Abstract
In recent times, there has been a revolutionary surge in antioxidant research, with a focus on harnessing microalgae to enhance wellness and extend human longevity. Microalgae, a diverse group of unicellular photosynthetic organisms, have emerged as promising sources of natural antioxidants due to their ability to synthesize various bioactive compounds, including carotenoids, polyphenols, and tocopherols. These antioxidants play a pivotal role in scavenging free radicals and reducing oxidative stress, known contributors to aging and chronic diseases. This review provides an over-view of recent advancements in understanding microalgae’s antioxidant potential, covering their biochemical composition, extraction techniques, and purification methods. Moreover, it delves into compelling in vitro and in vivo studies showcasing microalgae-derived antioxidants’ protective effects against oxidative damage, inflammation, cardiovascular diseases, and neurodegenerative disorders. The sustainable cultivation of microalgae in controlled environments further supports the potential for large-scale production and commercialization of their antioxidant compounds. As microalgae continue to revolutionize antioxidant research, they hold immense promise in developing novel preventive and therapeutic strategies to promote human health and wellbeing.
Keywords
Microalgae, antioxidants, wellness, longevity, health benefits, natural antioxidants, sustainable production, bioactive compoundsIntroduction
Antioxidants are crucial molecules that play a pivotal role in neutralizing harmful free radicals, thereby preventing oxidative damage to biomolecules, including lipids, proteins, and nucleic acids. Oxidative stress resulting from an imbalance between free radicals and the body’s antioxidant defense system has been implicated in various chronic diseases, including cardiovascular disorders, neurodegenerative conditions, and cancer. As the global burden of these diseases continues to rise, there is a growing urgency to explore novel and sustainable sources of antioxidants to improve human health and promote longevity [1, 2].
At the same time, due to concerns about the safety and efficacy of synthetic antioxidant molecules, the quest for natural antioxidants has escalated. Natural antioxidants produced from plant sources have sparked considerable attention due to their potential health advantages and lack of side effects. Among them, microalgae have emerged as a potential and underutilized resource [3]. Microalgae are microscopic, single-celled organisms that inhabit various aquatic environments, from freshwater lakes to oceans. These remarkable organisms have long been recognized for their ability to convert solar energy into valuable biomass through photosynthesis. Recent studies have unveiled the impressive diversity of bioactive compounds synthesized by microalgae, many of which exhibit potent antioxidant properties [4, 5].
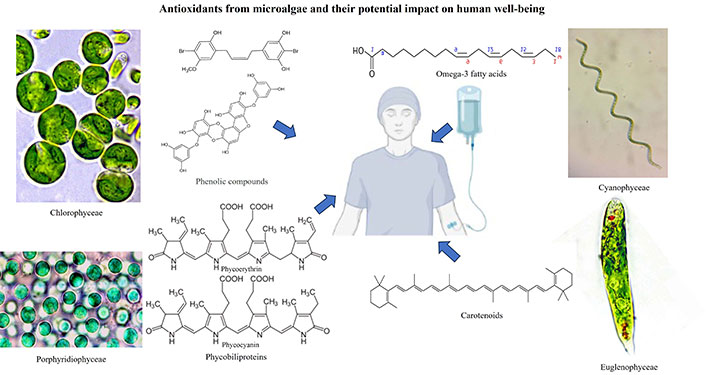
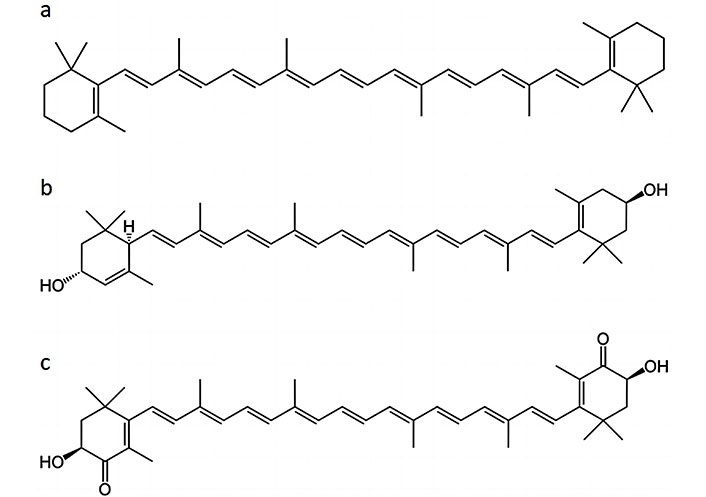
Notably, microalgae produce an extensive array of antioxidant molecules, including carotenoids, polyphenols, phycobilins, tocopherols, and essential fatty acids. Carotenoids, such as β-carotene, lutein, and astaxanthin (Figure 1), are well-known for their efficient scavenging of free radicals and singlet oxygen species, contributing to cellular protection against oxidative damage [6–9]. Polyphenols, on the other hand, possess versatile antioxidant properties and can chelate metal ions, further enhancing their efficacy in neutralizing reactive oxygen species (ROS) [10]. The antioxidant capacity of microalgae is closely linked to their biochemical composition, which varies across different species, growth conditions, and environmental factors. This variability opens a vast reservoir of potential antioxidant sources, each with unique bioactive profiles and health-promoting properties [11].
This review aims to shed light on the green revolution in antioxidant research, with a specific focus on harnessing microalgae for enhancing wellness and extending human longevity. By understanding the biochemical composition of microalgae and their antioxidant potential, we can unlock a sustainable and nature-based solution to combat oxidative stress and its associated health challenges. Moreover, investigating the various extraction, purification, and cultivation techniques for microalgae-derived antioxidants will pave the way for their integration into preventive and therapeutic approaches for improved human health and wellbeing. Through this exploration, we hope to inspire further research and innovation in the field of microalgae-based antioxidants and propel the green revolution towards a healthier and more sustainable future [5, 6].
Microalgae as a potential natural source of antioxidants
Microalgae, as a diverse group of microscopic unicellular and pluricellular photo-synthetic organisms, have garnered significant attention as a potential and promising source of natural antioxidants. These microscopic organisms thrive in a wide range of aquatic environments, from freshwater bodies to marine ecosystems, exhibiting an extraordinary capacity to synthesize an array of bioactive compounds with antioxidant properties [7]. One of the primary reasons microalgae have become a focal point in antioxidant research is their unique ability to convert solar energy into valuable biomass through photosynthesis. During this process, microalgae accumulate various bioactive compounds as part of their defense mechanisms against environmental stressors, including high light intensities and fluctuations in nutrient availability [8].
Harnessing microalgae as a source of antioxidants also aligns with the principles of sustainability. These organisms exhibit rapid growth rates and have the potential for large-scale cultivation with minimal environmental impact. Additionally, they do not compete for arable land, as their growth is primarily aquatic, thereby avoiding conflicts with agricultural practices and food production [12]. Overall, microalgae represent a sustainable, nature-based solution for addressing the increasing demand for natural antioxidants [13, 14]. By exploring their diverse bioactive compounds and elucidating their antioxidant mechanisms, we can unlock the full potential of microalgae as a valuable resource in the quest for enhanced health and wellbeing [5]. The subsequent sections of this review will delve into the specific bioactive compounds found in microalgae and their potential health benefits, further substantiating their role as a potent source of antioxidants for human health and longevity.
The fundamental goal of this study is to present a thorough and up-to-date review of the green revolution in antioxidant research, with a particular emphasis on utilizing microalgae for boosting health and prolonging human longevity. The review will look at the biochemical makeup of microalgae and their potential as a source of natural antioxidants, as well as their role in promoting human overall well-being. This complete revision’s information may be a critical aspect in understanding the entire process, from picking the microalgae species to human health and welfare.
Diversity and characteristics of microalgae
Microalgae are a diverse group of unicellular or simple multicellular photosynthetic organisms that can be found in various aquatic environments, such as oceans, freshwater bodies, and even in damp soil. They are classified under the group of microorganisms called phytoplankton. Despite their tiny size, microalgae play a crucial role in the ecosystem and have significant potential in various applications, including acting as natural antioxidant factories [15, 16].
