping mechanisms, improve outcomes, and decrease disability in patients with spine surgeries [9, 10].
Non-opioid drugs and interventions for pain management in spine surgery
| Non-opioid medications | Other interventions | Non-pharmacologic strategies |
|---|---|---|
| Gabapentin, pregabalin, ketamine, nonsteroidal anti-inflammatory drug (NSAID), acetaminophen | Epidural, intravenous (IV) lidocaine, transcutaneous electrical nerve stimulation (TENS) | Physical therapy, cognitive behavioral therapy (CBT) |
Transcutaneous electrical nerve stimulation (TENS) is a non-invasive treatment that delivers low-voltage electrical impulses through electrodes placed on the skin to the area impacted by pain (Table 1) [11]. TENS-induced analgesia is postulated to be mediated through peripheral, spinal, and supraspinal mechanisms [12, 13]. TENS has been researched since the 1980s and has been shown to reduce the need for narcotics in postoperative pain relief following spine surgeries [14].
While there will always be a need for some intravenous (IV) or oral pain medication for these neurosurgical patients, non-pharmaceutical approaches possibly decrease the number of drugs these patients need and improve their recovery and quality of life.
Pharmaceutically, many different approaches and combinations of drugs are varied among surgeons and surgery types. A recent study showed that administering morphine alongside other drugs such as ketamine and combination therapy of nonsteroidal anti-inflammatory drugs (NSAIDs) and other non-opiate-like drugs had been shown to maximize pain control while not solely relying on opiates to control pain [15]. There are a lot of new multi-modality pain management regimens that are being studied to remove reliance upon just one drug, specifically opiates. Other examples of non-opiate medications that are used for neurosurgical pain management can be anticonvulsants, tramadol, acetaminophen, lidocaine, scalp blocks, dexmedetomidine, parecoxib, and dexamethasone [16]. All these drugs have been shown to help patients with perioperative pain, and some have been shown to help postoperative pain. Epidural injections and facet injections have been proven to improve spinal cord pain. These modalities can be used for patients who do not want to undergo surgery and have also been shown to help with post-surgical pain that is not alleviating with time [17]. A recent study showed that post-surgical lumbar canal decompression patients showed some relief with bolus epidural fentanyl injection, which improved their pain for a short time [18]. This type of fentanyl injection could help resolve acute short-term pain in patients who undergo spinal surgery, which in turn could decrease the amount of opiates given to these patients [18]. Despite using multiple modalities to help manage pain in neurosurgical patients, it is hard to eliminate opiates due to their ability to help with such acute post-surgical pain [19].
Opiate dependency is one of the leading reasons research aims to find other pharmaceutical pain management medications [20, 21]. A recent study showed, that “26.8 million people were estimated to be living with opioid use disorder (OUD) globally in 2016, with > 100,000 opioid overdose deaths annually, including > 47,000 in the USA in 2017” [22]. Due to the opioid pandemic, the medical community is facing, there is a high demand for further research into how the surgical community can help address postoperative pain without overprescribing potentially addictive medications.
Spinal surgery has been rated among the top six procedures causing severe pain in the initial postoperative period [23]. Consequently, substantial prescription of pain medications is critical for favorable patient outcomes [23–25]. Orthopedic and neurosurgery are the two surgical specialties with a higher prevalence of opioid use, and coincidentally, spinal procedures overlap [26]. Opioid therapy has traditionally been the recommended approach for treating pain associated with chronic spinal disorders and spinal surgeries [27]. Opioid use and recommendations for back pain can be traced back to the 1960s. Brown et al. [28] encourage a strong consideration for chronic opioid analgesic therapy in patients with chronic lower back pain. Over the past two decades, the number of spinal fusion surgeries reported has tripled nationwide [29]. Many of these patients have chronic pain and tend to receive long-term opioids even before surgery, with the risk of persistent postoperative opioid use [30]. Deyo et al. [29] conducted a study regarding opioid use in lumbar fusion surgeries, with conclusions that prescriptions for opioids post-spinal fusion surgeries were higher than before the procedure, with an increase in cumulative dosage post-procedure. The historical use of opioids in spinal disorders and procedures does not predate the concerns regarding risks associated with long-term opioid consumption. Data and justifiable trials and outcomes date back to 1996, showing the need for controlled prescription of opioids [25].