Diversity
Microalgae exhibit a vast diversity, with thousands of species identified so far. They can be classified into different taxonomic groups based on their cellular characteristics, pigments, and other morphological features [4, 8, 12, 15, 16]. Some of the major taxonomic groups of microalgae include:
Bacillariophyceae: Diatoms are unicellular algae with intricate siliceous cell walls, known as frustules. They are highly diverse and contribute significantly to global oxygen production.
Cyanophyceae (blue-green algae): These are a type of photosynthetic bacteria that are often considered as microalgae due to their similar ecological role. They are known for their ability to fix atmospheric nitrogen and produce oxygen as a byproduct of photosynthesis.
Chlorophyta: Green microalgae are some of the most common and well-studied microalgae. They contain chlorophyll a and b, as well as other pigments like β-carotene and xanthophylls.
Coccolithophyceae: Also known as coccolithophores, are a distinct group of marine phytoplankton belonging to the phylum Haptophyta. These tiny single-celled algae are characterized by their unique calcium carbonate plates called coccoliths, which surround their cell walls. The presence of coccoliths gives these organisms a remarkable appearance and makes them easily identifiable under the microscope.
Cryptophyceae: Often referred to as cryptophytes, are a group of unicellular algae belonging to the phylum Cryptophyta. These microorganisms are primarily found in fresh-water and marine environments, where they play essential roles in aquatic ecosystems.
Euglenophyceae: Commonly known as euglenoids, are a diverse group of unicellular flagellated microorganisms that belong to the phylum Euglenophyta. These organisms are found in various aquatic environments, including freshwater, brackish water, and sometimes marine habitats. Euglenoids are unique in that they share characteristics of both plants and animals, making their classification a subject of historical debate.
Eustigmatophyceae: Also known as eustigmatophytes, are a small group of unicellular microalgae belonging to the phylum Ochrophyta. These algae are found in various aquatic environments, including freshwater, marine, and soil habitats. Eustigmatophytes are photosynthetic organisms and play important roles in aquatic ecosystems.
Haptophyta (haptophytes): These microalgae are characterized by the presence of unique scales or plates made of calcium carbonate. They are abundant in marine environments.
Miozoa: Dinoflagellates are characterized by two flagella, which enable them to move through the water. Some species are bioluminescent and responsible for “red tides” events.
Porphyridiophyceae: Is a class of red algae (Rhodophyta) that includes a group of unicellular or filamentous marine algae. They are commonly found in various marine environments, from tropical to temperate regions. As part of the red algae, they have distinctive pigments, such as phycoerythrin and phycocyanin, which give them their characteristic red color and allow them to photosynthesize efficiently in deeper waters where blue and green light are scarce.
Thus, there is a vast number of species of microalgae which can be further exploited.
Characteristics
Microalgae possess several characteristics that make them attractive for various applications, including their role as natural antioxidant factories. Microalgae are photosynthetic organisms, utilizing sunlight to produce energy-rich compounds such as carbohydrates and lipids. During photosynthesis, they also produce antioxidants to protect themselves from ROS generated as byproducts of their metabolic processes [17, 18]. Many microalgae species have rapid growth rates, allowing for efficient biomass production in a relatively short time. This characteristic makes them suitable for large-scale cultivation and commercial use. Microalgae are also rich in proteins, essential fatty acids, vitamins, minerals, and other bioactive compounds. They have a huge potential to serve as a sustainable source of high-quality nutrition for humans and animals [19]. However, due to biochemical variation, there is a need to select the species by their growth potential, bio-chemical profiling, economic output, and sustainability.
Microalgae like Dunaliella (Figure 2a), Chlorella (Figure 2b), Haematococcus (Figure 2c), Scenedesmus (Figure 2d), and Trentepohlia (Figure 2e) (Chlorophyta), and Euglena (Euglenophyceae), produce a range of antioxidants, such as carotenoids (e.g., β-carotene, lutein, astaxanthin), tocopherols (vitamin E), and phycobiliproteins, produced by Porphyridium (Figure 2f) (Rhodophyta). These antioxidants can help neutralize free radicals and protect cells from oxidative damage, making them valuable for various health and cosmetic applications. In recent years, researchers and industries have been exploring microalgae’s potential as natural antioxidant factories, harnessing their ability to produce valuable antioxidants for various applications in food, pharmaceuticals, and cosmetics, among others [13]. Additionally, ongoing research aims to improve cultivation techniques and optimize antioxidant production to make microalgae-based products more economically viable and sustainable [20]. Microalgae play a crucial role in carbon fixation and oxygen production, contributing to global carbon and oxygen cycles. Additionally, they have the potential to be utilized in wastewater treatment and carbon capture technologies [21, 22].
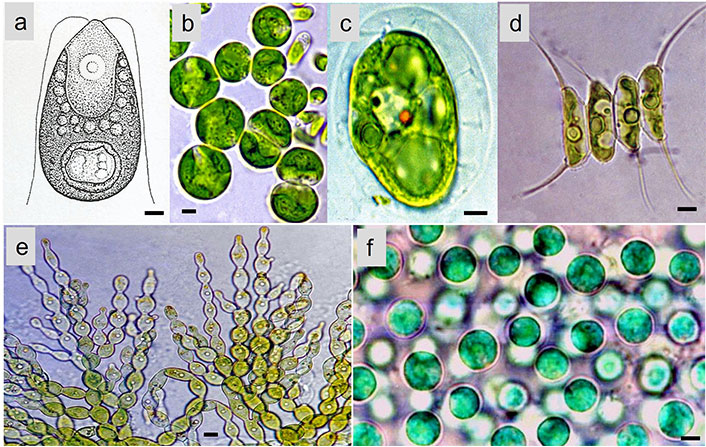
Some common genera of microalgae. (a) Dunaliella; (b) Chlorella; (c) Haematococcus; (d) Scenedesmus; (e) Trentepohlia (Chlorophyta); (f) Porphyridium (Rhodophyta). Scale bar: 20 μm
Bioactive compounds in microalgae with antioxidant properties
Thus, as above mentioned, microalgae have a wide range of compounds, some of which have an innate antioxidant activity. These compounds play a significant role in protecting cells from oxidative stress and damage caused by ROS. Microalgae are rich in carotenoids, which are responsible for their characteristic colors (green, yellow, orange, red). Carotenoids, such as β-carotene, astaxanthin, lutein, and zeaxanthin, are potent antioxidants that can neutralize free radicals and protect cells from oxidative damage (see Table 1) [13, 20, 23].
| Microalgae species | Antioxidant production | Stress factor |
|---|---|---|
| Arthrospira platensis (Cyan) | Ascorbic acid (AA) | pH alteration and nutrient limitation |
| Mycosporine-like amino acids (MAAs) | ||
| Polyphenols | ||
| Chlamydomonas acidophila (C) | Carotenoids | Light intensity |
| Chlorella sorokiniana (C) | Ascorbate peroxidase (APX) | Metal exposure |
| Superoxide dismutase (SOD) | ||
| Ascorbate (AsA) | ||
| Proline | ||
| Tocopherols | ||
| MAAs | Ultraviolet-A (UV-A) and UV-B radiation and metal exposure | |
| Chlorella vulgaris (C) | SOD | UV-B radiation and metal exposure |
| AA | UV-B radiation and nutrient limitation | |
| Carotenoids | Temperature, UV-A and UV-B radiation | |
| Proline | Metal exposure | |
| Tocopherols | Nutrient limitation | |
| Chlorococcum infusionum (as Chlorococcum humicola; C) | Peroxidase (POD) | Metal exposure |
| Coelastrella vacuolata (as Scenedesmus vacuolatus; C) | Catalase (CAT) | Metal exposure |
| AA | UV-A and UV-B radiation | |
| MAAs | pH alteration and nutrient limitation | |
| Diacronema viridis (as Pavlova viridis; Hap) | CAT | Metal exposure |
| Glutathione POD (GPX) | ||
| Glutathione (GSH) | ||
| Dunaliella salina (C) | SOD | UV-B radiation and metal exposure |
| MAAs | ||
| AA | UV-B radiation and nutrient limitation | |
| Phaeodactylum tricornutum (Bac) | AA | UV-B radiation and nutrient limitation |
| Tocopherols | Nutrient limitation | |
| Tetraselmis suecica (C) | AA | UV-B radiation and nutrient limitation |
| Tocopherols | Nutrient limitation | |
| Tetradesmus dimorphus (as Acutodesmus dimorphus; C) | CAT | pH alteration and nutrient limitation |
| SOD | ||
| Proline | Nutrient limitation and salinity | |
| Tetradesmus lagerheimii (as Scenedesmus acuminatus; C) | APX | Metal exposure |
| GPX | ||
| POD | ||
| Proline | ||
| MAAs | UV-A and UV-B radiation and metal exposure | |
| Polyphenols |
Bac: Bacillariophyceae; C: Chlorophyta; Cyan: Cyanophyceae; Hap: Haptophyta
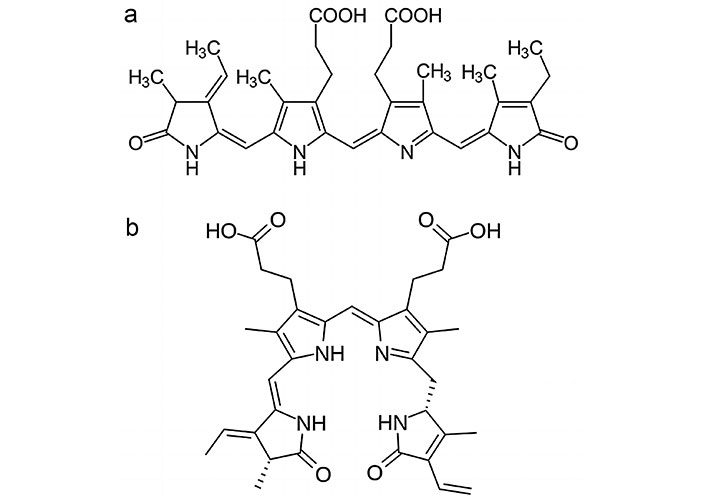
Chlorophylls are essential pigments for photosynthesis in microalgae. Besides their role in photosynthesis, they also possess antioxidant properties, helping to scavenge free radicals and reduce oxidative stress [24]. Phycobiliproteins are water-soluble pigments found in some groups of microalgae, like Anabaena (Figure 3a), Nostoc (Figure 3b), and Tolypothrix (Figure 3c) (Cyanophyceae), and red microalga like Porphyridium. These pigments, including phycocyanin (Figure 4a) and phycoerythrin (Figure 4b), have strong antioxidant activities and can protect cells from oxidative damage [25, 26].
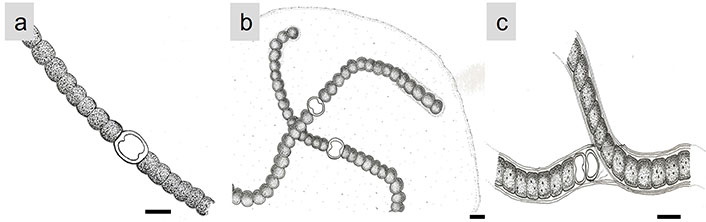
Some filamentous cyanobacteria. (a) Anabaena; (b) Nostoc; (c) Tolypothrix (Cyanophyceae). Scale bar: 20 μm
Some microalgae species like Chlorella, Scenedesmus, Trentepohlia, and Euglena (Figure 5a) (Euglenophyceae), produce polyphenolic compounds, including flavonoids and phenolic acids, which are well-known for their antioxidant properties. These compounds can neutralize free radicals and exhibit various health benefits [6, 13]. Microalgae like Dunaliella and Porphyridium can also contain vitamin E compounds, such as tocopherols and tocotrienols, which act as antioxidants to protect cellular membranes from lipid peroxidation [27]. Some microalgae, as is the case with the green alga Dunaliella and the blue-green Spirulina/Arthrospira (Figure 5b), produce antioxidant enzymes like superoxide dismutase (SOD), which catalyze the breakdown of super-oxide radicals, reducing oxidative stress in cells. Microalgae also contain glutathione (GSH), a tripeptide antioxidant that helps in maintaining cellular redox balance by scavenging free radicals [28].
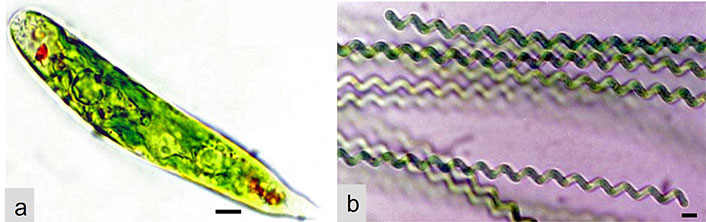
Two common genera of microalgae. (a) Euglena (Euglenophyceae); (b) Spirulina/Arthrospira (Cyanophyceae). Scale bar: 20 μm
The antioxidant properties of these bioactive compounds make microalgae potential candidates for various applications in the nutraceutical, pharmaceutical, and cosmetic industries. Extracts and supplements derived from microalgae are being explored for their health-promoting effects and their potential to combat oxidative stress-related diseases [6]. It’s important to note that the antioxidant potential of microalgae can vary depending on the species, growing conditions, and extraction methods. Researchers continue to study and explore microalgae to harness their antioxidant capabilities and unlock their potential as natural antioxidant factories [29].
Factors influencing antioxidant production in microalgae
The production of antioxidants in microalgae is influenced by various factors, which can either enhance or inhibit their synthesis. Understanding these factors is crucial for optimizing antioxidant production in microalgae for different applications. Some of the key factors influencing antioxidant production in microalgae include light is a critical factor for photosynthesis in microalgae, and it also affects the production of antioxidants [30]. Adequate light intensity can stimulate the synthesis of pigments like carotenoids and phycobiliproteins, which have strong antioxidant properties. However, excessive light exposure may lead to oxidative stress and reduce antioxidant production [31].
The availability of essential nutrients such as nitrogen, phosphorus, sulfur, and trace elements influences the growth and metabolism of microalgae. Optimal nutrient conditions can enhance antioxidant production, while nutrient deficiencies may limit their synthesis. However, sometimes, unsaturated fatty acids are produced in cases of nutrient deficiency [32]. Temperature plays a significant role in the growth and physiological activities of microalgae. Moderate temperatures generally support higher antioxidant production, but extreme temperatures can lead to oxidative stress and affect antioxidant levels [33]. Microalgae have different tolerance levels for salinity. Changes in salinity can affect the production of antioxidants in marine and halotolerant microalgae [34].
Exposure to certain environmental stressors, such as high light, ultraviolet (UV) radiation, heavy metals, or pollutants, can induce oxidative stress in microalgae. As a response, microalgae may increase their antioxidant production to counteract the harmful effects of ROS [35]. Factors such as pH, aeration, agitation, and culture medium composition can influence antioxidant production in microalgae. Maintaining optimal culture conditions is crucial for promoting the synthesis of antioxidants. Microalgae activate specific stress responses and signaling pathways in response to various environmental cues. These pathways regulate the expression of genes involved in antioxidant synthesis [18].
The stage of growth and cell density can influence antioxidant production in microalgae. Antioxidant levels may vary during different growth phases, and higher cell densities may lead to higher antioxidant synthesis due to increased metabolic activity. The genetic makeup of microalgae can significantly impact their ability to synthesize antioxidants. Different species and strains may have varying antioxidant capacities based on their genetic characteristics [36].