Confronted with the epidemic, spine surgeons thus faced the challenge of evolving their prescription practices and developing alternative strategies for pain management in their patients. Implementing innovative minimally invasive surgical techniques has been proven to minimize tissue damage, while enhanced preoperative and recovery protocols have helped reduce the need for opioids after surgery [31].
Multiple non-opioid drugs are effective in reducing complications and providing satisfactory pain relief [26, 32]. There has been increasing emphasis on adequate perioperative pain management for spine surgeries, ultimately leading to a decreased need for opioids post-surgery. So, an optimal opioid-sparing approach to pain control involves an individualized multimodal strategy involving perioperative management, non-opioid medications, and other post-surgical interventions (Table 1) [23, 33]. Every available non-opioid pain therapy may not be appropriate for each patient, and patient preference should also be considered.
Narcotic analgesics are a central component in the management of postoperative spine pain. However, opioid-based analgesics can lead to dangerous side effects such as tolerance, respiratory depression, nausea, vomiting, and diminished wound healing [34]. Non-opioid and combined therapies are important strategies for postoperative spine pain management. Multimodal analgesia (MMA) combines the use of 2 or more analgesics with different mechanisms, allowing for a synergistic effect while decreasing individual dosage and side effects. MMA is recommended for postoperative pain in many clinical situations by the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine (ASRA), and the American Society of Anesthesiologists [35]. A meta-analysis of MMA for adults undergoing spine surgery established that patients who received maximal MMA had lower pain scores and decreased hospital stays [36]. This review will focus on opiate alternatives for postoperative spine pain management that can be used in MMA or as standalone agents, which have been shown to reduce the use of opiates. In addition to discussing new agents with therapeutic potential for spine pain.
NSAIDs reduce pain and inflammation by inhibiting the production of prostaglandins by blocking cyclooxygenase enzymes [37]. Oral NSAIDs are often used in MMA regimens and are effective agents in postoperative analgesia after lumbar spine surgery [38]. An important consideration with prolonged NSAID use in postoperative spine pain management is that NSAIDs have dose and duration-dependent effects on fusion rates [39].
Acetaminophen is metabolized to p-aminophenol, which crosses the blood-brain barrier and is converted to N-acylphenolamine. N-acylphenolamine blocks cyclooxygenase enzymes and other receptors within the central nervous system inducing analgesia [40]. Acetaminophen enhances MMA in experimental and clinical pain states [41], and perioperative acetaminophen administration can reduce post-surgical pain and narcotic consumption [42].
Gabapentin and pregabalin reduce the excitability of neurons in the brain by decreasing the activation and insertion of calcium channels containing an α2δ subunit, which plays a role in transmitting pain signals [43, 44]. Perioperative administration of gabapentin decreases pain and narcotics consumption [45].
Ketamine is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist. The antagonism of NMDA receptors decreases the release of glutamate, which modulates central sensitization [46]. Ketamine decreases postoperative pain scores and opioid requirements [47] and postoperative analgesia was enhanced by the combination of ketamine and methadone in patients who had spine surgery [48].
Lidocaine’s analgesic effect is multifactorial. Lidocaine primarily exerts its analgesic effect on acute pain by blocking voltage-gated sodium channels and preventing depolarization [49]. IV lidocaine increases the concentration of acetylcholine in the cerebrospinal fluid and enhances the pain inhibitor pathway [50]. It also selectively decreases the conduction of unmyelinated C-fibers by reducing the excitability of the spinal dorsal horn [51, 52]. Intraoperative IV lidocaine infusion has proven effective in diminishing the severity of postoperative pain in patients with spinal fusion surgery [53].
Cebranopadol is an opioid-like agent developed by Tris Pharma, which is not yet FDA-approved but has completed phase 3 trials. Cebranopadol is a mu opioid (MOP) receptor and nociceptin opioid peptide (NOP) receptor agonist (Figure 1) [54]. An abuse potential analysis developed by Tris Pharma suggests that cebranopadol has a lower potential for abuse than tramadol and oxycodone.
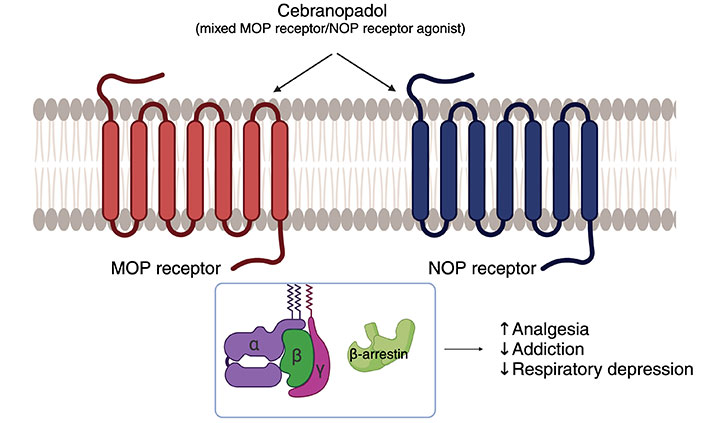
The mechanism of action of cebranopadol. ↑: promoting effect; ↓: inhibitory effect. MOP: mu opioid; NOP: nociceptin opioid peptide. Created with BioRender.com
Tanezumab is a monoclonal antibody that interferes with pain signals by inhibiting nerve growth factor (NGF) from binding to its receptor (Figure 2) [55]. Tanezumab was in development for the treatment of osteoarthritis pain and was shown to have a potential effect on pain modulation in low-back pain [55] but was rejected by the FDA in March of 2021 due to the high risk for joint destruction and rapidly progressive osteoarthritis at high doses. Despite this medication being rejected by the FDA due to side effects, the pathway used requires further research into its use as an effective option for pain regulation.
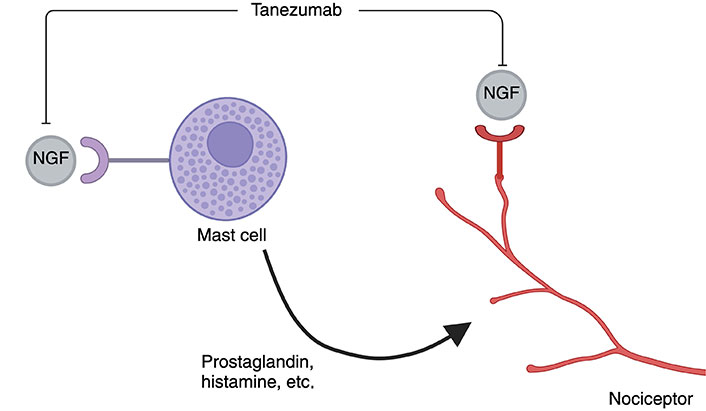
The mechanism of action of Tanezumab. NGF: nerve growth factor. Created with BioRender.com
In addition to the initial pain that leads patients to undergo spinal procedures, the postoperative and long-term pain management of spinal issues is complex and unique to every patient. The operation can cause a stress response in the body contributing to pain [56]. There is a need to utilize a personalized medical approach in treating pain for neurosurgical patients undergoing spine procedures. Countless individual contributing factors, such as genetics, family history, personal medication use, and pain tolerance, should impact the overall decision on prescribing medications [57]. Admittedly, the opioid crisis has impacted pain management and patient care. A rightful concern for addiction should not hinder adequate patient care. Increased readmissions, morbidity, mortality, and decreased patient satisfaction have all been associated with inadequate pain management postoperatively [58]. If patients can find adequate pain relief post-surgery, then their ability to return to a quality of life that is manageable and does not require further care is possible.
Due to the current climate of opioid addiction worldwide in medical pain management, there is a push to move away from opioid-focused pain management. Non-opioid-based novel therapies are being studied to relieve pain with less addictive potential, providing a new outlook on the possibilities for pain management in spinal procedures. Outside of pharmaceutical approaches to pain relief for spinal patients, non-pharmaceutical approaches such as spinal cord stimulators have also been shown to provide pain relief [6, 7]. There is a need for further research into pharmaceutical and non-pharmaceutical options for pain management post-spinal procedures to aid patients in returning to a sustainable quality of life that does not involve debilitating acute or chronic pain.
IV: intravenous
MMA: multimodal analgesia
NSAIDs: nonsteroidal anti-inflammatory drugs
SCI: spinal cord injury
TENS: transcutaneous electrical nerve stimulation
AMC and SY equally contributed to: Conceptualization, Writing—original draft, Writing—review & editing. JT, SP, and BLW: Conceptualization, Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