The methods used for harvesting and post-harvest processing of microalgae can affect the stability and preservation of antioxidants. To maximize antioxidant production in microalgae, researchers and cultivators need to optimize these factors based on the specific microalgal species and their intended applications. Understanding the interplay of these factors can lead to the development of sustainable and efficient strategies for producing antioxidant-rich microalgae products [37]. To know the target compound, there is a need to observe and select very well a microalgae species and do a preliminary biochemical characterization to observe if the targeted compound or compounds are feasible, even before the compound extraction and purification. Thus, a technique known as microalgae cultivation has been evolving to ensure the quality and safety of microalgae and microalgae compounds. However, in order to fully use this methodology, cultivation methods must be developed from a multidisciplinary standpoint to guarantee the quality and stability of the microalgae targeted compound [29].
Extraction and purification techniques of microalgae-derived antioxidants
Although, these beneficial antioxidant compounds that microalgae species have, there is a need to isolate from the microalgae biomass, after selecting the target compound(s). Thus, before testing the compounds in vitro and in vivo assays, there is a need to extract and purify the compounds with low impurity rates to be accepted for further analysis. This is a key vital point to reduce impurities and to promote the compound chemical and bioactivity stability to be further exploited.
Various extraction methods for efficient recovery
Extraction of antioxidants from microalgae involves separating the bioactive compounds from the algal biomass. Several extraction methods are employed to efficiently recover antioxidants from microalgae [38]. Each method has its advantages and limitations, and the choice of technique depends on factors such as the type of antioxidant, microalgal species, and the intended application. Some common extraction methods are listed in the next paragraphs.
Solvent extraction is one of the most common methods for extracting antioxidants from microalgae. Solvents such as ethanol, methanol, acetone, or a mixture of organic solvents are used to dissolve the antioxidants from the microalgal biomass. The solvent extracts are then concentrated and purified through further processing [39].
In supercritical fluid extraction (SFE), supercritical carbon dioxide (CO2) is used as the extraction solvent. This method offers advantages like lower environmental impact and mild extraction conditions, avoiding the use of potentially harmful organic solvents. SFE has been applied to extract carotenoids and other lipophilic antioxidants from microalgae [40].
Pressurized liquid extraction (PLE), also known as accelerated solvent extraction, employs high pressure and temperature with solvents to extract antioxidants efficiently. It allows for shorter extraction times compared to traditional solvent extraction [41].
In microwave-assisted extraction (MAE), microwave irradiation is used to accelerate the extraction process by heating the solvent and microalgal matrix. This method can significantly reduce extraction time and improve extraction efficiency [42].
Ultrasound-assisted extraction (UAE) utilizes ultrasonic waves to enhance the extraction process. The cavitation effect generated by ultrasound disrupts the cell walls of microalgae, facilitating the release of antioxidants into the solvent [43].
In the enzyme-assisted extraction (EAE) method, enzymes can be used to degrade the cell walls of microalgae, aiding the release of antioxidants. EAE can improve the yield and bio-accessibility of antioxidants [44].
Subcritical water extraction (SWE) employs hot water under high pressure as an extraction medium. It can efficiently extract both hydrophilic and lipophilic antioxidants from microalgae [45].
Ionic liquid (IL)-based extraction—ILs are salts in a liquid state at low temperatures, and they have been used as alternative solvents for the extraction of antioxidants due to their unique properties and ability to solubilize a wide range of compounds [46].
Solid-phase extraction (SPE) is a purification technique used to concentrate and purify antioxidants from crude extracts. It employs a solid sorbent material to selectively retain target compounds, allowing unwanted compounds to be washed away [47].
Fractionation methods such as chromatography [e.g., column chromatography, high-performance liquid chromatography (HPLC)] and thin-layer chromatography (TLC) can be used to separate and isolate specific antioxidants from complex mixtures [48].
Optimizing extraction parameters such as temperature, time, solvent-to-sample ratio, and the use of multiple extraction techniques in combination can enhance the efficiency of antioxidant recovery from microalgae. It is essential to evaluate the antioxidant activity and purity of the extracts to ensure their suitability for various applications, such as functional foods, nutraceuticals, and cosmetic formulations [49].
Purification techniques for concentrating antioxidant compounds
After the initial extraction, further purification is often required to concentrate and isolate specific antioxidant compounds from the crude extracts. Various purification techniques are employed to achieve this goal. For example, fractionation involves the use of different solvents of varying polarities to partition the crude extract into different fractions. By selecting appropriate solvents, specific antioxidant compounds can be concentrated into separate fractions based on their polarity and solubility [50].
Column chromatography is a widely used technique for the purification of antioxidants. The crude extract is loaded onto a column filled with a stationary phase (e.g., silica gel or a resin) and eluted with a solvent or a solvent gradient. Antioxidant compounds with different polarities will elute at different rates, allowing for their separation and concentration [39].
HPLC is a powerful chromatographic technique that provides high resolution and precise control over the separation of antioxidant compounds. It involves passing the extract through a high-pressure column packed with a stationary phase. The elution is performed with a solvent gradient, and specific antioxidants can be collected in pure form based on their retention times [51, 52].
Preparative of TLC can be used for preparative purposes to separate and concentrate antioxidant compounds from the crude extract. The bands corresponding to the desired antioxidants are scraped off the TLC plate and further processed to obtain purified compounds.
Techniques such as salting-out, protein precipitation, and solvent precipitation can be used to concentrate antioxidant compounds. These methods involve adding specific agents or changing the solvent conditions to cause the precipitation of the desired antioxidants, which can then be collected and further purified [53].
SPE can be utilized as a purification technique to selectively retain antioxidant compounds on a solid sorbent material while washing away unwanted components. The retained antioxidants are then eluted and concentrated for further analysis or applications [54].
Membrane-based techniques, such as ultrafiltration and nanofiltration, can be used to concentrate antioxidant compounds based on their molecular size. Smaller antioxidant molecules pass through the membrane, while larger impurities are retained, resulting in a concentrated fraction [55].
Centrifugation can be used to separate and concentrate antioxidant compounds based on their density and size. Differential centrifugation or density gradient centrifugation can be employed to achieve the purification of specific antioxidants. Crystallization involves the controlled precipitation of antioxidant compounds from a solution, leading to their concentration and purification [39].
Combining multiple purification techniques in a sequential manner can enhance the purity and concentration of antioxidant compounds from microalgae extracts. The choice of the most suitable purification method depends on the specific properties of the antioxidants of interest and the complexity of the crude extract. Proper characterization and analysis of the purified antioxidants are crucial to ensure their identity and quality for various applications [56].
These two steps (extraction and purification) need to be very controlled due to being vital for the bioactive assay, where impurities or chemical conformation of the molecule can affect the assay directly with false positives or contradictory results.
Benefits from microalgae-derived antioxidants
Because of their abundance of primary and secondary bioactive metabolites including carbohydrates, proteins, lipids, vitamins, and pigments, these photosynthetic microorganisms are recognized as one of the greatest renewable resources for several therapeutic substances. However, their isolated compounds or extracts need to be accessed beforehand using a two-step technique:
In vitro studies to observe antioxidant and cytotoxic potential, which can be further explored to the compound mechanism of action and pharmacokinetics.
In vivo studies if the in vitro results are good, the compound can be studied in organisms to understand their potential and collect data for the preclinical assay, mostly pharmacokinetics and pharmacodynamics, and side effects.
In vitro studies on microalgae-derived antioxidants
In vitro studies on microalgae-derived antioxidants often involve evaluating their antioxidant activity in cell-free assays. These assays are conducted outside a living organism and involve isolated cellular components or chemical systems that mimic the oxidative stress conditions that antioxidants may encounter in biological systems [21].
Evaluation of antioxidant activity in cell-free assays
The purpose of these assays is to assess the ability of microalgae-derived antioxidants to scavenge free radicals and reduce oxidative damage [13, 20]. The main assess tests used are described below.
DPPH (2,2-diphenyl-1-picrylhydrazyl) assay is one of the most widely used methods for evaluating antioxidant activity. DPPH is a stable free radical that appears purple in solution. When an antioxidant is added to the DPPH solution, it reduces the free radical, resulting in a color change from purple to yellow. The degree of discoloration indicates the scavenging ability of the antioxidant [57, 58].
ABTS [2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] is another stable free radical that is used to assess the antioxidant capacity of compounds. The ABTS assay measures the reduction of the ABTS radical, which is accompanied by a color change. The decrease in absorbance at a specific wavelength indicates the antioxidant activity of the tested compound [58].
The ferric reducing antioxidant power (FRAP) assay evaluates the ability of antioxidants to reduce ferric ions (Fe3+) to ferrous ions (Fe2+) in the presence of a reducing agent. The reduction is measured spectrophotometrically and provides information on the antioxidant potential of the test sample [59].
The total antioxidant capacity (TAC) assay measures the overall antioxidant capacity of a sample. It involves generating free radicals in the presence of the antioxidant, and the decrease in free radicals is determined by using a suitable marker. The TAC assay provides a comprehensive evaluation of the cumulative antioxidant activity of the tested compound [60].
Oxygen radical absorbance capacity (ORAC) assay is one method to measure antioxidant capacity. The fluorescence decay rate is monitored, and the antioxidant capacity is calculated based on the degree of inhibition of the fluorescence decay [61].
Lipid peroxidation inhibition assay is an assay that assesses the ability of antioxidants to prevent lipid peroxidation, a process that damages cell membranes and other lipids. The inhibition of lipid peroxidation is determined by measuring the formation of thiobarbituric acid reactive substances (TBARS) or other lipid peroxidation markers [62].
SOD assay is an enzyme that plays a crucial role in the defense against oxidative stress by converting superoxide radicals into hydrogen peroxide and oxygen. The SOD assay measures the inhibition of superoxide-mediated reduction of a tetrazolium dye, providing insights into the antioxidant activity of the test compound [63].
These cell-free assays help researchers identify microalgae-derived antioxidants with strong free radical scavenging and antioxidant potential. However, it’s essential to validate the results from cell-free assays with further studies, including in vivo experiments and clinical trials, to determine their effectiveness and safety in living systems before potential applications in the pharmaceutical or nutraceutical industries [64].
Mechanisms of action of microalgae-derived antioxidants
Microalgae-derived antioxidants exhibit their protective effects against oxidative stress through various mechanisms of action. These mechanisms are attributed to the specific chemical compounds present in the microalgae, which act as antioxidants or promote the antioxidant defense system within cells. Some of the key mechanisms of action of microalgae-derived antioxidants are described below.
Microalgae-derived antioxidants, such as carotenoids (e.g., astaxanthin, β-carotene) and phycobiliproteins (e.g., phycocyanin, phycoerythrin), can directly neutralize free radicals and ROS. They donate electrons to ROS, thereby stabilizing and reducing their damaging effects on cellular components like lipids, proteins, and DNA. Some microalgae-derived antioxidants possess metal-chelating properties, meaning they can bind to transition metal ions (e.g., iron, copper) that contribute to the generation of ROS through Fenton reactions. By chelating these metals, antioxidants prevent ROS formation and reduce oxidative damage [65–67].
Microalgae-derived antioxidants may stimulate the activity of endogenous antioxidant enzymes, such as SOD, catalase (CAT), and GSH peroxidase (POD; GPX). These enzymes work together to neutralize ROS and maintain redox balance in cells. Certain microalgae-derived antioxidants, like omega-3 fatty acids and polyphenols, have anti-inflammatory properties. By reducing inflammation, they indirectly contribute to the reduction of oxidative stress, as inflammation is closely linked to the generation of ROS [1, 68].
Various microalgae-derived antioxidants can activate the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Nrf2 is a transcription factor that regulates the expression of various antioxidant and detoxification genes, enhancing the cellular defense against oxidative stress. Microalgae-derived antioxidants may protect mitochondria, the cellular powerhouses, from oxidative damage. Preserving mitochondrial function helps maintain cellular energy production and reduces the release of ROS from malfunctioning mitochondria [69].
Autophagy is a cellular process that removes damaged components and helps maintain cellular homeostasis. Certain microalgae-derived antioxidants have been found to regulate autophagy, which can contribute to cellular protection against oxidative stress. Oxidative stress can damage DNA, leading to mutations and potentially harmful consequences. Microalgae-derived antioxidants may aid in DNA repair mechanisms, preventing long-term damage and maintaining genetic integrity [70].
It’s important to note that the specific mechanisms of action can vary depending on the type of microalgae, the particular antioxidant compounds present, and the biological context in which they are applied. Additionally, the interactions among different antioxidants and their combined effects may further enhance their overall antioxidant capacity. Further research and studies are needed to fully elucidate the precise mechanisms of action of microalgae-derived antioxidants and their potential therapeutic applications [6, 13, 20].
In vivo studies and health benefits
Animal models are essential tools in scientific research for investigating the health effects of microalgae-derived antioxidants [6]. These models allow researchers to study the physiological and biochemical responses of living organisms to the consumption of microalgae or their isolated antioxidant compounds.
Animal models investigating the health effects of microalgae-derived antioxidants
Animal studies provide valuable insights into the potential health benefits, safety, and mechanisms of action of these antioxidants. Below are listed some of the common types of animal models used in such research [5, 8, 20].
Mice and rats are commonly used in animal studies due to their small size, short reproductive cycle, and genetic similarity to humans. These models allow researchers to investigate various health aspects, such as antioxidant activity, anti-inflammatory effects, lipid metabolism, and immune response, after administering microalgae-derived antioxidants through diet or other means [71, 72].
Zebrafish and other fish species are used as models to study the effects of microalgae-derived antioxidants on aquatic organisms. Fish models can provide insights into oxidative stress, neuroprotection, cardiovascular health, and other relevant parameters in aquatic environments [73].
Pigs are anatomically and physiologically more similar to humans than rodents, making them valuable models for studying nutritional interventions and health outcomes. Pigs can help researchers understand the impact of microalgae-derived antioxidants on gut health, lipid metabolism, and overall systemic effects [74].
Non-human primates, such as monkeys, share significant genetic and physiological similarities with humans. While their use is less common due to ethical considerations and cost, primate models offer valuable data regarding the potential effects of microalgae-derived antioxidants on human health [75].
Birds, such as quails or chickens, are also used as models in some studies to investigate the health effects of microalgae-derived antioxidants. These models can provide insights into antioxidant activity, egg quality, and other relevant parameters [76].
Some key health benefits that have been studied in animal models regarding microalgae-derived antioxidants include animal studies that can confirm the antioxidant capacity of microalgae-derived compounds and their ability to reduce oxidative stress in various tissues and organs. Microalgae-derived antioxidants may modulate inflammatory responses in animals, potentially providing benefits for inflammatory conditions [5, 6, 20].
Animal models can be used to evaluate the impact of microalgae-derived antioxidants on blood pressure, lipid profiles, and other cardiovascular parameters. Also, research on animal models helps understand the potential neuroprotective effects of microalgae-derived antioxidants in conditions like Alzheimer’s, Parkinson’s, or stroke [13, 77]. Animal studies can provide insights into the effects of microalgae-derived antioxidants on liver function and hepatic health. Research on animals may reveal the impact of microalgae-derived antioxidants on immune function and overall immune system support [27, 78].
It’s important to note that while animal studies provide valuable preliminary data, further research, including human clinical trials, is essential to validate the health benefits and safety of microalgae-derived antioxidants for human consumption. Animal studies serve as an important steppingstone in the research process, helping to identify promising compounds and potential health effects that warrant further investigation in human subjects [5, 6, 77].
Protective effects against oxidative damage, inflammation, and chronic diseases
Microalgae-derived antioxidants have been studied for their potential protective effects against oxidative damage, inflammation, and chronic diseases. These protective effects are attributed to their ability to scavenge free radicals and reduce oxidative stress, as well as their anti-inflammatory properties. Here are some of the chronic diseases and health conditions where microalgae-derived antioxidants have shown promise in animal and in vitro studies [79].
Oxidative stress plays a significant role in the development of cardiovascular diseases, including atherosclerosis and hypertension. Microalgae-derived antioxidants, such as astaxanthin and omega-3 fatty acids, have demonstrated potential in reducing oxidative damage to blood vessels, lowering blood pressure, and improving lipid profiles in animal models [80].
Chronic inflammation and oxidative stress are implicated in neurodegenerative conditions like Alzheimer’s and Parkinson’s diseases. Studies on microalgae-derived antioxidants, particularly astaxanthin and phycocyanin, have shown neuroprotective effects and the potential to mitigate neuroinflammation in animal models [81, 82].
The liver is susceptible to oxidative damage due to its role in detoxification processes. Microalgae-derived antioxidants, such as Spirulina and Chlorella extracts, have demonstrated hepatoprotective effects in animal models, reducing liver injury induced by toxins and oxidative stress [83, 84].
Oxidative stress and inflammation are involved in the development of insulin resistance and metabolic syndrome. Certain microalgae-derived compounds, such as fucoxanthin from brown algae, have shown potential in improving insulin sensitivity and reducing markers of inflammation in animal studies related to diabetes and metabolic syndrome [85]. Oxidative stress is also associated with DNA damage and the promotion of cancer development. Microalgae-derived antioxidants, such as carotenoids and polyphenols, have exhibited anti-cancer properties in animal and cell culture studies by reducing oxidative damage and inhibiting tumor cell growth [86]. Some microalgae-derived compounds, such as fucoxanthin, have been studied for their potential in reducing body weight and adipose tissue accumulation in animal models of obesity [87, 88].
Microalgae-derived antioxidants like astaxanthin and β-carotene have shown promise in protecting the skin from UV-induced oxidative damage and promoting skin health in animal studies. Microalgae-derived compounds, particularly polysaccharides and phycobiliproteins, have also been investigated for their potential prebiotic and anti-inflammatory effects on gut health [27, 80].
It’s important to emphasize that while animal and in vitro studies provide valuable insights into the potential health benefits of microalgae-derived antioxidants, human clinical trials are necessary to establish their efficacy and safety for therapeutic use [74, 89]. The bioavailability, dosage, and long-term effects in humans need to be thoroughly investigated before these compounds can be recommended for the prevention or treatment of chronic diseases. Additionally, individual responses to antioxidants may vary based on genetic factors, diet, and lifestyle, further highlighting the need for human studies to validate their effects on specific health conditions [90].
Microalgae-based antioxidants for skin health, immune system modulation, and cellular rejuvenation
Microalgae-based antioxidants have gained attention for their potential benefits in promoting skin health, modulating the immune system, and supporting cellular rejuvenation. These antioxidants are rich in various bioactive compounds that can help protect the skin from oxidative damage, boost the immune response, and promote the regeneration of cells [91].
Skin health
Microalgae-derived antioxidants, such as astaxanthin and β-carotene (Figure 1), have been studied for their ability to scavenge free radicals induced by UV radiation. By reducing UV-induced oxidative stress, these antioxidants may help protect the skin from sunburn, premature aging (wrinkles, fine lines), and other UV-related damage. Certain microalgae-derived compounds, like phycocyanin and polysaccharides, have shown potential in promoting collagen synthesis and cellular regeneration, contributing to improved skin elasticity and texture. Some microalgae extracts contain polysaccharides that help retain moisture in the skin, improving hydration and reducing dryness [20, 92].
Some microalgae-based antioxidants possess anti-inflammatory properties, which can be beneficial for skin conditions characterized by inflammation, such as acne, eczema, and psoriasis. By reducing inflammation, these antioxidants may help soothe irritated skin and promote its overall health [93].
Immune system modulation
Microalgae-based antioxidants, particularly polysaccharides and phycobiliproteins extracted from Dunaliella, Chlorella, and Haematococcus (Chlorophyta), and Arthrospira/Spirulina (Cyanophyceae) have been investigated for their immunomodulatory effects. They may help enhance immune function by stimulating the activity of immune cells, such as macrophages and lymphocytes, thereby supporting the body’s defense against infections and diseases. By reducing inflammation, microalgae-derived antioxidants may help regulate the immune response and prevent excessive immune reactions that can lead to chronic inflammatory conditions [6, 29, 93].
Microalgae-based antioxidants can neutralize free radicals and reduce oxidative stress, which is a major contributor to cellular aging. By protecting cells from oxidative damage, these antioxidants may help maintain cellular health and delay the aging process. Some microalgae-derived compounds have also been shown to promote cell repair and regeneration processes, which are essential for maintaining tissue health and function [6, 94, 95].
It’s important to note that while in vitro and animal studies have provided promising results regarding the potential benefits of microalgae-based antioxidants for skin health, immune system modulation, and cellular rejuvenation, human clinical trials are necessary to validate these effects in humans [96]. Additionally, individual responses to antioxidants may vary, and factors like dosage, formulation, and application method need to be carefully considered in potential therapeutic applications. Always consult with a healthcare professional or dermatologist before using any microalgae-based antioxidant products or supplements for specific health concerns [96].
Sustainable production and cultivation of microalgae
Even, with all the information above where microalgae can be useful as antioxidants, there is a question, how to mass produce microalgae with the same quality and yield? By using cultivation techniques, which can be a feasible way to promote new natural raw sources for antioxidant compounds.
Microalgae selected compounds that obtained positive results in in vitro and in vivo assays can be further exploited. However, microalgae cultivation scale-up and compound safety difficulties continue to be significant barriers to the cost-effective commercialization of microalgal compounds. However, ways for overcoming these obstacles and effectively marketing microalgae-derived goods in the pharmaceutical sectors have been created.
Photobioreactors and open ponds for large-scale cultivation
The sustainable production and cultivation of microalgae involve the use of various cultivation systems, with photobioreactors and open ponds being the two primary methods for large-scale production [97].
Photobioreactors
Photobioreactors (Figure 6) are enclosed systems designed to optimize microalgae growth by controlling various environmental factors. These closed cultivation systems offer several advantages. Photobioreactors allow precise control of temperature, light intensity, pH, and nutrient concentration, which helps maximize microalgae growth and productivity [97, 98].

Photobioreactors. (a) Laminate; (b) tubular (made up of 11 overlapping tubes); (c) tubular (made up of 24 overlapping tubes)
The closed nature of photobioreactors minimizes the risk of contamination from external sources, such as competing microorganisms or pollutants. So, the controlled environment in photobioreactors can lead to higher microalgae biomass and better quality of target compounds. Photobioreactors enable year-round cultivation, making them suitable for locations with varying climatic conditions [99].
However, photobioreactors also have some limitations since the construction and maintenance of photobioreactors can be more expensive compared to open pond systems. Operating a photobioreactor requires energy input for controlling various parameters, leading to higher operational costs. Scaling up photobioreactors to very large volumes can be challenging due to engineering and cost constraints [100].
Open ponds
Open ponds (Figure 7a) are large, shallow basins where microalgae are cultivated under natural sunlight. They are more straightforward and cost-effective compared to photobioreactors and are commonly used for large-scale microalgae cultivation [101].

Microalgae cultivation in open systems. (a) Open ponds; (b) overview of a raceway; (c) detail of the rotating blades of a raceway
Open ponds are relatively simple to construct and operate, resulting in lower capital and energy expenses. Expanding the cultivation area in open ponds is more feasible than scaling up photobioreactors. Open ponds use natural sunlight, which is abundant and does not require additional energy input. The open raceway pond system (Figure 7b and c) has a very simple structure consisting of a closed raceway and paddle wheel. However, open systems are susceptible to contamination from competing microorganisms, which can affect microalgae growth and product purity. Also, the productivity of open ponds can be influenced by weather conditions like temperature, sunlight availability, and rainfall. In regions with severe winters or extreme climates, open ponds may not be suitable for year-round cultivation [102].
Hybrid systems
To optimize the advantages and mitigate the limitations of both photobioreactors and open ponds, some large-scale microalgae cultivation facilities use hybrid systems. These hybrid systems combine the advantages of controlled conditions in photobioreactors with the scalability and lower cost of open ponds. For instance, microalgae can be initially grown in photobioreactors and then transferred to open ponds for further cultivation and biomass expansion [103].
Overall, the choice between photobioreactors and open ponds depends on factors such as the specific microalgae species, intended application, location, available resources, and economic considerations. Sustainable cultivation practices, efficient resource utilization, and continuous advancements in cultivation technology play crucial roles in the successful large-scale production of microalgae for various applications, including food, feed, biofuels, pharmaceuticals, and more [104].
Environmental and economic considerations for sustainable production
Sustainable production of microalgae involves careful consideration of environmental and economic factors to ensure responsible and efficient cultivation practices. Microalgae cultivation requires water, and in water-scarce regions, sustainable practices must be employed to optimize water usage. Techniques like water recycling and using brackish or saline water can reduce freshwater consumption [105].
Selecting suitable land for microalgae cultivation is essential to avoid encroaching on ecologically sensitive areas. Utilizing non-arable land or integrating microalgae cultivation with existing facilities (e.g., wastewater treatment plants) can minimize land use conflicts. Efficient nutrient utilization is crucial to prevent nutrient runoff and eutrophication in surrounding ecosystems. Proper nutrient recycling and balancing nutrient inputs to match microalgae requirements can reduce environmental impacts [105, 106].
Energy-intensive cultivation methods can result in higher carbon emissions. Adopting renewable energy sources or using waste CO2 from industrial processes can help minimize the carbon footprint of microalgae cultivation [107].
Care must be taken to prevent the introduction of non-native microalgae species into natural ecosystems, as they can lead to unintended ecological disruptions. Controlling contamination by unwanted microorganisms is crucial to maintain the purity of microalgae cultures and prevent potential ecological disruptions if released into the environment [108].
Sustainable microalgae production should be economically viable. The choice of cultivation system, resource utilization, and product marketability play a significant role in determining the economic feasibility of the operation. Exploring a range of high-value products from microalgae, such as nutraceuticals, biofuels, and specialty chemicals, can enhance the economic viability of the production process. Identifying efficient value chains, from cultivation to downstream processing and marketing, can reduce production costs and increase overall profitability [4, 109, 110].
Understanding market demand for microalgae-derived products is crucial for ensuring a stable and profitable market for cultivated biomass. Supportive policies and incentives from governments and institutions can encourage investments in sustainable microalgae production and help promote its commercial success [111].
Ongoing research and technological advancements can lead to more cost-effective cultivation methods and higher-value product development. Exploring ways to utilize by-products and waste streams from microalgae cultivation can add value to the overall production process and reduce waste disposal costs [112]. Balancing environmental sustainability with economic viability is a critical aspect of sustainable microalgae production. By implementing environmentally responsible practices and optimizing economic considerations, microalgae cultivation can contribute to various industries while minimizing its impact on natural resources and ecosystems [113].
Microalgae potential therapeutics and challenges
Although microalgae can be utilized to manufacture potential therapeutics, safety and regulatory difficulties remain important concerns, and more research is needed to make microalgae a commercial success in the future. Making the microalgal pharmaceutical and biomedical sector economically viable involves several practical challenges.
Potential applications in preventive and therapeutic strategies
Microalgae-based antioxidants and bioactive compounds hold great potential for various preventive and therapeutic strategies in medicine, nutrition, and biotechnology. As research in this area continues to progress, the following are some potential applications where microalgae-derived compounds could play a significant role [6, 11, 20].
Microalgae-derived antioxidants, such as astaxanthin and phycocyanin, have demonstrated potent free-radical scavenging abilities and anti-aging effects in preclinical studies. These compounds could find applications in skincare products, dietary supplements, and pharmaceutical formulations targeting age-related conditions [67, 88].
Certain microalgae-derived compounds, such as omega-3 fatty acids and phycobiliproteins, have shown promise in improving lipid profiles, reducing inflammation, and supporting overall cardiovascular health. These compounds could be used as preventive measures or complementary therapies for managing cardiovascular diseases [74, 114].
Microalgae-based antioxidants have been investigated for their potential neuro-protective effects against neurodegenerative diseases. Astaxanthin, in particular, has shown promise in preclinical studies as a potential therapeutic agent for conditions like Alzheimer’s and Parkinson’s disease [79]. Hesperidin has also proven to be effective in protecting against neurodegenerative diseases like Alzheimer’s. The discovery of innovative therapies for numerous diseases has been successfully accomplished using naturally occurring substances. Hesperidin has the potential to act as a structural model for the creation of novel treatments and appears to be therapeutically involved in Alzheimer’s signaling pathways. Several microalgae produce a wide variety of secondary metabolites, however, the distribution of hesperidin is poorly understood. Chlorella vulgaris, Chlorococcum hypnosporum (Chlorophyta), and Arthrospira platensis (Cyanophyceae) are microalgae species that can produce hesperidin. Additionally, hesperidin was found in the species of Chlamydomonas sp. (Chlorophyta), Nostoc sp., Anabaena sp., and Tolypothrix sp. (Cyanophyceae) [115].
Polysaccharides and polyphenols, which are derived from the microalgae C. vulgaris, Auxenochlorella pyrenoidosa, Dunaliella spp. (Chlorophyta), Porphyridium spp. (Rhodophyta), and Arthrospira spp. (Cyanophyceae), have shown anti-inflammatory potential. These substances could be researched for their potential value in treating autoimmune disorders and chronic inflammatory illnesses [74, 86]. C. vulgaris, Dunaliella salina, Tribonema sp. (Chlorophyta), and Euglena gracilis (Euglenophyceae) all produce chemicals that have been demonstrated to have immunomodulatory effects, increase immune responses, or prevent excessive inflammation. These substances may be used to strengthen the immune system and support immunity in a variety of medical disorders [116].
Some microalgae-derived compounds, like fucoxanthin, have been studied for their potential effects on metabolism and weight management. These compounds could be investigated further as part of strategies to combat obesity and metabolic syndrome [117]. Microalgae are an abundant and sustainable source of bioactive compounds, and advances in biotechnology could enable the large-scale production of these compounds for pharmaceutical and nutraceutical applications [38].
Challenges
While the potential applications of microalgae-derived compounds in preventive and therapeutic strategies are exciting, several challenges need to be addressed for successful translation from research to practical use. For example, ensuring sufficient bioavailability of microalgae-derived compounds in the human body remains a challenge, as their absorption, metabolism, and distribution can vary significantly [118].
Standardizing the cultivation, harvesting, and processing of microalgae is essential to maintain consistent quality and ensure the presence of bioactive compounds in the final products. Microalgae-derived compounds must go through rigorous testing and regulatory approval processes to ensure their safety and efficacy before they can be used in medical or pharmaceutical applications [119].
Making microalgae-derived compounds economically viable for large-scale production and commercialization is a significant hurdle that needs to be addressed. Raising public awareness and acceptance of microalgae-based products as preventive and therapeutic agents will be crucial for their successful integration into healthcare and nutrition practices [120].
Despite these challenges, ongoing research and advancements in biotechnology, along with a deeper understanding of microalgae biology and biochemistry, are likely to drive the realization of the potential applications of microalgae-derived compounds in preventive and therapeutic strategies, improving human health and well-being in the future [121].
Regulatory considerations for microalgae-derived antioxidant products
When it comes to microalgae-derived antioxidant products, navigating the regulatory landscape is a crucial aspect of their development and marketing. These products, which capitalize on the natural antioxidant properties of microalgae compounds, must comply with a range of regulations to ensure their safety, efficacy, and accurate labeling [6, 13, 20]. Depending on the jurisdiction, these considerations might encompass aspects such as ingredient sourcing, production processes, quality control measures, and potential health claims. Regulatory bodies like the Food and Drug Administration (FDA) in the United States or the European Food Safety Authority (EFSA) in Europe play pivotal roles in assessing the scientific evidence supporting antioxidant claims, determining acceptable dosage levels, and evaluating potential adverse effects. As the popularity of microalgae-derived antioxidant products grows, manufacturers must work closely with regulatory experts to ensure compliance and provide consumers with reliable and safe offerings that meet the highest standards of quality and transparency [122–124].
Challenges and opportunities in the field
The field of microalgae research and applications presents a dynamic landscape filled with both challenges and opportunities. One of the major challenges lies in optimizing cultivation techniques to ensure consistent and high biomass yields, which requires addressing issues related to contamination, nutrient supply, and growth conditions [125, 126]. Additionally, cost-effective harvesting and extraction methods for valuable compounds, such as antioxidants, remain areas of active exploration. Regulatory complexities also pose challenges, as the development of microalgae-based products must align with evolving guidelines. However, amidst these challenges, there are promising opportunities [101, 127].
Microalgae hold immense potential as sustainable sources of bioactive compounds, including antioxidants, with applications in pharmaceuticals, cosmetics, and functional foods. Their rapid growth rates and ability to thrive in diverse environments offer an avenue for addressing food security and environmental sustainability [128]. Research into novel cultivation systems, genetic modification, and biotechnological advancements opens doors to unlocking the full potential of microalgae [126]. Collaborations between researchers, industries, and regulatory bodies are key to harnessing these opportunities and overcoming the obstacles on the path to realizing the transformative potential of microalgae-derived products [119, 121, 129].
Future perspectives
The exploration of microalgae as a source of antioxidants heralds a promising era in wellness and longevity research, embodying the essence of a green revolution. The multi-faceted benefits of microalgae-derived antioxidants extend beyond their potential to combat oxidative stress, reaching into various sectors such as healthcare, cosmetics, and nutraceuticals [18]. However, this transformative journey is not devoid of challenges. While scientific advancements propel us toward harnessing the full potential of these microorganisms, hurdles related to scalability, cost-effectiveness, and regulatory compliance underscore the need for continued interdisciplinary collaboration. As microalgae pave the way for sustainable solutions, bridging the gap between research, industry, and policy will be pivotal in realizing the profound impact of this green revolution on human health and the environment. As we stand at the nexus of innovation, there is a palpable sense that the symbiotic relationship between microalgae and human wellness is poised to shape a healthier, more sustainable future [130].
Microalgae as a rich source of antioxidants with far-reaching implications. These antioxidants hold promise in combating oxidative stress and its associated health concerns. The study emphasizes the versatility of microalgae-derived antioxidants, not only in traditional healthcare but also across industries like cosmetics and nutraceuticals [6, 13, 20, 93]. However, it underscores challenges related to scalability, cost-effectiveness, and regulatory alignment, which demand concerted efforts from researchers, industries, and policymakers. The findings elucidate the pressing need for collaborative initiatives to bridge scientific advancements with practical applications, while recognizing the transformative power of microalgae in catalyzing a sustainable green revolution for human health and the environment [131].
The significance of microalgae-based antioxidants in promoting health and longevity cannot be overstated. Astaxanthin, zeaxanthin, lutein, β-cryptoxanthin, fucoxanthin, and canthaxanthin serve variously as anti-inflammatory, antioxidant, and anti-tumor agents [132]. Astaxanthin gained approval from the United States FDA in 1987 as a feed additive within the aquaculture industry. Subsequently, in 1999, it received additional approval for utilization as a dietary supplement [133]. The EFSA has reconfirmed its 2017 sanction permitting the utilization of astaxanthin-rich oleoresin derived from Haematococcus lacustris (as Haematococcus pluvialis) microalgae in food supplements, endorsing levels of up to 8 mg per day. This approval aligns with the growing demand among consumers for natural ingredients and products bearing clear labels. Notably, EFSA’s approval pertains specifically to microalgae-derived astaxanthin, omitting its synthetic and yeast-based counterparts due to insufficient safety data and limited established human clinical trials [134]. Safety of a change in specifications of the novel food oleoresin from H. lacustris containing astaxanthin pursuant to regulation [European Union (EU)] 2015/2283 [135].
These natural compounds offer a unique and potent approach to combating the detrimental effects of oxidative stress, a key contributor to aging and various chronic diseases. Microalgae, as a source of diverse antioxidant molecules, possess the capacity to neutralize harmful free radicals, reduce cellular damage, and enhance overall cellular health. Their potential to support various physiological processes, including immune function, cardiovascular health, and cognitive function, underscores their role in extending healthy lifespans [136].
Moreover, microalgae-derived antioxidants introduce an eco-friendly and sustainable avenue for wellness. By harnessing these compounds, industries can reduce reliance on synthetic antioxidants, contributing to environmentally responsible practices. The cultivation of microalgae can also address food security and resource scarcity concerns, offering a viable source of nutritionally rich ingredients for functional foods and supplements. The expansive applications of microalgae-based antioxidants, ranging from pharmaceuticals to cosmetics, amplify their significance in modern healthcare and well-being. As research continues to unravel the intricate mechanisms behind their health benefits, integrating these natural wonders into daily routines could potentially redefine the way we approach aging and longevity, paving the way for a healthier and more vibrant future [137, 138].
Exploring synergies between microalgae-based antioxidants and other bioactive compounds could lead to novel combinations with enhanced health benefits. Integrating these compounds into functional foods, personalized nutrition plans, and preventive healthcare strategies holds the potential to revolutionize how we approach longevity and well-being [27, 74]. Lastly, as we advance in this field, considerations of environmental sustainability must remain at the forefront. Research into cultivating microalgae using renewable energy sources and wastewater treatment could align with broader ecological goals while enhancing the economic viability of microalgae-based industries [139].
So, the future of microalgae-based antioxidants is ripe with possibilities, spanning scientific, industrial, and ecological realms. As research continues to unfold, these prospects have the potential to reshape health paradigms, rejuvenate industries, and contribute to a more sustainable and health-conscious global future [4, 140].
Conclusions
The exploration of microalgae-based antioxidants opens the door to exciting future directions and research implications. Firstly, refining cultivation techniques and optimizing growth conditions will be crucial for enhancing biomass yield and the concentration of valuable compounds. Developing cost-effective and scalable harvesting and extraction methods is another avenue for innovation. Genetic modification and strain selection offer the potential to tailor microalgae for higher antioxidant production and improved nutritional profiles.
Because of their high variety, quick growth, and product contributions in many industries, microalgae are vital resources for human needs as an alternative to terrestrial plants. The efficiency and success of biomedical products obtained from microalgal biomass, or its metabolites are mostly determined by the technology utilized in microalgal biomass production, harvesting, drying, and extraction. Innovations, ideas, and technologies that improve and expand the economic viability of algal biomass production and accompanying technologies are required. Where there is no counterpart in terrestrial organisms, technology can be utilized in the extraction and subsequent phases.
In-depth mechanistic studies are needed to unravel the precise ways in which microalgae-derived antioxidants interact with cellular processes and mitigate oxidative stress. Understanding these mechanisms will provide a foundation for targeted therapeutic interventions and personalized approaches to wellness. Collaborative efforts between researchers, industries, and regulatory bodies will be vital to establish standardized quality control measures and safety assessments for microalgae-derived products. This will ensure consumer confidence and facilitate their integration into mainstream health and wellness markets.
Abbreviations
| ABTS: | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH: | 2,2-diphenyl-1-picrylhydrazyl |
| EFSA: | European Food Safety Authority |
| ROS: | reactive oxygen species |
| SOD: | superoxide dismutase |
| TLC: | thin-layer chromatography |
| UV: | ultraviolet |
Declarations
Author contributions
LP, JC, and AV: Conceptualization, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.
 A
A